Role of the Slug Transcription Factor in Chemically-Induced Skin Cancer
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal Studies
2.2. Histology and Image Analysis
2.3. Cell Studies
2.4. RNA Isolation and RT-PCR
2.5. Western Blotting
2.6. Prostaglandin E2 Levels
2.7. Statistics
3. Results and Discussion
3.1. Slug and Snail Are Expressed at Increased Levels in DMBA/TPA-Induced Tumors

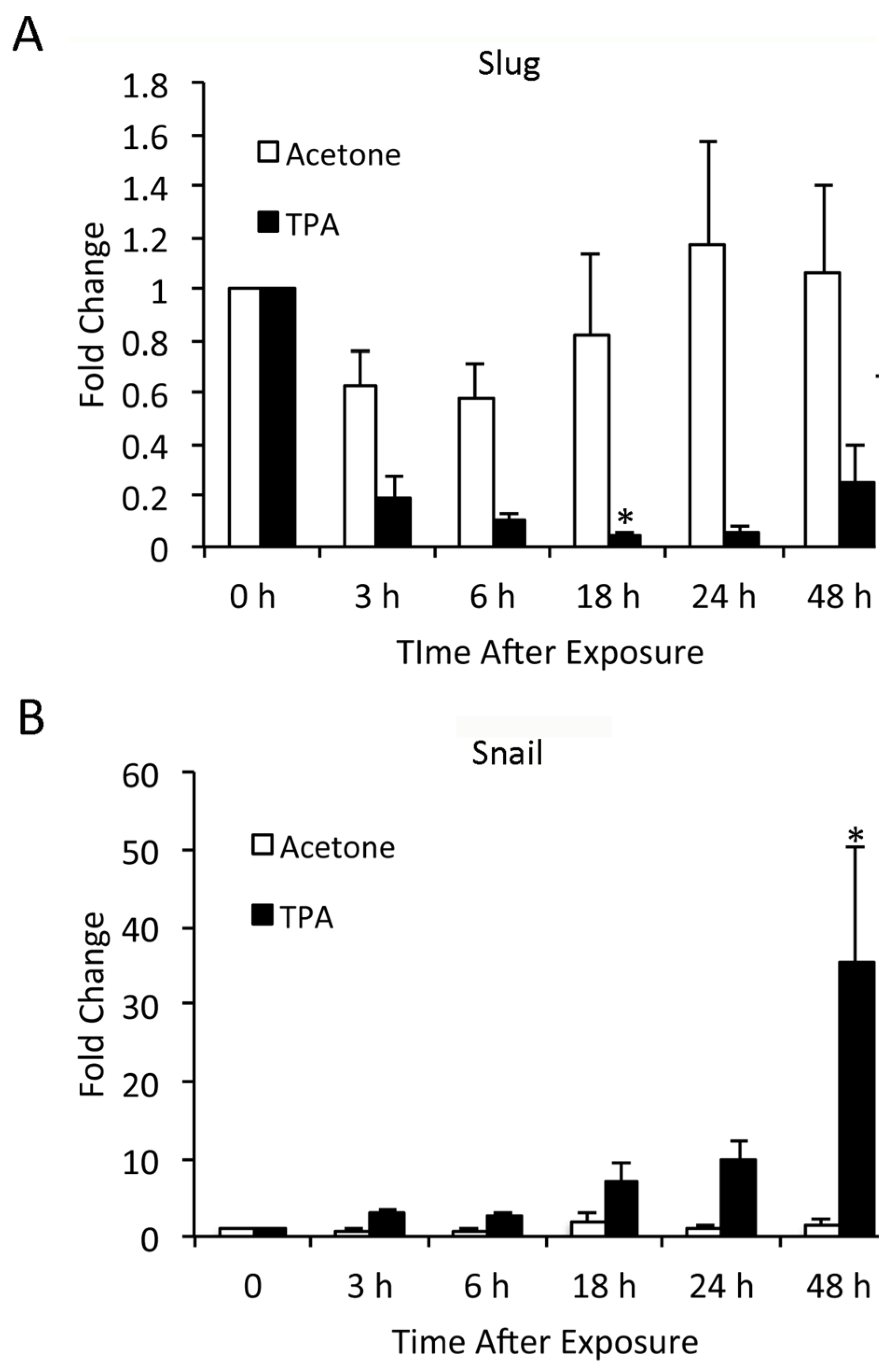
3.2. TPA Induces Expression of Slug and Snail in Mouse Epidermis
3.3. Slug Modulates TPA-Induced Epidermal Hyperplasia
3.4. Slug Plays a Role in TPA-Induced Acute Inflammation


3.5. COX-2 Expression is Unrelated to SLUG and Snail Induction by TPA
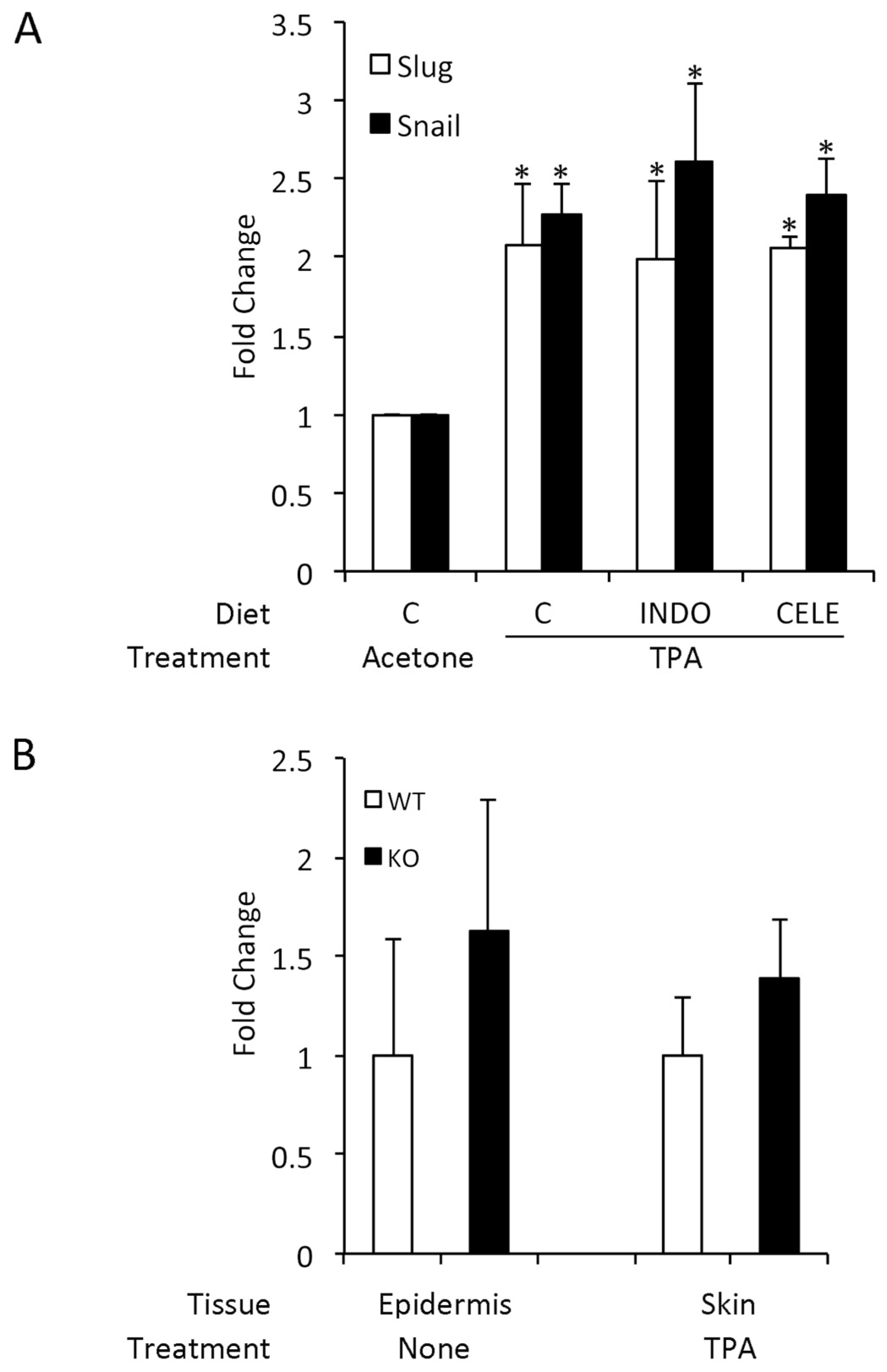
3.6. TPA-Induced Changes in Slug and Snail Expression in Keratinocytes In Vitro Differ from In Vivo Findings

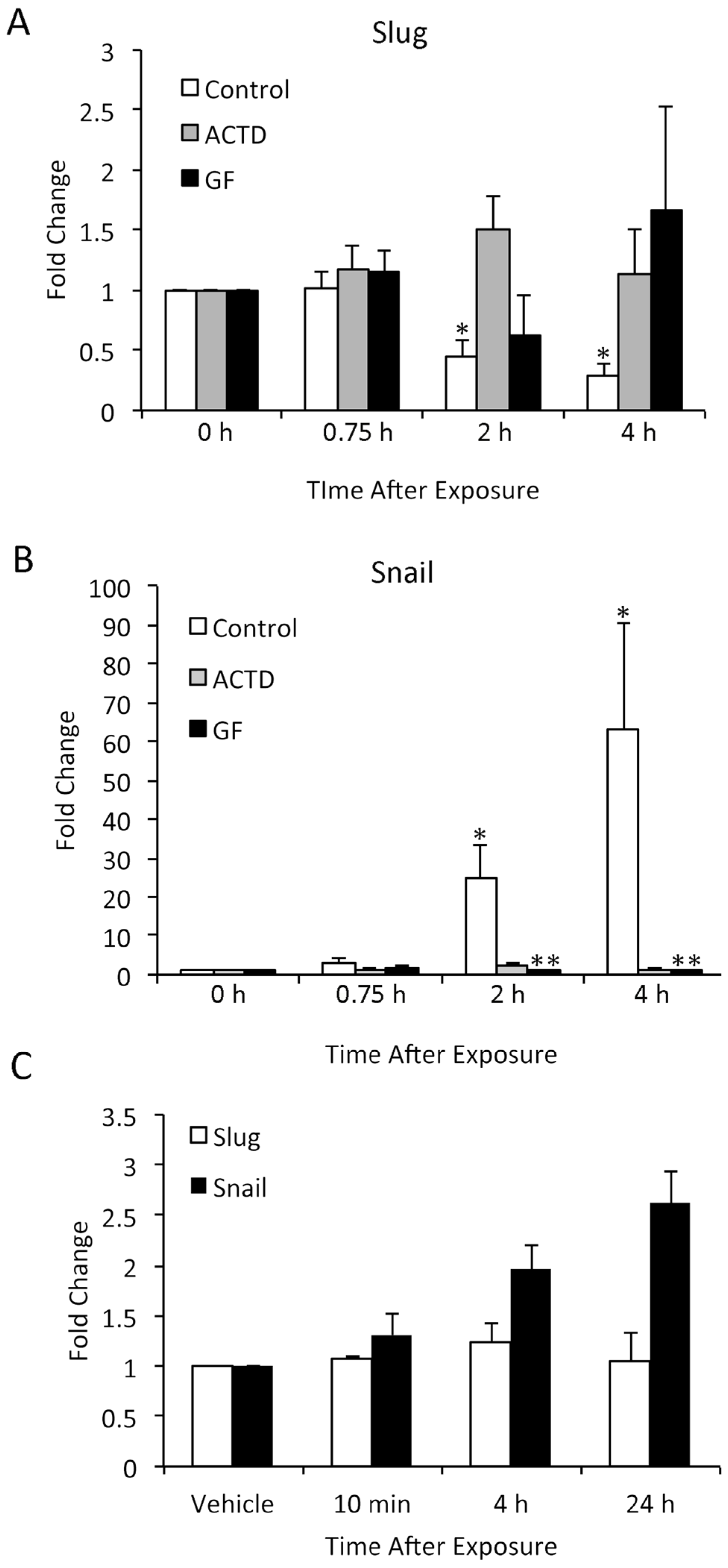
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hemavathy, K.; Ashraf, S.I.; Ip, Y.T. Snail/slug family of repressors: Slowly going into the fast lane of development and cancer. Gene 2000, 257, 1–12. [Google Scholar] [CrossRef]
- Murray, S.A.; Oram, K.F.; Gridley, T. Multiple functions of Snail family genes during palate development in mice. Development 2007, 134, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Franci, C.; Takkunen, M.; Dave, N.; Alameda, F.; Gomez, S.; Rodriguez, R.; Escriva, M.; Montserrat-Sentis, B.; Baro, T.; Garrido, M.; et al. Expression of Snail protein in tumor-stroma interface. Oncogene 2006, 25, 5134–5144. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. Leaving the neighborhood: Molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays 2001, 23, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.G.; Choi, C.; Newkirk, K.M.; Parkhani, J.; Cooper, K.L.; Lu, P.; Kusewitt, D.F. Ultraviolet radiation stimulates expression of Snail family transcription factors in keratinocytes. Mol. Carcinog. 2007, 46, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Parent, A.E.; Fossey, S.L.; Choi, C.; Chandler, H.L.; Rajala-Schultz, P.J.; Kusewitt, D.F. Snai2 expression enhances ultraviolet radiation-induced skin carcinogenesis. Am. J. Pathol. 2007, 171, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Nakamura, Y.; Tan, T.L.; Lee, P.; Lee, R.; Yu, B.; Jamora, C. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010, 70, 10080–10089. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Duncan, F.J.; Brannick, E.M.; Chandler, H.L.; Parent, A.E.; Kusewitt, D.F. The acute cutaneous inflammatory response is attenuated in Slug-knockout mice. Lab. Investig. 2008, 88, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Muller-Decker, K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: Pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011, 30, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E.; Fischer, S.M. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem. Photobiol. 2008, 84, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Judson, B.L.; Miyaki, A.; Kekatpure, V.D.; Du, B.; Gilleaudeau, P.; Sullivan-Whalen, M.; Mohebati, A.; Nair, S.; Boyle, J.O.; Granstein, R.D.; et al. UV radiation inhibits 15-hydroxyprostaglandin dehydrogenase levels in human skin: Evidence of transcriptional suppression. Cancer Prev. Res. 2010, 3, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; Backlund, M.G.; Buchanan, F.G.; Daikoku, T.; Holla, V.R.; Rosenberg, D.W.; Dey, S.K.; DuBois, R.N. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006, 66, 6649–6656. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.J.; Cha, W.H.; Lee, K.S. Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human bladder transitional cell carcinomas. Virchows Arch. 2010, 457, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Liu, C. Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin. Exp. Metastasis 2011, 28, 721–731. [Google Scholar] [CrossRef] [PubMed]
- St. John, M.A.; Dohadwala, M.; Luo, J.; Wang, G.; Lee, G.; Shih, H.; Heinrich, E.; Krysan, K.; Walser, T.; Hazra, S.; et al. Proinflammatory mediators upregulate snail in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.R.; Johnson, K.M.; Nemenoff, R.A.; Schiemann, W.P. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis 2008, 29, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Berry, J.A.; Shoher, A.; Ramakrishnan, V.; Lucci, A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int. J. Oncol. 2005, 26, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.K.; Kusewitt, D.F.; Hudson, L.G. Glyoxal leads to defective keratinocyte migration and down-regulation of Snai2. J. Dermatol. Sci. 2014, 73, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lan, Y.; Norton, C.R.; Sundberg, J.P.; Gridley, T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev. Biol. 1998, 198, 277–285. [Google Scholar] [CrossRef]
- Nicholson, L.J.; Pei, X.F.; Watt, F.M. Expression of E-cadherin, P-cadherin and involucrin by normal and neoplastic keratinocytes in culture. Carcinogenesis 1991, 12, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar] [PubMed]
