Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drug Administration
2.2. MicroCT Analysis
2.3. Histology
2.4. Immunohistochemistry
3. Results
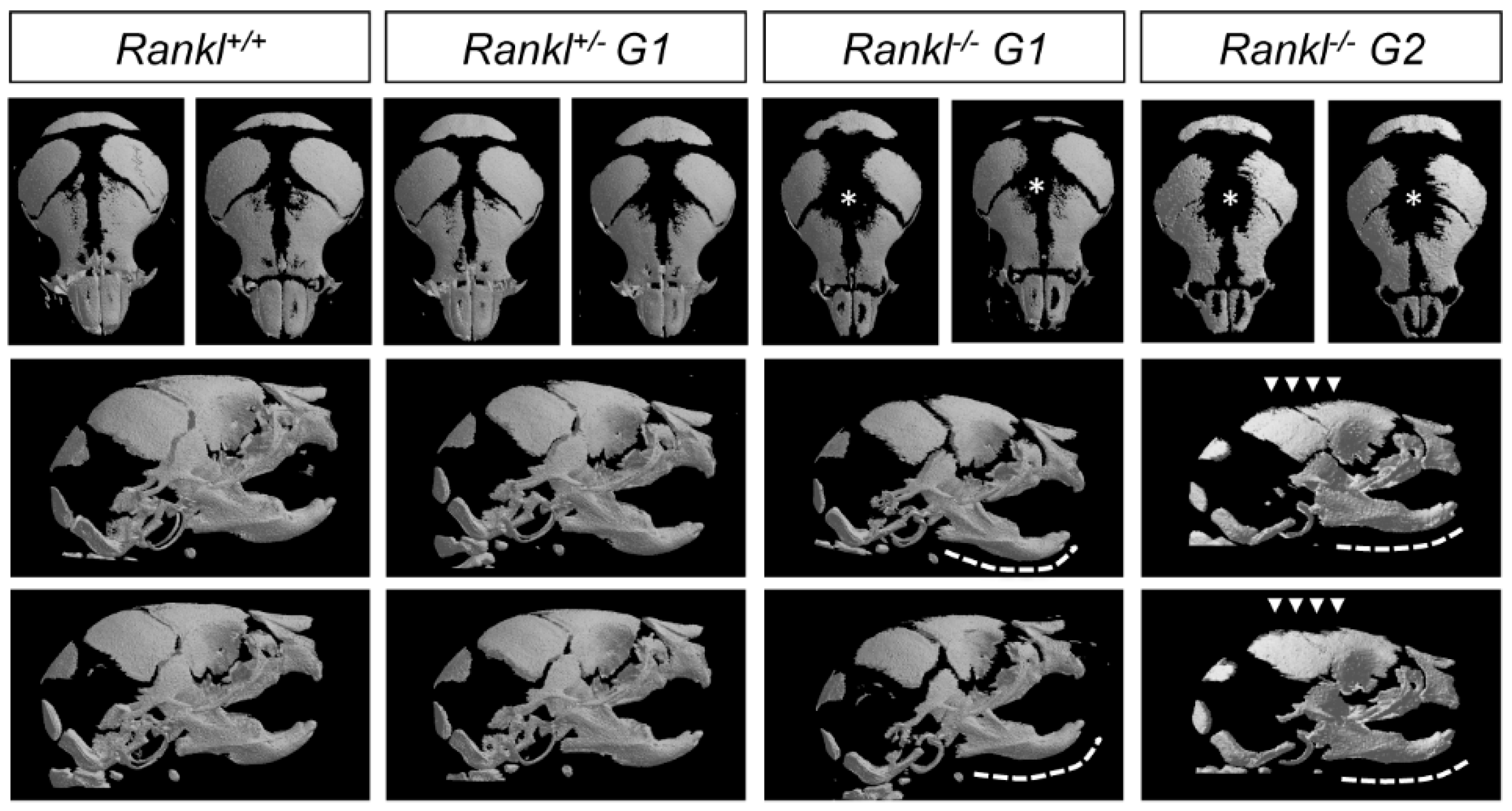
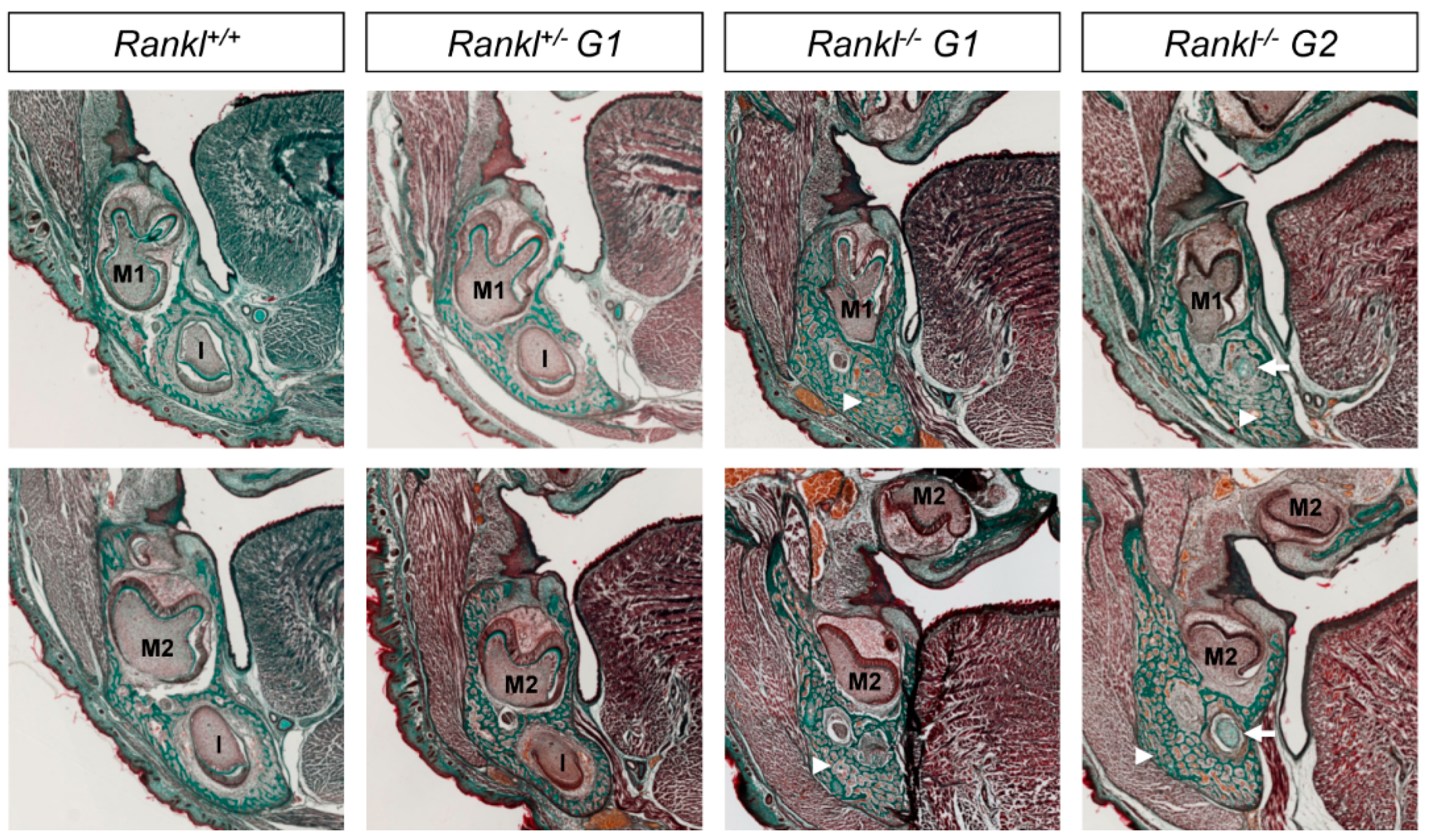
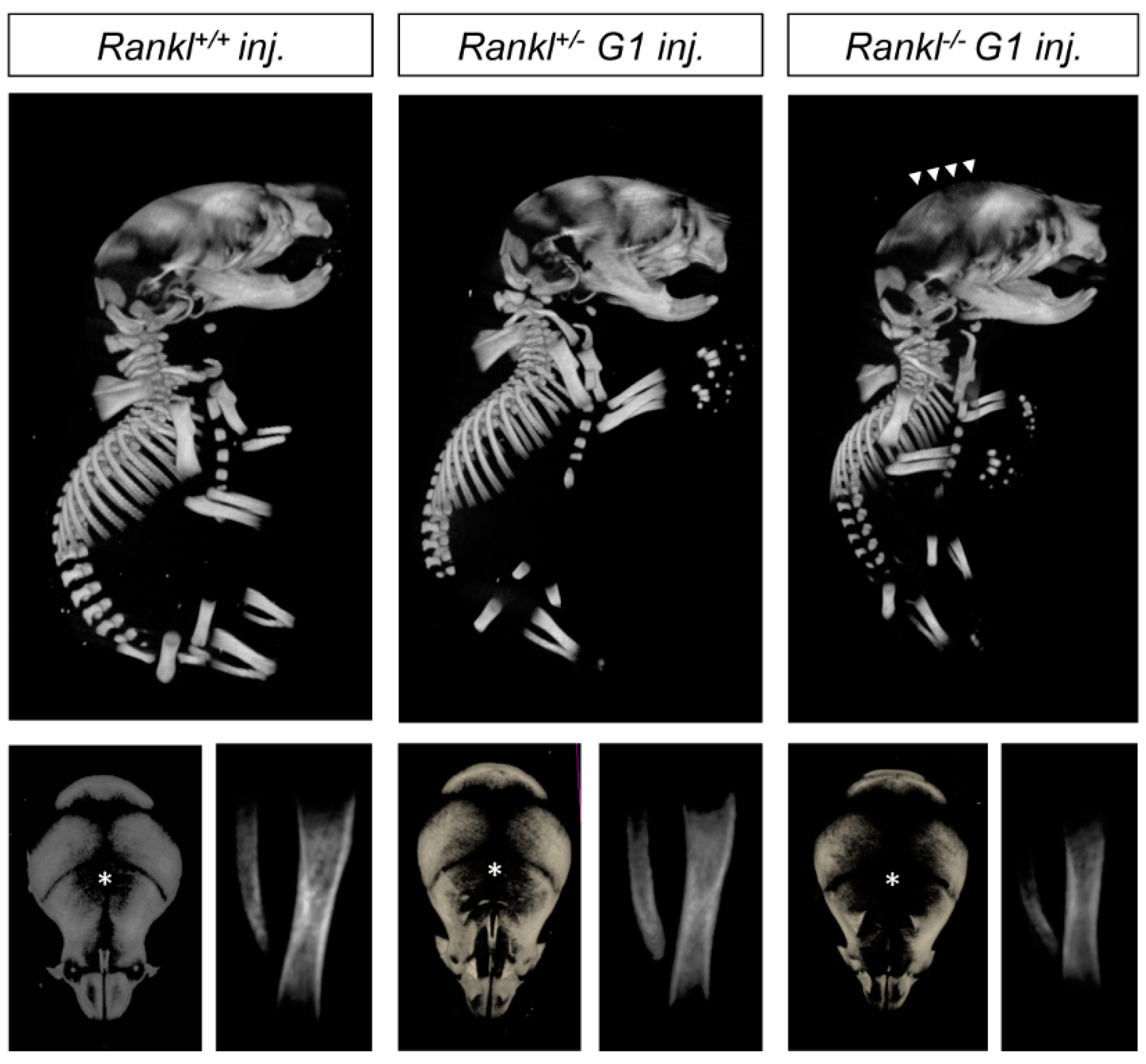
3.1. Second-Generation Rankl Null Mutants Had a More Severe Craniofacial Phenotype
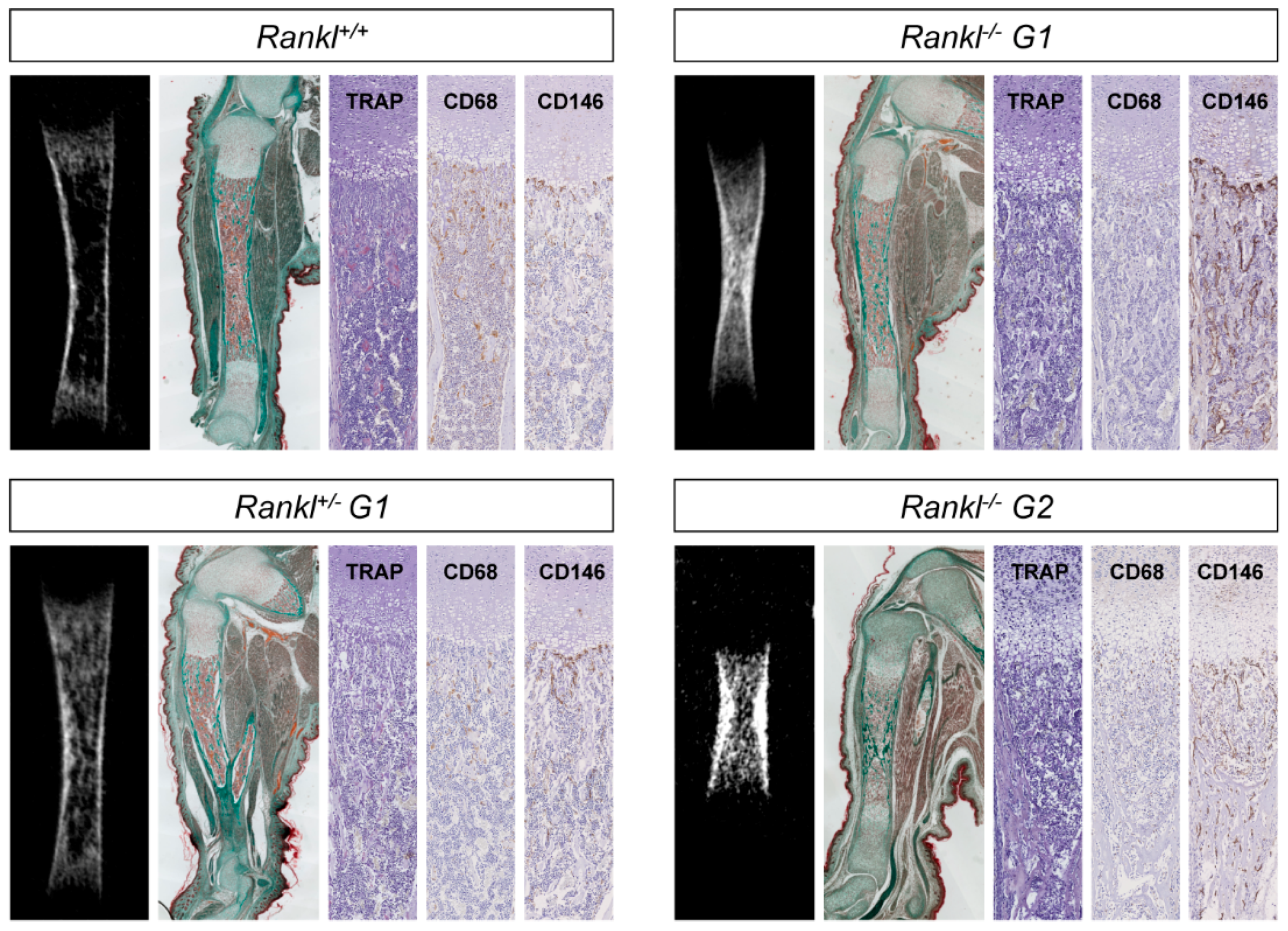
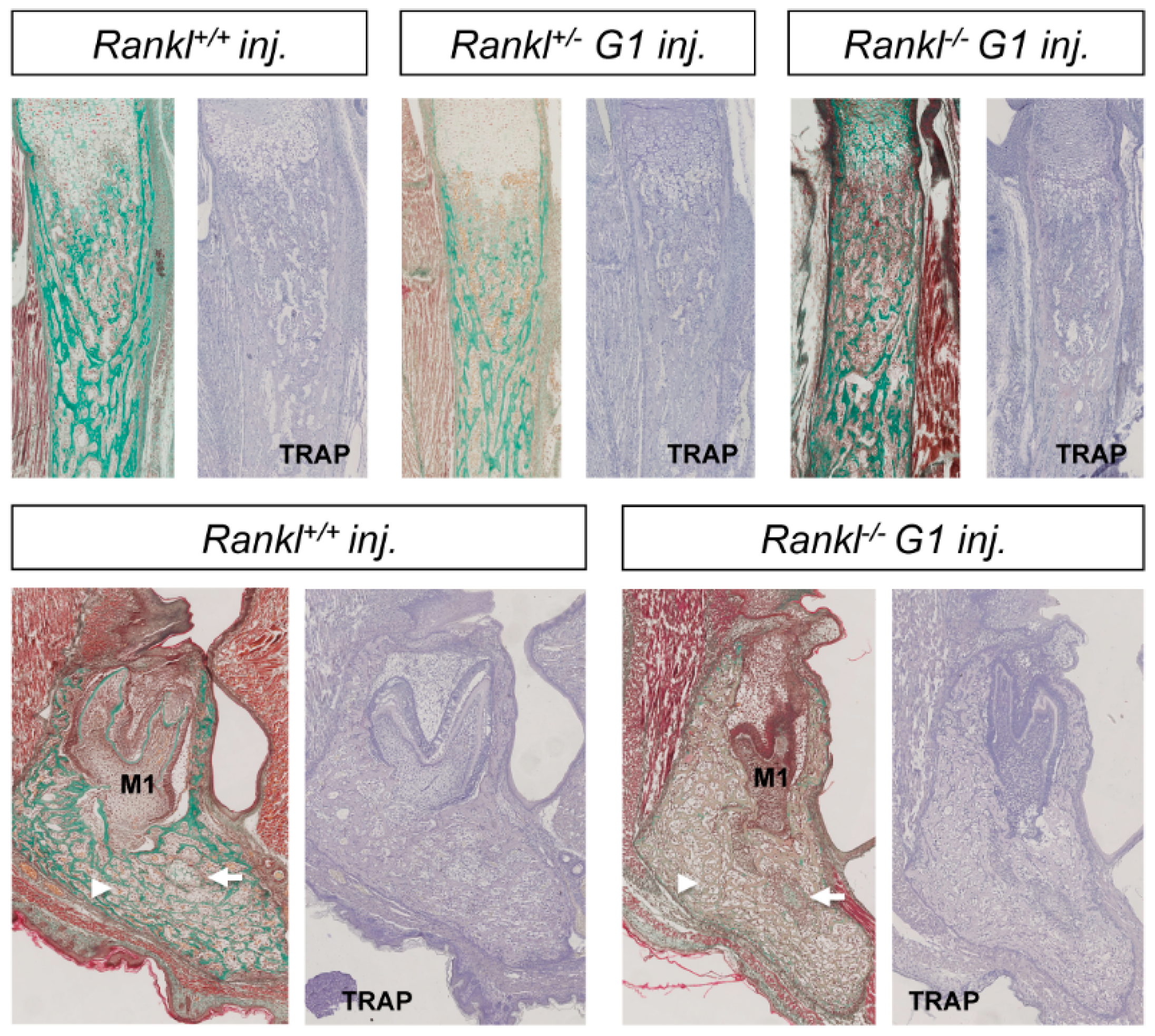
3.2. Second-Generation Rankl Null Mutants Had a More Severe Long Bone Phenotype
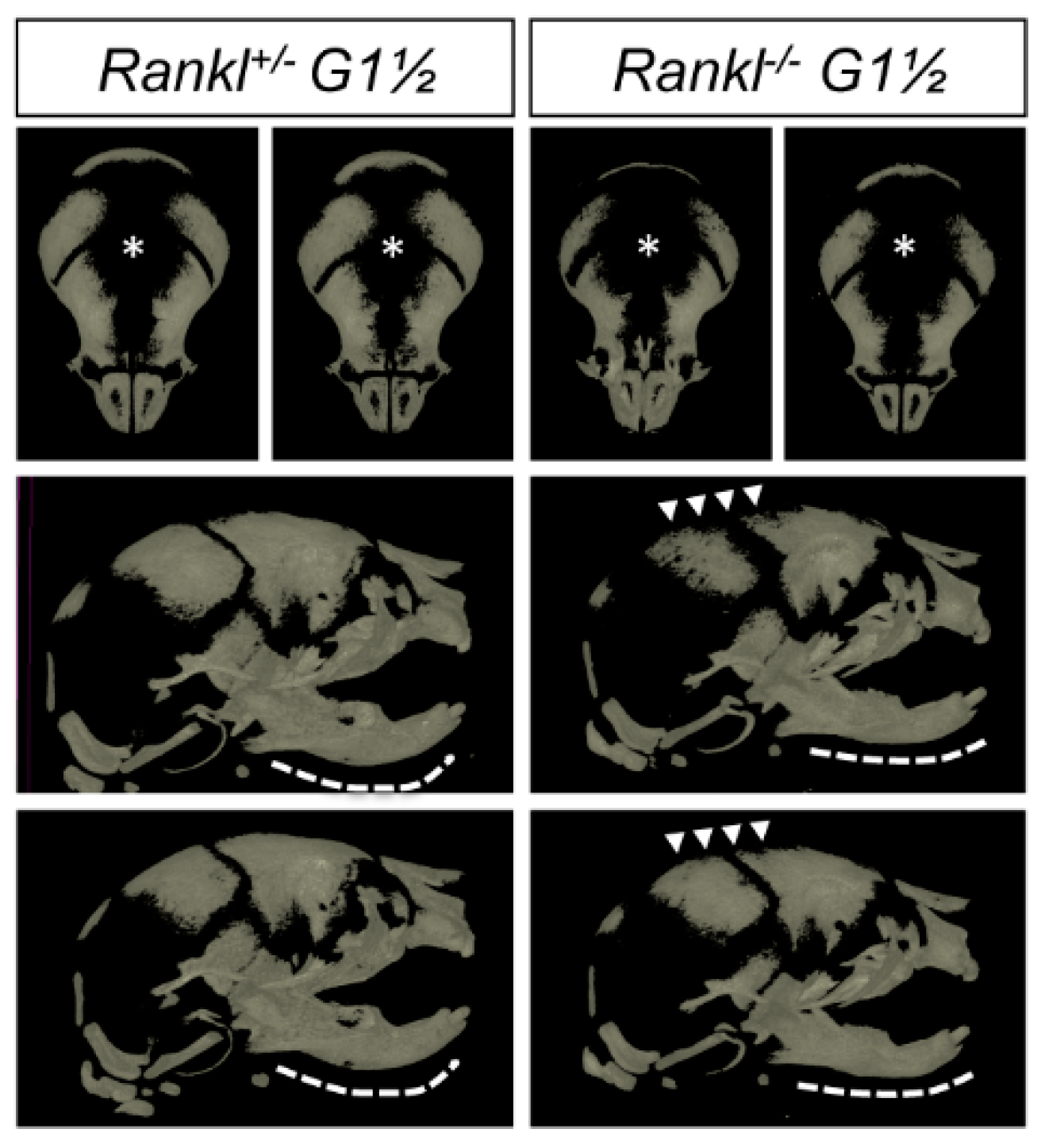
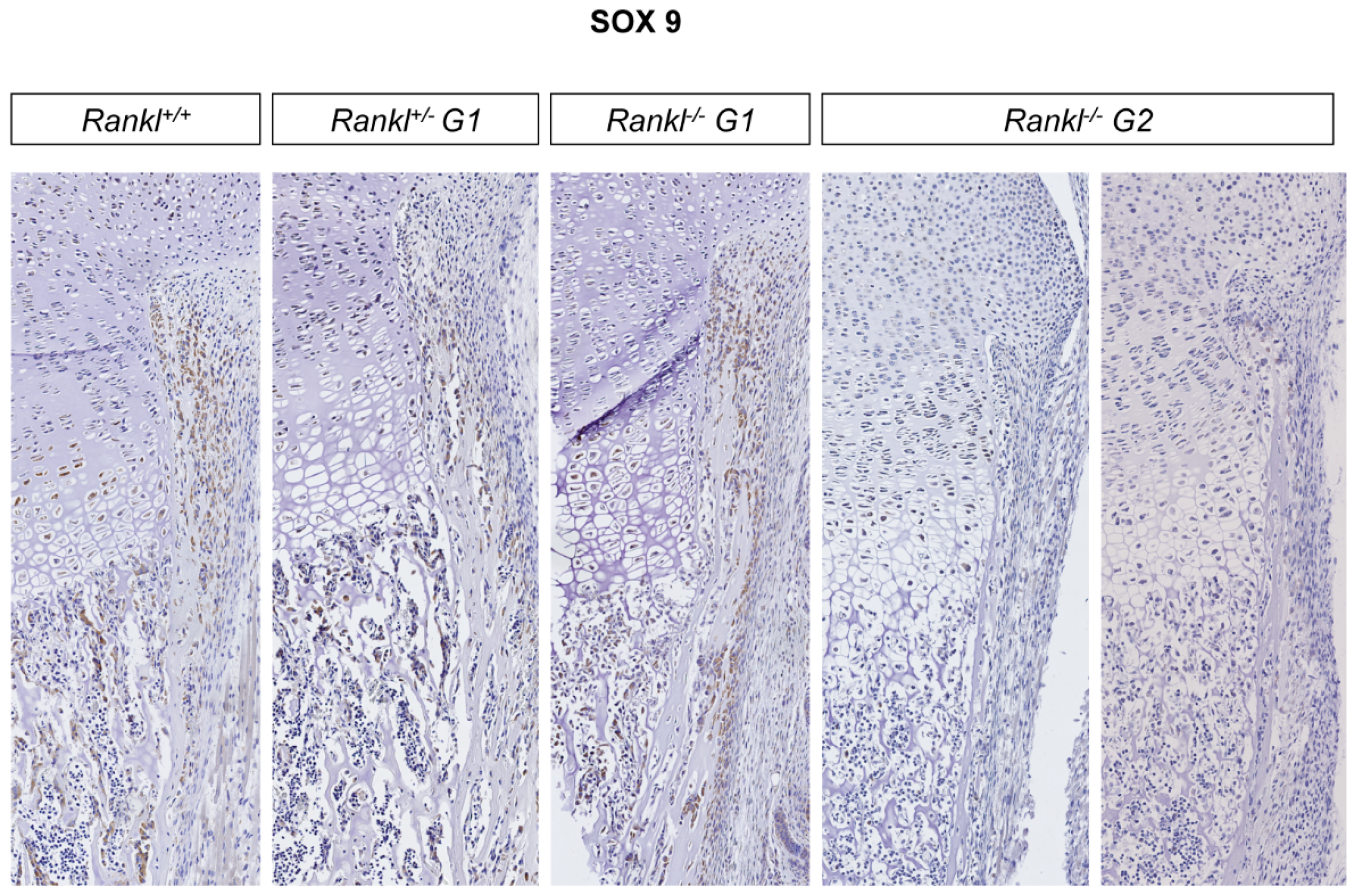
3.3. IK22-5 RANKL-Blocking Antibody Injections in Pregnant Heterozygous Mice Induced a Second-Generation-Like Phenotype in Null Mutant Pups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Boyle, W.J.; Penninger, J.M. Osteoprotegerin ligand: A common link between osteoclastogenesis, lymph node formation and lymphocyte development. Immunol. Cell Biol. 1999, 77, 188–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fata, J.E.; Kong, Y.Y.; Li, J.; Sasaki, T.; Irie-Sasaki, J.; Moorehead, R.A.; Elliott, R.; Scully, S.; Voura, E.B.; Lacey, D.L.; et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103, 41–50. [Google Scholar] [CrossRef]
- Kim, D.; Mebius, R.E.; MacMicking, J.D.; Jung, S.; Cupedo, T.; Castellanos, Y.; Rho, J.; Wong, B.R.; Josien, R.; Kim, N.; et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J. Exp. Med. 2000, 192, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Odgren, P.R.; Kim, D.K.; Marks, S.C.; Choi, Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc. Natl. Acad. Sci. USA 