Maternal Serum Screening Markers and Adverse Outcome: A New Perspective
Abstract
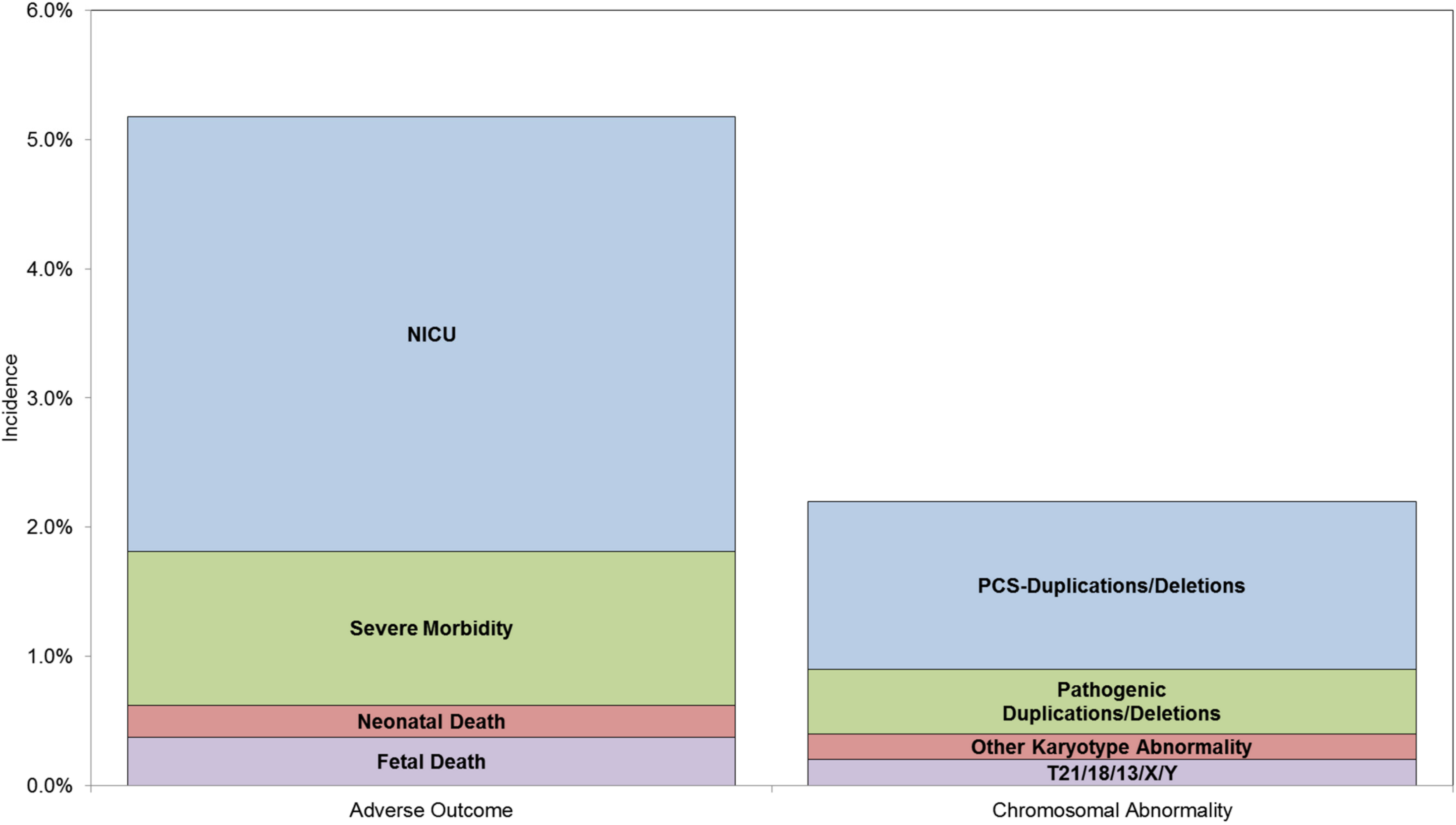
:1. Introduction
2. Preeclampsia
3. Intrauterine Growth Restriction

| Marker | Birth Weight Percentiles | p-Value * | ||
|---|---|---|---|---|
| ≤5th | ≤10th | 6th–10th | ||
| PAPP-A | 12.2 | 10.5 | 9.1 | 0.006 |
| AFP | 7.2 | 4.9 | 3.1 | <0.001 |
| hCG | 11.9 | 10.7 | 9.7 | 0.06 |
| uE3 | 2.7 | 2.2 | 1.8 | 0.104 |
| Inhibin | 13.1 | 10.5 | 8.5 | <0.001 |
4. Preterm Birth
| Author | Early Preterm | Preterm | ||||||
|---|---|---|---|---|---|---|---|---|
| GA at Delivery | DR (%) * | LR+ | LR− | GA at Delivery | DR (%) * | LR+ | LR− | |
| Ong et al. [54] | <34 | 14.9 | 2.98 | 0.90 | 34–36 | 5.5 | 1.10 | 0.99 |
| Smith et al. [53] | <32 | 14.0 | 2.80 | 0.91 | 32–36 | 9.5 | 1.90 | 0.95 |
| Krantz et al. [16] | <34 | 9.4 | 1.88 | 0.95 | - | - | - | - |
| Dugoff et al. [17] | <33 | 9.5 | 1.90 | 0.95 | 33–36 | 8.4 | 1.68 | 0.96 |
| Spencer et al. [78] | <34 | 12.4 | 2.48 | 0.92 | 34–36 | 9.2 | 1.84 | 0.96 |
| Spencer et al. [78] | <32 | 15.0 | 3.00 | 0.89 | 32–36 | 9.6 | 1.92 | 0.95 |
| Goetzinger et al. [79] | <34 | 20 | 2.00 | 0.89 | 34–36 | 24 | 2.40 | 0.84 |
| Goetzinger et al. [79] *,† | <34 | 38 | 3.80 | 0.69 | 34–36 | 38 | 3.80 | 0.69 |
5. Fetal Loss
6. Placenta Accreta
| Study | Protocol | Unaffected (n) | Cases (n) | DR (%) @ 5% FPR | LR+ | LR− |
|---|---|---|---|---|---|---|
| Loss Prior to NT | ||||||
| Krantz et al. [85] | PAPP-A | 6464 | 55 | 36 | 7.1 | 0.68 |
| Krantz et al. [85] | Free hCGβ | 6464 | 55 | 47 | 9.5 | 0.56 |
| Krantz et al. [85] | Free hCGβ + PAPP-A | 6464 | 55 | 49 | 9.8 | 0.54 |
| Early Fetal Loss | ||||||
| Goetzl et al. [86] | PAPP-A | 7932 | 75 | 12 | 2.4 | 0.93 |
| Dugoff et al. [87] | PAPP-A | 33,395 | 389 | 12 | 2.4 | 0.93 |
| Spencer et al. [88] | Free hCGβ | 47,770 | 230 | 12 | 2.4 | 0.93 |
| Spencer et al. [88] | PAPP-A | 47,770 | 230 | 15 | 3.0 | 0.89 |
| Goetzl et al. [86] | Free hCGβ | 7932 | 75 | 17 | 3.4 | 0.87 |
| Dugoff et al. [87] | PAPP-A + Characteristics † | 32,631 | 194 | 23 | 4.6 | 0.81 |
| Dugoff et al. [87] | uE3 | 32,631 | 194 | 24 | 4.8 | 0.80 |
| Dugoff et al. [87] | AFP | 32,631 | 194 | 29 | 5.8 | 0.75 |
| Dugoff et al. [87] | uE3 + Characteristics † | 32,631 | 194 | 32 | 6.4 | 0.72 |
| Dugoff et al. [87] | AFP + uE3 + PAPP-A | 32,631 | 194 | 35 | 7.0 | 0.68 |
| Dugoff et al. [87] | AFP + Characteristics † | 32,631 | 194 | 36 | 7.2 | 0.67 |
| Dugoff et al. [87] | AFP + uE3 + PAPP-A + Characteristics † | 32,631 | 194 | 39 | 7.8 | 0.64 |
| Huang et al. [62] | AFP + uE3 + hCG | 141,698 | 296 | 42 | 8.4 | 0.61 |
| Late Fetal Loss | ||||||
| Goetzl et al. [86] | PAPP-A | 7932 | 75 | 3 | 0.6 | 1.02 |
| Goetzl et al. [86] | Free hCGβ | 7932 | 75 | 7 | 1.4 | 0.98 |
| Spencer et al. [88] | PAPP-A | 47,770 | 225 | 9 | 1.8 | 0.96 |
| Dugoff et al. [87] | PAPP-A | 33,395 | 389 | 11 | 2.2 | 0.94 |
| Spencer et al. [88] | Free hCGβ | 47,770 | 225 | 12 | 2.4 | 0.93 |
| Smith et al. [90] | Free hCGβ | 8817 | 22 | 14 | 2.8 | 0.91 |
| Dugoff et al. [87] | Inhibin | 32,631 | 194 | 17 | 3.4 | 0.87 |
| Smith et al. [90] | PAPP-A | 8817 | 22 | 18 | 3.6 | 0.86 |
| Dugoff et al. [87] | Inhibin + Characteristics ‡ | 32,631 | 194 | 20 | 4.0 | 0.84 |
7. Open Neural Tube Defects
8. Conclusions
| Preeclampsia | Preterm Birth | IUGR | ||||
|---|---|---|---|---|---|---|