- Hoot, K.E.; Lighthall, J.; Han, G.; Lu, S.L.; Li, A.; Ju, W.; Kulesz-Martin, M.; Bottinger, E.; Wang, X.J. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J. Clin. Investig. 2008, 118, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Nordenskjold, B.; Landberg, G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br. J. Cancer 2009, 101, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.H.; Hudson, L.G.; He, J.; Kusewitt, D.F. The skinny on Slug. Mol. Carcinog. 2010, 49, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Jamora, C.; Lee, P.; Kocieniewski, P.; Azhar, M.; Hosokawa, R.; Chai, Y.; Fuchs, E. A signaling pathway involving TGF-beta2 and snail in hair follicle morphogenesis. PLoS Biol. 2005, 3, e11. [Google Scholar]
- Maldve, R.E.; Fischer, S.M. Multifactor regulation of prostaglandin H synthase-2 in murine keratinocytes. Mol. Carcinog. 1996, 17, 207–216. [Google Scholar] [CrossRef]
- Mikulec, C.D.; Rundhaug, J.E.; Simper, M.S.; Lubet, R.A.; Fischer, S.M. The chemopreventive efficacies of nonsteroidal anti-inflammatory drugs: The relationship of short-term biomarkers to long-term skin tumor outcome. Cancer Prev. Res. 2013, 6, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Storci, G.; Sansone, P.; Mari, S.; D’Uva, G.; Tavolari, S.; Guarnieri, T.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Chieco, P.; et al. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J. Cell. Physiol. 