2000, 97, 10905–10910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, L.; Schwarz, E.M.; Boyce, B.F. Osteoclast precursors, RANKL/RANK, and immunology. Immunol. Rev. 2005, 208, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Nakato, G.; Jinnohara, T.; Akiba, H.; Okumura, K.; Ohno, H.; Yoshida, H. Expression pattern changes and function of RANKL during mouse lymph node microarchitecture development. Int. Immunol. 2012, 24, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Habbeddine, M.; Verthuy, C.; Rastoin, O.; Chasson, L.; Bebien, M.; Bajenoff, M.; Adriouch, S.; den Haan, J.M.M.; Penninger, J.M.; Lawrence, T. Receptor Activator of NF-κB Orchestrates Activation of Antiviral Memory CD8 T Cells in the Spleen Marginal Zone. Cell Rep. 2017, 21, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, Y.; Nitta, T.; Ohigashi, I.; Yano, K.; Ishimaru, N.; Hayashi, Y.; Matsumoto, M.; Matsuo, K.; Penninger, J.M.; Takayanagi, H.; et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 2008, 29, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Desanti, G.E.; Cowan, J.E.; Baik, S.; Parnell, S.M.; White, A.J.; Penninger, J.M.; Lane, P.J.L.; Jenkinson, E.J.; Jenkinson, W.E.; Anderson, G. Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J. Immunol. 2012, 189, 5519–5526. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.G.; Hess, E. Emerging Functions of RANKL in Lymphoid Tissues. Front. Immunol. 2012, 3, 261. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.; Duheron, V.; Decossas, M.; Lézot, F.; Berdal, A.; Chea, S.; Golub, R.; Bosisio, M.R.; Bridal, S.L.; Choi, Y.; et al. RANKL Induces Organized Lymph Node Growth by Stromal Cell Proliferation. J. Immunol. 2012, 188, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Duheron, V.; Hess, E.; Duval, M.; Decossas, M.; Castaneda, B.; Klöpper, J.E.; Amoasii, L.; Barbaroux, J.-B.; Williams, I.R.; Yagita, H.; et al. Receptor activator of NF-kappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc. Natl. Acad. Sci. USA 2011, 108, 5342–5347. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Courtney, J.-M.; Sharpe, P.T. Opg, Rank, and Rankl in tooth development: Co-ordination of odontogenesis and osteogenesis. J. Dent. Res. 2004, 83, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, B.; Simon, Y.; Jacques, J.; Hess, E.; Choi, Y.-W.; Blin-Wakkach, C.; Mueller, C.; Berdal, A.; Lézot, F. Bone resorption control of tooth eruption and root morphogenesis: Involvement of the receptor activator of NF-κB (RANK). J. Cell. Physiol. 2011, 226, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-S.; Kim, H.-J.; Koo, B.-K.; Kwon, M.-C.; Kim, Y.-W.; Cho, Y.; Yokota, Y.; Penninger, J.M.; Kong, Y.-Y. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 2006, 26, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Tanos, T.; Brisken, C. What signals operate in the mammary niche? Breast Dis. 2008, 29, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kartsogiannis, V.; Zhou, H.; Horwood, N.J.; Thomas, R.J.; Hards, D.K.; Quinn, J.M.; Niforas, P.; Ng, K.W.; Martin, T.J.; Gillespie, M.T. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 1999, 25, 525–534. [Google Scholar] [CrossRef]