| Description | Early Onset <34 Weeks | Late Onset ≥34 Weeks | <34 Weeks | 34–36 Weeks | Abnormal UA | Normal UA |
| Incidence | 0.4% | 2.7% | 2.1% | 8.2% | 3.1% | 6.3% |
| Rate of Severe Morbidity and Mortality | 25% | 2.5% | 10% | 2.6% | 13% | 1.4% |
Author Contributions
Conflicts of Interest
References
- Macri, J.N.; Weiss, R.R. Prenatal serum alpha-fetoprotein screening for neural tube defects. Obstet. Gynecol. 1982, 59, 633–639. [Google Scholar]
- UK Collaborative Study. Estimating an individual’s risk of having a fetus with open spina bifida and the value of repeat alpha-fetoprotein testing. Fourth report of the UK collaborative study on alpha-fetoprotein in relation to neural tube defects. J. Epidemiol. Community Health 1982, 36, 87–95. [Google Scholar] [CrossRef]
- Krantz, D.A.; Larsen, J.W.; Buchanan, P.D.; Macri, J.N. First-trimester Down syndrome screening: Free beta-human chorionic gonadotropin and pregnancy-associated plasma protein A. Am. J. Obstet. Gynecol. 1996, 174, 612–616. [Google Scholar] [CrossRef]
- Snijders, R.J.; Noble, P.; Sebire, N.; Souka, A.; Nicolaides, K.H. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10–14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet 1998, 352, 343–346. [Google Scholar]
- Orlandi, F.; Damiani, G.; Hallahan, T.W.; Krantz, D.A.; Macri, J.N. First-trimester screening for fetal aneuploidy: Biochemistry and nuchal translucency. Ultrasound Obstet. Gynecol. 1997, 10, 381–386. [Google Scholar]
- Krantz, D.A.; Hallahan, T.W.; Orlandi, F.; Buchanan, P.; Larsen, J.W., Jr.; Macri, J.N. First-trimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstet. Gynecol. 2000, 96, 207–213. [Google Scholar] [CrossRef]
- Wapner, R.; Thom, E.; Simpson, J.L.; Pergament, E.; Silver, R.; Filkins, K.; Platt, L.; Mahoney, M.; Johnson, A.; Hogge, W.A.; et al. First-trimester screening for trisomies 21 and 18. N. Engl. J. Med. 2003, 349, 1405–1413. [Google Scholar] [CrossRef]
- Malone, F.D.; Canick, J.A.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Bukowski, R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N. Engl. J. Med. 2005, 353, 2001–2011. [Google Scholar] [CrossRef]
- Spencer, K.; Nicolaides, K.H. A first trimester trisomy 13/trisomy 18 risk algorithm combining fetal nuchal translucency thickness, maternal serum free beta-hCG and PAPP-A. Prenat. Diagn. 2002, 22, 877–879. [Google Scholar] [CrossRef]
- Cuckle, H.; Benn, P.; Wright, D. Down syndrome screening in the first and/or second trimester: Model predicted performance using meta-analysis parameters. Semin. Perinatol. 2005, 29, 252–257. [Google Scholar] [CrossRef]
- Cicero, S.; Curcio, P.; Papageorghiou, A.; Sonek, J.; Nicolaides, K. Absence of nasal bone in fetuses with trisomy 21 at 11–14 weeks of gestation: An observational study. Lancet 2001, 358, 1665–1667. [Google Scholar] [CrossRef]
- Davenport, D.M.; Macri, J.N. The clinical significance of low maternal serum alpha-fetoprotein. Am. J. Obstet. Gynecol. 1983, 146, 657–661. [Google Scholar]
- Katz, V.L.; Chescheir, N.C.; Cefalo, R.C. Unexplained elevations of maternal serum alpha-fetoprotein. Obstet. Gynecol. Surv. 1990, 45, 719–726. [Google Scholar] [CrossRef]
- Gross, S.J.; Phillips, O.P.; Shulman, L.P.; Bright, N.L.; Dungan, J.S.; Simpson, J.L.; Elias, S. Adverse perinatal outcome in patients screen-positive for neural tube defects and fetal Down syndrome. Prenat. Diagn. 1994, 14, 609–613. [Google Scholar] [CrossRef]