2010, 225, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Falzon, M. Restoration of the anti-proliferative and anti-migratory effects of 1,25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells. Cancer Lett. 2015, 362, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Takahara, M.; Xie, L.; Takeuchi, S.; Tu, Y.; Nakahara, T.; Uchi, H.; Moroi, Y.; Furue, M. Levels of the EMT-related protein Snail/Slug are not correlated with p53/p63 in cutaneous squamous cell carcinoma. J. Cutan. Pathol. 2013, 40, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Hawley-Nelson, P.; Stanley, J.R.; Schmidt, J.; Gullino, M.; Yuspa, S.H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp. Cell Res. 1982, 137, 155–167. [Google Scholar] [CrossRef]
- Shih, J.Y.; Yang, P.C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011, 32, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Evers, B.M.; Zhou, B.P. Small C-terminal domain phosphatase enhances snail activity through dephosphorylation. J. Biol. Chem. 2009, 284, 640–648. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Maltzan, K.; Li, Y.; Rundhaug, J.E.; Hudson, L.G.; Fischer, S.M.; Kusewitt, D.F. Role of the Slug Transcription Factor in Chemically-Induced Skin Cancer. J. Clin. Med. 2016, 5, 21. https://doi.org/10.3390/jcm5020021
Von Maltzan K, Li Y, Rundhaug JE, Hudson LG, Fischer SM, Kusewitt DF. Role of the Slug Transcription Factor in Chemically-Induced Skin Cancer. Journal of Clinical Medicine. 2016; 5(2):21. https://doi.org/10.3390/jcm5020021
Chicago/Turabian StyleVon Maltzan, Kristine, Yafan Li, Joyce E. Rundhaug, Laurie G. Hudson, Susan M. Fischer, and Donna F. Kusewitt. 2016. "Role of the Slug Transcription Factor in Chemically-Induced Skin Cancer" Journal of Clinical Medicine 5, no. 2: 21. https://doi.org/10.3390/jcm5020021