- Sakakura, Y.; Tsuruga, E.; Irie, K.; Hosokawa, Y.; Nakamura, H.; Yajima, T. Immunolocalization of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) in Meckel’s cartilage compared with developing endochondral bones in mice. J. Anat. 2005, 207, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odgren, P.R.; Witwicka, H.; Reyes-Gutierrez, P. The cast of clasts: Catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect. Tissue Res. 2016, 57, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Kostakis, P.; Pan, B.; Farrugia, A.; Gronthos, S.; Evdokiou, A.; Harrison, K.; Findlay, D.M.; Zannettino, A.C.W. RANKL expression is related to the differentiation state of human osteoblasts. J. Bone Miner. Res. 2003, 18, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Frattini, A.; Guerrini, M.M.; Abinun, M.; Pangrazio, A.; Susani, L.; Bredius, R.; Mancini, G.; Cant, A.; Bishop, N.; et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 2007, 39, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Boyce, R.W.; Varela, A.; Chouinard, L.; Bussiere, J.L.; Chellman, G.J.; Ominsky, M.S.; Pyrah, I.T. Infant cynomolgus monkeys exposed to denosumab in utero exhibit an osteoclast-poor osteopetrotic-like skeletal phenotype at birth and in the early postnatal period. Bone 2014, 64, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Lézot, F.; Chesneau, J.; Navet, B.; Gobin, B.; Amiaud, J.; Choi, Y.; Yagita, H.; Castaneda, B.; Berdal, A.; Mueller, C.G.; et al. Skeletal consequences of RANKL-blocking antibody (IK22-5) injections during growth: Mouse