- Spencer, K. Second-trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenat. Diagn. 2000, 20, 652–656. [Google Scholar]
- Krantz, D.; Goetzl, L.; Simpson, J.L.; Thom, E.; Zachary, J.; Hallahan, T.W.; Silver, R.; Pergament, E.; Platt, L.D.; Filkins, K.; et al. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2004, 191, 1452–1458. [Google Scholar] [CrossRef]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D.; et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451. [Google Scholar] [CrossRef]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Vidaver, J.; Sullivan, L.; Canick, J.A.; Lambert-Messerlian, G.M.; Porter, T.F.; Luthy, D.A.; Comstock, C.H.; et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet. Gynecol. 2005, 106, 260–267. [Google Scholar] [CrossRef]
- Dugoff, L.; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet. Gynecol. 2010, 115, 1052–1061. [Google Scholar] [CrossRef]
- Kuc, S.; Wortelboer, E.J.; van Rijn, B.B.; Franx, A.; Visser, G.H.; Schielen, P.C. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: A systematic review. Obstet. Gynecol. Surv. 2011, 66, 225–239. [Google Scholar] [CrossRef]
- Goetzl, L. Adverse pregnancy outcomes after abnormal first-trimester screening for aneuploidy. Clin. Lab. Med. 2010, 30, 613–628. [Google Scholar] [CrossRef]
- Gagnon, A.; Wilson, R.D.; Audibert, F.; Allen, V.M.; Blight, C.; Brock, J.A.; Désilets, V.A.; Johnson, J.A.; Langlois, S.; Summers, A.; et al. Obstetrical complications associated with abnormal maternal serum markers analytes. J. Obstet. Gynaecol. Can. 2008, 30, 918–949. [Google Scholar]
- Morris, R.K.; Cnossen, J.S.; Langejans, M.; Robson, S.C.; Kleijnen, J.; Ter Riet, G.; Mol, B.W.; van der Post, J.A.; Khan, K.S. Serum screening with Down’s syndrome markers to predict pre-eclampsia and small for gestational age: Systematic review and meta-analysis. BMC Pregnancy Childbirth 2008, 8. [Google Scholar] [CrossRef]
- Goetzinger, K.R.; Odibo, A.O. Screening for abnormal placentation and adverse pregnancy outcomes with maternal serum biomarkers in the second trimester. Prenat. Diagn. 2014. [Google Scholar] [CrossRef]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar]
- Ehrich, M.; Deciu, C.; Zwiefelhofer, T.; Tynan, J.A.; Cagasan, L.; Tim, R.; Lu, V.; McCullough, R.; McCarthy, E.; Nygren, A.O.; et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: A study in a clinical setting. Am. J. Obstet. Gynecol. 2011, 204. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E.; Neveux, L.M.; Ehrich, M.; van den Boom, D.; Bombard, A.T.; Deciu, C.; Grody, W.W.; et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet. Med. 2011, 13, 913–920. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Platt, L.D.; Goldberg, J.D.; Abuhamad, A.Z.; Sehnert, A.J.; Rava, R.P.; MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet. Gynecol. 2012, 119, 890–901. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Deciu, C.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E.; Neveux, L.M.; Ehrich, M.; van den Boom, D.; Bombard, A.T.; Grody, W.W.; et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet. Med. 2012, 14, 296–305. [Google Scholar] [CrossRef]
- Srinivasan, A.; Bianchi, D.W.; Huang, H.; Sehnert, A.J.; Rava, R.P. Noninvasive detection of fetal subchromosome abnormalities via deep sequencing of maternal plasma. Am. J. Hum. Genet. 2013, 92, 167–176. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet. Gynecol. 2012, 120, 1532–1534. [Google Scholar] [CrossRef]
- Cuckle, H.; Benn, P.; Pergament, E. Maternal cfDNA screening for Down syndrome—A cost sensitivity analysis. Prenat. Diagn. 2013, 33, 636–642. [Google Scholar] [CrossRef]
- Petersen, O.; Vogel, I.; Ekelund, C.; Hyett, J.; Tabor, A. Potential diagnostic consequences of applying non-invasive prenatal testing (NIPT); a population-based study from a country with existing first trimester screening. Ultrasound Obstet. Gynecol. 2014, 43, 265–271. [Google Scholar] [CrossRef]
- Poon, L.C.; Musci, T.; Song, K.; Syngelaki, A.; Nicolaides, K.H. Maternal plasma cell-free fetal and maternal DNA at 11–13 weeks’ gestation: Relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn. Ther. 2013, 33, 215–223. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209. [Google Scholar] [CrossRef]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef]
- Askie, L.M.; Duley, L.; Henderson-Smart, D.J.; Stewart, L.A. Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet 2007, 369, 1791–1798. [Google Scholar] [CrossRef]
- Bujold, E.; Roberge, S.; Lacasse, Y.; Bureau, M.; Audibert, F.; Marcoux, S.; Forest, J.C.; Giguère, Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet. Gynecol. 2010, 116, 402–414. [Google Scholar] [CrossRef]
- Roberge, S.; Villa, P.; Nicolaides, K.; Giguère, Y.; Vainio, M.; Bakthi, A.; Ebrashy, A.; Bujold, E. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: A systematic review and meta-analysis. Fetal Diagn. Ther. 2012, 31, 141–146. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.H.; Demers, S.; Villa, P.; Bujold, E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 491–499. [Google Scholar] [CrossRef]
- Meis, P.J.; Klebanoff, M.; Thom, E.; Dombrowski, M.P.; Sibai, B.; Moawad, A.H.; Spong, C.Y.; Hauth, J.C.; Miodovnik, M.; Varner, M.W.; et al. Prevention of recurrent preterm delivery by 17 alpha-ydroxyprogesterone caproate. N. Engl. J. Med. 2003, 348, 2379–2385. [Google Scholar] [CrossRef]
- Da Fonseca, E.B.; Bittar, R.E.; Carvalho, M.H.; Zugaib, M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double-blind study. Am. J. Obstet. Gynecol. 2003, 188, 419–424. [Google Scholar] [CrossRef]
- Fonseca, E.B.; Celik, E.; Parra, M.; Singh, M.; Nicolaides, K.H.; Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N. Engl. J. Med. 2007, 357, 462–469. [Google Scholar] [CrossRef]
- Owen, J.; Hankins, G.; Iams, J.D.; Berghella, V.; Sheffield, J.S.; Perez-Delboy, A.; Egerman, R.S.; Wing, D.A.; Tomlinson, M.; Silver, R.; et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am. J. Obstet. Gynecol. 2009, 201. [Google Scholar] [CrossRef]
- Berghella, V.; Rafael, T.J.; Szychowski, J.M.; Rust, O.A.; Owen, J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: A meta-analysis. Obstet. Gynecol. 2011, 117, 663–671. [Google Scholar] [CrossRef]
- Bruner, J.P.; Tulipan, N.; Paschall, R.L.; Boehm, F.H.; Walsh, W.F.; Silva, S.R.; Hernanz-Schulman, M.; Lowe, L.H.; Reed, G.W. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA 1999, 282, 1819–1825. [Google Scholar] [CrossRef]