strain disparities and synergic effect with zoledronic acid. Bone 2015, 73, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu, N.; Sakai, N.; Karakawa, A.; Kouyama, N.; Sato, Y.; Inagaki, K.; Kiuchi, Y.; Oguchi, K.; Negishi-Koga, T.; Takami, M. Biological effects of anti-RANKL antibody administration in pregnant mice and their newborns. Biochem. Biophys. Res. Commun. 2017, 491, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, N.; Böcker, W.; Schönitzer, V.; Bähr, A.; Radic, T.; Fröhlich, T.; Wünsch, A.; Keßler, B.; Kurome, M.; Schilling, E.; et al. First inducible transgene expression in porcine large animal models. FASEB J. 2012, 26, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Kanno, T.; Hoshi, M.; Shibata, O.; Yano, K.; Fujise, N.; Kinosaki, M.; Yamaguchi, K.; Tsuda, E.; Murakami, A.; et al. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J. Bone Miner. Metab. 2002, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; Ralston, S.H.; Marken, J.; Bell, C.; MacPherson, H.; Wallace, R.G.; van Hul, W.; Whyte, M.P.; Nakatsuka, K.; Hovy, L.; et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat. Genet. 2000, 24, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Palenzuela, L.; Vives-Bauza, C.; Fernández-Cadenas, I.; Meseguer, A.; Font, N.; Sarret, E.; Schwartz, S.; Andreu, A.L. Familial expansile osteolysis in a large Spanish kindred resulting from an insertion mutation in the TNFRSF11A gene. J. Med. Genet. 2002, 39, E67. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, B.; Simon, Y.; Ferbus, D.; Robert, B.; Chesneau, J.; Mueller, C.; Berdal, A.; Lézot, F. Role of RANKL (TNFSF11)-dependent osteopetrosis in the dental phenotype of Msx2 null mutant mice. PLoS ONE 2013, 8, e80054. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kasai, M.; Utsuyama, M.; Hirokawa, K. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology 2001, 142, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Sojod, B.; Chateau, D.; Mueller, C.G.; Babajko, S.; Berdal, A.; Lézot, F.; Castaneda, B. RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model. Front. Physiol. 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Penninger, J.M. RANK/RANKL: Regulators of immune responses and bone physiology. Ann. N. Y. Acad. Sci. 2008, 1143, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Chen, D.; Boyce, B.F. Mice Deficient in NF-κB p50 and p52 or RANK Have Defective Growth Plate Formation and Post-natal Dwarfism. Bone Res. 2013, 1, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.-H.; Zhou, W.-J.; Jin, L.-P.; Liu, L.-B.; Chang, K.-K.; Mei, J.; Li, H.; Wang, J.; Li, D.-J.; Li, M.-Q. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. 2017, 8, e3105. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, M.; Zaki, S.; Ramzi, A.-M.; Salem, M.E.; El-Minawi, A.M. Feto-maternal bone remodeling in normal pregnancy and preeclampsia. J. Soc. Gynecol. Investig. 2005, 12, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Gourgiotis, D.; Malamitsi-Puchner, A. Circulating osteoprotegerin and sRANKL concentrations in the perinatal period at term. The impact of intrauterine growth restriction. Neonatology 2009, 96, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Lambrinoudaki, I.; Rizos, D.; Armeni, E.; Alexandrou, A.; Creatsas, G. Maternal circulating osteoprotegerin and soluble RANKL in pre-eclamptic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Gong, Y.; Wang, T.; Chen, Y.; Jia, J.; Ni, S.; Zhou, B.; Song, Y.; Zhang, L.; Zhou, R. Expression of osteoprotegerin in placenta and its association with preeclampsia. PLoS ONE 2012, 7, e44340. [Google Scholar] [CrossRef] [PubMed]
- Tenta, R.; Bourgiezi, I.; Aliferis, E.; Papadopoulou, M.; Gounaris, A.; Skouroliakou, M. Bone metabolism compensates for the delayed growth in small for gestational age neonates. Organogenesis 2013, 9, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepka, R.; Dołęgowska, B.; Sałata, D.; Rajewska, A.; Budkowska, M.; Domański, L.; Kwiatkowski, S.; Mikołajek-Bedner, W.; Torbé, A. Soluble receptors for advanced glycation end products and receptor activator of NF-κB ligand serum levels as markers of premature labor. BMC Pregnancy Childbirth 2015, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; von der Mark, H.; et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Dev. Camb. Engl. 2010, 137, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orestes-Cardoso, S.; Nefussi, J.R.; Lezot, F.; Oboeuf, M.; Pereira, M.; Mesbah, M.; Robert, B.; Berdal, A. Msx1 is a regulator of bone formation during development and postnatal growth: In vivo investigations in a transgenic mouse model. Connect. Tissue Res. 2002, 43, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, N.; Peters, H.; Tucker, A.S. Species-specific