- Sutton, L.N.; Adzick, N.S.; Bilaniuk, L.T.; Johnson, M.P.; Crombleholme, T.M.; Flake, A.W. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA 1999, 282, 1826–1831. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Desai, N.; Krantz, D.; Roman, A.; Fleischer, A.; Boulis, S.; Rochelson, B. Elevated first trimester PAPP-A is associated with increased risk of placenta accreta. Prenat. Diagn. 2014, 34, 159–162. [Google Scholar] [CrossRef]
- Zelop, C.; Nadel, A.; Frigoletto, F.D.; Pauker, S.; MacMillan, M.; Benacerraf, B.R. Placenta accreta/percreta/increta: A cause of elevated maternal serum alpha-fetoprotein. Obstet. Gynecol. 1992, 80, 693–694. [Google Scholar]
- Kupferminc, M.J.; Tamura, R.K.; Wigton, T.R.; Glassenberg, R.; Socol, M.L. Placenta accreta is associated with elevated maternal serum alpha-fetoprotein. Obstet. Gynecol. 1993, 82, 266–269. [Google Scholar]
- Yaron, Y.; Heifetz, S.; Ochshorn, Y.; Lehavi, O.; Orr-Urtreger, A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat. Diagn. 2002, 22, 778–782. [Google Scholar] [CrossRef]
- Smith, G.C.; Stenhouse, E.J.; Crossley, J.A.; Aitken, D.A.; Cameron, A.D.; Connor, J.M. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, pre eclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002, 87, 1762–1767. [Google Scholar] [CrossRef]
- Ong, C.Y.; Liao, A.W.; Spencer, K.; Munim, S.; Nicolaides, K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. Br. J. Obstet. Gynaecol. 2000, 107, 1265–1270. [Google Scholar] [CrossRef]
- Goetzinger, K.R.; Singla, A.; Gerkowicz, S.; Dicke, J.M.; Gray, D.L.; Odibo, A.O. Predicting the risk of pre-eclampsia between 11 and 13 weeks’ gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat. Diagn. 2010, 30, 1138–1142. [Google Scholar] [CrossRef]
- Spencer, K.; Cowans, N.J.; Nicolaides, K.H. Low levels of maternal serum PAPP-A in the first trimester and the risk of pre-eclampsia. Prenat. Diagn. 2008, 28, 7–10. [Google Scholar] [CrossRef]
- Kang, J.H.; Farina, A.; Park, J.H.; Kim, S.H.; Kim, J.Y.; Rizzo, N.; Elmakky, A.; Jun, H.S.; Hahn, W.B.; Cha, D.H. Down syndrome biochemical markers and screening for preeclampsia at first and second trimester: Correlation with the week of onset and the severity. Prenat. Diagn. 2008, 28, 704–709. [Google Scholar] [CrossRef]
- Olsen, R.N.; Woelkers, D.; Dunsmoor-Su, R.; Lacoursiere, D.Y. Abnormal second-trimester serum analytes are more predictive of preterm preeclampsia. Am. J. Obstet. Gynecol. 2012, 207. [Google Scholar] [CrossRef]
- Akolekar, R.; Syngelaki, A.; Sarquis, R.; Zvanca, M.; Nicolaides, K.H. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat. Diagn. 2011, 31, 66–74. [Google Scholar] [CrossRef]
- D’Antonio, F.; Rijo, C.; Thilaganathan, B.; Akolekar, R.; Khalil, A.; Papageourgiou, A.; Bhide, A. Association between first-trimester maternal serum pregnancy-associated plasma protein-A and obstetric complications. Prenat. Diagn. 2013, 33, 839–847. [Google Scholar] [CrossRef]
- Kuc, S.; Koster, M.P.; Franx, A.; Schielen, P.C.; Visser, G.H. Maternal characteristics, mean arterial pressure and serum markers in early prediction of preeclampsia. PLoS One 2013, 8, e63546. [Google Scholar]
- Huang, T.; Hoffman, B.; Meschino, W.; Kingdom, J.; Okun, N. Prediction of adverse pregnancy outcomes by combinations of first and second trimester biochemistry markers used in the routine prenatal screening of Down syndrome. Prenat. Diagn. 2010, 30, 471–477. [Google Scholar]
- Akolekar, R.; Syngelaki, A.; Poon, L.; Wright, D.; Nicolaides, K.H. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn. Ther. 2013, 33, 8–15. [Google Scholar] [CrossRef]
- Poon, L.C.; Syngelaki, A.; Akolekar, R.; Lai, J.; Nicolaides, K.H. Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther. 2013, 33, 16–27. [Google Scholar] [CrossRef]
- Cuckle, H.S. Screening for pre-eclampsia—Lessons from aneuploidy screening. Placenta 2011, 32, S42–S48. [Google Scholar] [CrossRef]
- Kagan, K.O.; Hoopmann, M.; Abele, H.; Alkier, R.; Lüthgens, K. First-trimester combined screening for trisomy 21 with different combinations of placental growth factor, free β-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. 2012, 40, 530–535. [Google Scholar] [CrossRef]
- Donalson, K.; Turner, S.; Morrison, L.; Liitti, P.; Nilsson, C.; Cuckle, H. Maternal serum placental growth factor and α-fetoprotein testing in first trimester screening for Down syndrome. Prenat. Diagn. 2013, 33, 457–461. [Google Scholar] [CrossRef]
- Johnson, J.; Pastuck, M.; Metcalfe, A.; Connors, G.; Krause, R.; Wilson, D.; Cuckle, H. First-trimester Down syndrome screening using additional serum markers with and without nuchal translucency and cell-free DNA. Prenat. Diagn. 2013, 33, 1044–1049. [Google Scholar] [CrossRef]
- Lausman, A.; Mccarthy, F.P.; Walker, M.; Kingdom, J. Screening, diagnosis, and management of intrauterine growth restriction. J. Obstet. Gynaecol. Can. 2012, 34, 17–28. [Google Scholar]
- Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Optimizing the definition of intrauterine growth restriction: The multicenter prospective PORTO Study. Am. J. Obstet. Gynecol. 2013, 208. [Google Scholar] [CrossRef]
- Pilalis, A.; Souka, A.P.; Antsaklis, P.; Daskalakis, G.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler and PAPP-A at 11–14 weeks’ gestation. Ultrasound Obstet. Gynecol. 2007, 29, 135–140. [Google Scholar]
- Carbone, J.F.; Tuuli, M.G.; Bradshaw, R.; Liebsch, J.; Odibo, A.O. Efficiency of first-trimester growth restriction and low pregnancy-associated plasma protein-A in predicting small for gestational age at delivery. Prenat. Diagn. 2012, 32, 724–729. [Google Scholar] [CrossRef]
- Spencer, K.; Cowans, N.J.; Avgidou, K.; Molina, F.; Nicolaides, K.H. First-trimester biochemical markers of aneuploidy and the prediction of small-for-gestational age fetuses. Ultrasound Obstet. Gynecol. 2008, 31, 15–19. [Google Scholar] [CrossRef]
- Kavak, Z.N.; Basgul, A.; Elter, K.; Uygur, M.; Gokaslan, H. The efficacy of first-trimester PAPP-A and free beta hCG levels for predicting adverse pregnancy outcome. J. Perinat. Med. 2006, 34, 145–148. [Google Scholar]
- Leung, T.Y.; Sahota, D.S.; Chan, L.W.; Law, L.W.; Fung, T.Y.; Leung, T.N.; Lau, T.K. Prediction of birth weight by fetal crown-rump length and maternal serum levels of pregnancy-associated plasma protein-A in the first trimester. Ultrasound Obstet. Gynecol. 2008, 31, 10–14. [Google Scholar] [CrossRef]
- Roman, A.; Desai, N.; Krantz, D.; Liu, H.P.; Rosner, J.; Vohra, N.; Rochelson, B. Maternal serum analytes as predictors of IUGR with different degrees of placental vascular dysfunction. Prenat. Diagn. 2014. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Health Statistics. Gestational Length and Birthweight 2012. Available online: http://nchs/vitalstats.htm (accessed on 12 March 2014).