modifications of mandible shape reveal independent mechanisms for growth and initiation of the coronoid. EvoDevo 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Lézot, F.; Thomas, B.L.; Blin-Wakkach, C.; Castaneda, B.; Bolanos, A.; Hotton, D.; Sharpe, P.T.; Heymann, D.; Carles, G.F.; Grigoriadis, A.E.; et al. Dlx homeobox gene family expression in osteoclasts. J. Cell. Physiol. 2010, 223, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.A.; Majithia, A.; Booker, T.; Henderson, J.E.; Loomis, C.A. The homeoprotein engrailed 1 has pleiotropic functions in calvarial intramembranous bone formation and remodeling. Dev. Camb. Engl. 2006, 133, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navet, B.; Vargas-Franco, J.W.; Gama, A.; Amiaud, J.; Choi, Y.; Yagita, H.; Mueller, C.G.; Rédini, F.; Heymann, D.; Castaneda, B.; et al. Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups. J. Clin. Med. 2018, 7, 426. https://doi.org/10.3390/jcm7110426
Navet B, Vargas-Franco JW, Gama A, Amiaud J, Choi Y, Yagita H, Mueller CG, Rédini F, Heymann D, Castaneda B, et al. Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups. Journal of Clinical Medicine. 2018; 7(11):426. https://doi.org/10.3390/jcm7110426
Chicago/Turabian StyleNavet, Benjamin, Jorge William Vargas-Franco, Andrea Gama, Jérome Amiaud, Yongwon Choi, Hideo Yagita, Christopher G. Mueller, Françoise Rédini, Dominique Heymann, Beatriz Castaneda, and et al. 2018. "Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups" Journal of Clinical Medicine 7, no. 11: 426. https://doi.org/10.3390/jcm7110426