- Spencer, K.; Cowans, N.J.; Molina, F.; Kagan, K.O.; Nicolaides, K.H. First-trimester ultrasound and biochemical markers of aneuploidy and the prediction of preterm or early preterm delivery. Ultrasound Obstet. Gynecol. 2008, 31, 147–152. [Google Scholar] [CrossRef]
- Goetzinger, K.R.; Cahill, A.G.; Kemna, J.; Odibo, L.; Macones, G.A.; Odibo, A.O. First-trimester prediction of preterm birth using ADAM12, PAPP-A, uterine artery Doppler, and maternal characteristics. Prenat. Diagn. 2012, 32, 1002–1007. [Google Scholar] [CrossRef]
- Werner, E.F.; Han, C.S.; Pettker, C.M.; Buhimschi, C.S.; Copel, J.A.; Funai, E.F.; Thung, S.F. Universal cervical-length screening to prevent preterm birth: A cost-effectiveness analysis. Ultrasound Obstet. Gynecol. 2011, 38, 32–37. [Google Scholar]
- Heath, V.C.F.; Southall, T.R.; Souka, A.P.; Elisseou, A.; Nicolaides, K.H. Cervical length at 23 weeks of gestation: Prediction of spontaneous preterm delivery. Ultrasound Obstet. Gynecol. 1998, 12, 312–317. [Google Scholar]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef]
- Iams, J.D.; Creasy, R.K. Preterm labor and delivery. In Maternal-Fetal Medicine Principals and Practice, 5th ed.; Creasy, R.K., Resnik, R., Eds.; Saunders: Philadelphia, PA, USA, 2004; pp. 623–661. [Google Scholar]
- Cuckle, H.S.; Sehmi, I.K.; Jones, R.G.; Mason, G. Low maternal serum PAPP-A and fetal viability. Prenat. Diagn. 1999, 19, 788–790. [Google Scholar] [CrossRef]
- Krantz, D.; Hallahan, T.; Ishack, S.; Macri, V.J.; Macri, J.N. First-trimester maternal dried blood Down syndrome screening marker levels in early pregnancy loss. Prenat. Diagn. 2006, 26, 1137–1141. [Google Scholar] [CrossRef]
- Goetzl, L.; Krantz, D.; Simpson, J.L.; Silver, R.K.; Zachary, J.M.; Pergament, E.; Platt, L.D.; Mahoney, M.J.; Wapner, R.J. Pregnancy-associated plasma protein A, free beta-hCG, nuchal translucency, and risk of pregnancy loss. Obstet. Gynecol. 2004, 104, 30–36. [Google Scholar] [CrossRef]
- Dugoff, L.; Cuckle, H.S.; Hobbins, J.C.; Malone, F.D.; Belfort, M.A.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Eddleman, K.A.; Dar, P.; et al. Prediction of patient-specific risk for fetal loss using maternal characteristics and first- and second-trimester maternal serum Down syndrome markers. Am. J. Obstet. Gynecol. 2008, 199. [Google Scholar] [CrossRef]
- Spencer, K.; Cowans, N.J.; Avgidou, K.; Nicolaides, K.H. First-trimester ultrasound and biochemical markers of aneuploidy and the prediction of impending fetal death. Ultrasound Obstet. Gynecol. 2006, 28, 637–643. [Google Scholar] [CrossRef]
- Benn, P.A.; Craffey, A.; Horne, D.; Ramsdell, L.; Rodis, J.F. Elevated maternal serum alpha-fetoprotein with low unconjugated estriol and the risk for lethal perinatal outcome. J. Matern. Fetal Med. 2000, 9, 165–169. [Google Scholar]
- Smith, G.C.S.; Crossley, J.A.; Aitken, D.A.; Pell, J.P.; Cameron, A.D.; Connor, J.M.; Dobbie, R. First trimester placentation and the risk of antepartum stillbirth. JAMA 2004, 292, 2249–2254. [Google Scholar] [CrossRef]
- Silver, R.M.; Landon, M.B.; Rouse, D.J.; Leveno, K.J.; Spong, C.Y.; Thom, E.A.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006, 107, 1226–1232. [Google Scholar] [CrossRef]
- Warshak, C.R.; Ramos, G.A.; Eskander, R.; Benirschke, K.; Saenz, C.C.; Kelly, T.F.; Moore, T.R.; Resnik, R. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet. Gynecol. 2010, 115, 65–69. [Google Scholar] [CrossRef]
- Wright, J.D.; Herzog, T.J.; Shah, M.; Bonanno, C.; Lewin, S.N.; Cleary, K.; Simpson, L.L.; Gaddipati, S.; Sun, X.; D’Alton, M.E.; et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet. Gynecol. 2010, 115, 1194–1200. [Google Scholar] [CrossRef]
- Briery, C.M.; Rose, C.H.; Hudson, W.T.; Lutgendorf, M.A.; Magann, E.F.; Chauhan, S.P.; Morrison, J.C. Planned vs. emergent cesarean hysterectomy. Am. J. Obstet. Gynecol. 2007, 197. [Google Scholar] [CrossRef]
- Eller, A.G.; Bennett, M.A.; Sharshiner, M.; Masheter, C.; Soisson, A.P.; Dodson, M.; Silver, R.M. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet. Gynecol. 2011, 117, 331–337. [Google Scholar] [CrossRef]
- Hung, T.H.; Shau, W.Y.; Hsieh, C.C.; Chiu, T.H.; Hsu, J.J.; Hsieh, T.T. Risk factors for placenta accreta. Obstet. Gynecol. 1999, 93, 545–550. [Google Scholar] [CrossRef]
- Dreux, S.; Salomon, L.J.; Muller, F.; Goffinet, F.; Oury, J.F.; ABA Study Group; Sentilhes, L. Second-trimester maternal serum markers and placenta accreta. Prenat. Diagn. 2012, 32, 1010–1012. [Google Scholar] [CrossRef]
- Johnson, S.P.; Sebire, N.J.; Snijders, R.J.; Tunkel, S.; Nicolaides, K.H. Ultrasound screening for anencephaly at 10–14 weeks of gestation. Ultrasound Obstet. Gynecol. 1997, 9, 14–16. [Google Scholar]
- Sebire, N.J.; Noble, P.L.; Thorpe-Beeston, J.G.; Snijders, R.J.; Nicolaides, K.H. Presence of the “lemon” sign in fetuses with spina bifida at the 10–14-week scan. Ultrasound Obstet. Gynecol. 1997, 10, 403–405. [Google Scholar]
- Chaoui, R.; Benoit, B.; Heling, K.S.; Kagan, K.O.; Pietzsch, V.; Sarut Lopez, A.; Tekesin, I.; Karl, K. Prospective detection of open spina bifida at 11–13 weeks by assessing intracranial translucency and posterior brain. Ultrasound Obstet. Gynecol. 2011, 38, 722–726. [Google Scholar] [CrossRef]
- Lennon, C.A.; Gray, D.L. Sensitivity and specificity of ultrasound for the detection of neural tube and ventral wall defects in a high-risk population. Obstet. Gynecol. 1999, 94, 562–566. [Google Scholar] [CrossRef]
- Crane, J.P.; LeFevre, M.L.; Winborn, R.C.; Evans, J.K.; Ewigman, B.G.; Bain, R.P.; Frigoletto, F.D.; McNellis, D. A randomized trial of prenatal ultrasonographic screening: Impact on the detection, management, and outcome of anomalous fetuses. The RADIUS Study Group. Am. J. Obstet. Gynecol. 1994, 171, 392–399. [Google Scholar] [CrossRef]
- Cheschier, N.; ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. Int. J. Gynaecol. Obstet. 2003, 83, 123–133. [Google Scholar] [CrossRef]
- Milunsky, A.; Jick, S.S.; Bruell, C.L.; MacLaughlin, D.S.; Tsung, Y.K.; Jick, H.; Rothman, K.J.; Willett, W. Predictive values, relative risks, and overall benefits of high and low maternal serum alpha-fetoprotein screening in singleton pregnancies: New epidemiologic data. Am. J. Obstet. Gynecol. 1989, 161, 291–297. [Google Scholar] [CrossRef]
- Wald, N.J.; Hackshaw, A.K.; George, L.M. Assay precision of serum alpha fetoprotein in antenatal screening for neural tube defects and Down’s syndrome. J. Med. Screen. 2000, 7, 74–77. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Use of folic acid for prevention of spina bifida and other neural tube defects—1983–1991. MMWR Morb. Mortal. Wkly. Rep. 1991, 40, 513–516. [Google Scholar]
- Moster, D.; Lie, R.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef]
- Clements, K.; Barfield, W.; Ayadi, M.; Wilber, N. Preterm birth associated cost of early intervention services: An analysis by gestational age. Pediatrics 2007, 119, 866–874. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krantz, D.; Hallahan, T.; Janik, D.; Carmichael, J. Maternal Serum Screening Markers and Adverse Outcome: A New Perspective. J. Clin. Med. 2014, 3, 693-712. https://doi.org/10.3390/jcm3030693
Krantz D, Hallahan T, Janik D, Carmichael J. Maternal Serum Screening Markers and Adverse Outcome: A New Perspective. Journal of Clinical Medicine. 2014; 3(3):693-712. https://doi.org/10.3390/jcm3030693
Chicago/Turabian StyleKrantz, David, Terrence Hallahan, David Janik, and Jonathan Carmichael. 2014. "Maternal Serum Screening Markers and Adverse Outcome: A New Perspective" Journal of Clinical Medicine 3, no. 3: 693-712. https://doi.org/10.3390/jcm3030693




