Short Term Culture of Human Mesenchymal Stem Cells with Commercial Osteoconductive Carriers Provides Unique Insights into Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Orthopedic Substrates
2.3. Cell Retention
| Gene | Forward primer sequence | Reverse primer sequence | Notes |
|---|---|---|---|
| GAPDH | CTCTCTGCTCCTCCTGTTCGAC | TGAGCGATGTGGCTCGGCT | 55 °C anneal [49] |
| Runx2 | GCAAGGTTCAACGATCTGAGA | TCCCCGAGGTCCATCTACTG | 55 °C anneal [51] |
| ALP | GACCCTTGACCCCCACAAT | GCTCGTACTGCATGTCCCCT | 55 °C anneal [52] |
| Collagen I | GAACGCGTGTCATCCCTTGT | GAACGAGGTAGTCTTTCAGCAACA | 50 °C anneal [50] |
2.4. Osteogenic Gene Expression for Mineralized Substrates
2.5. BMSC Expansion and Osteogenic Gene Expression with DBM and DBM-Poloxamer Putties
2.6. Resorption and pH
2.7. Statistics
3. Results
3.1. Cell Retention
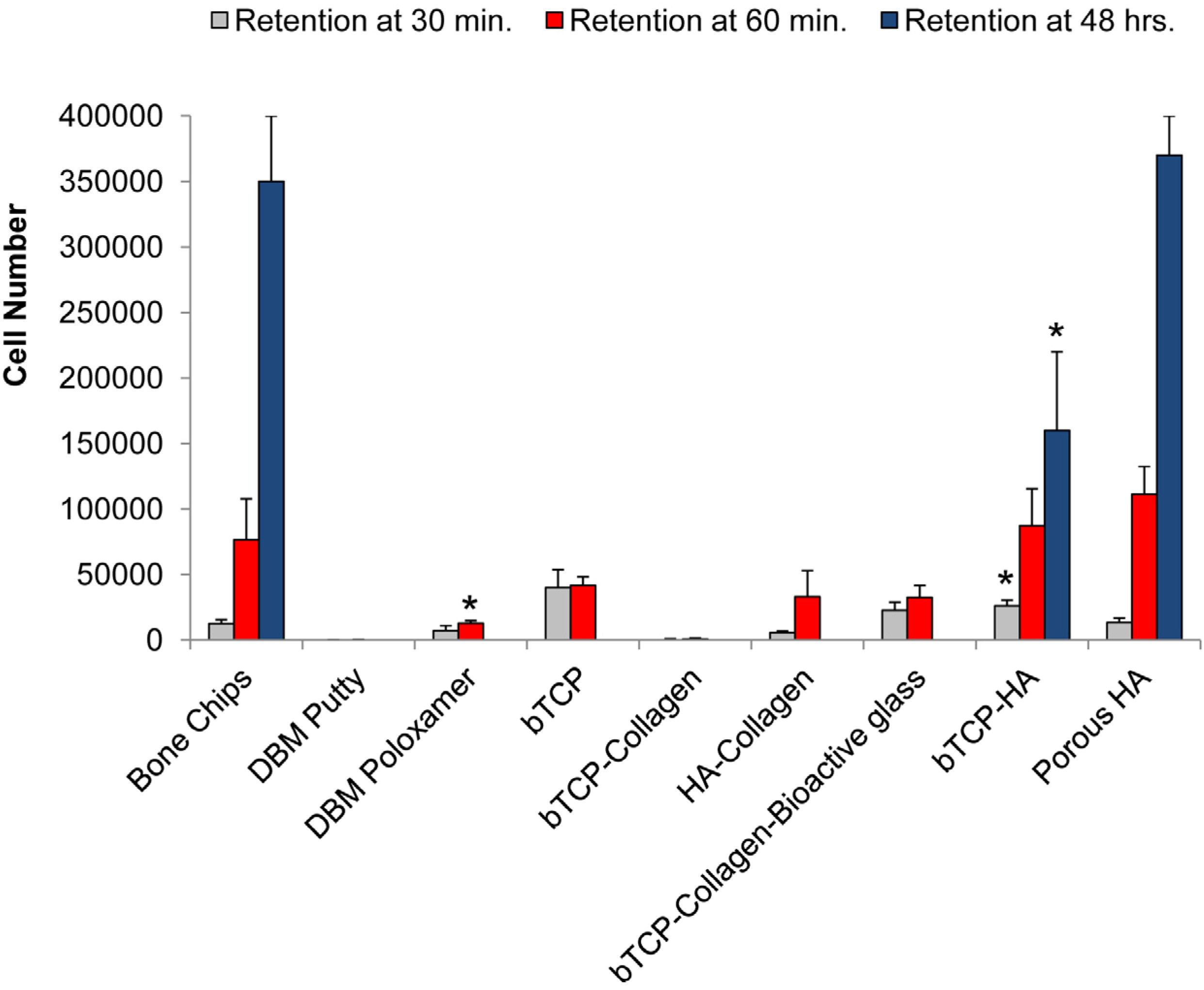
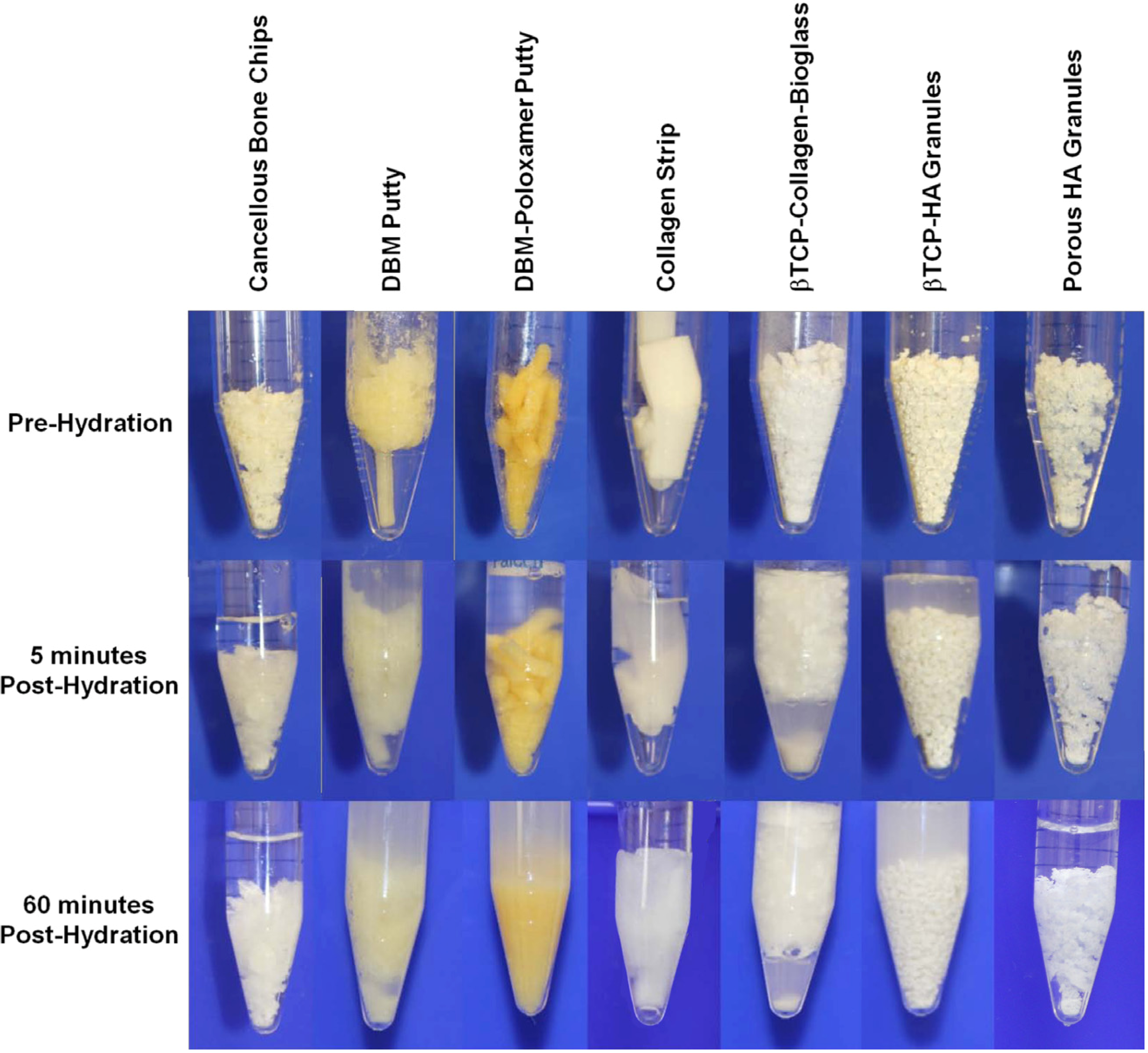
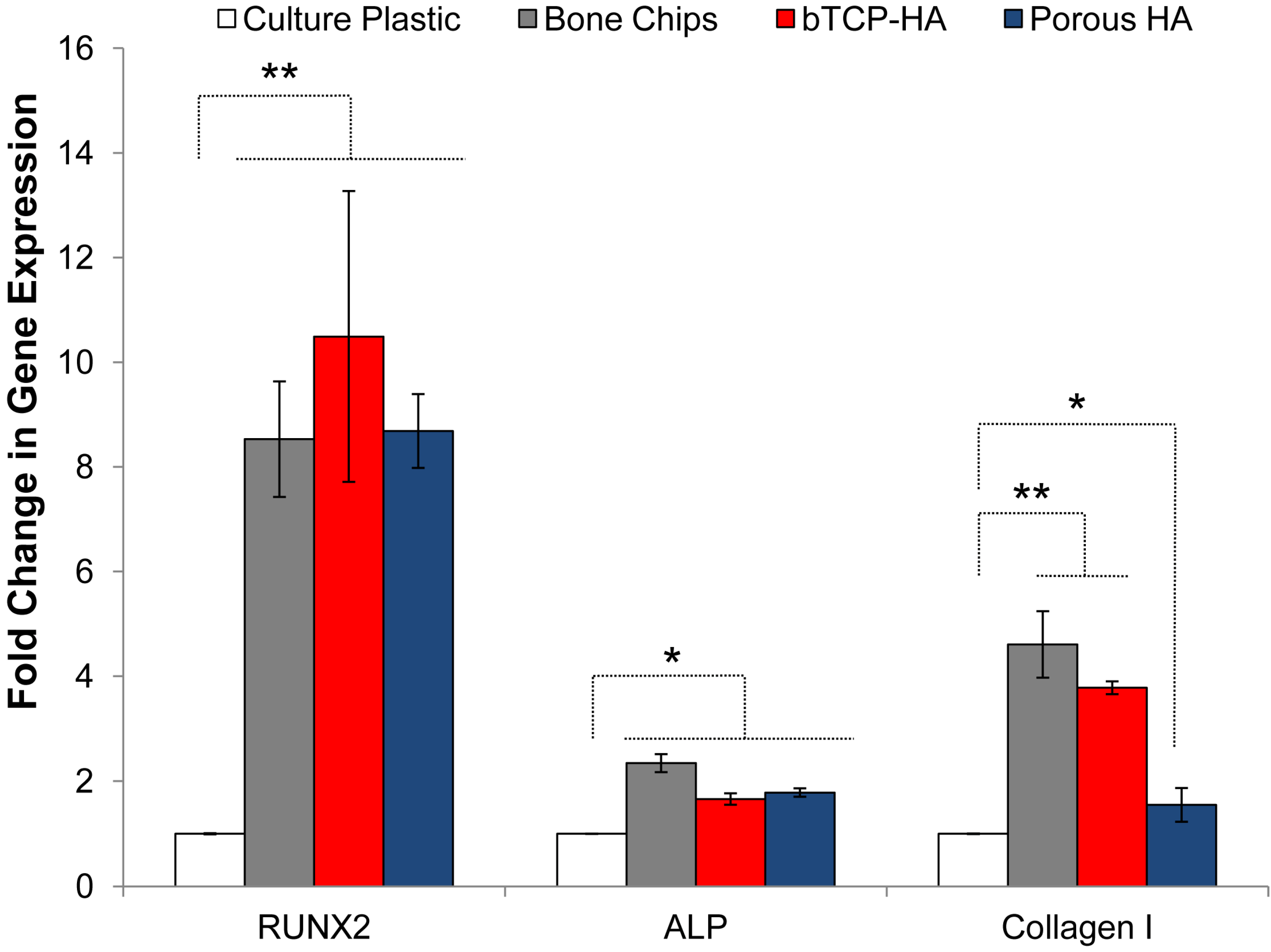
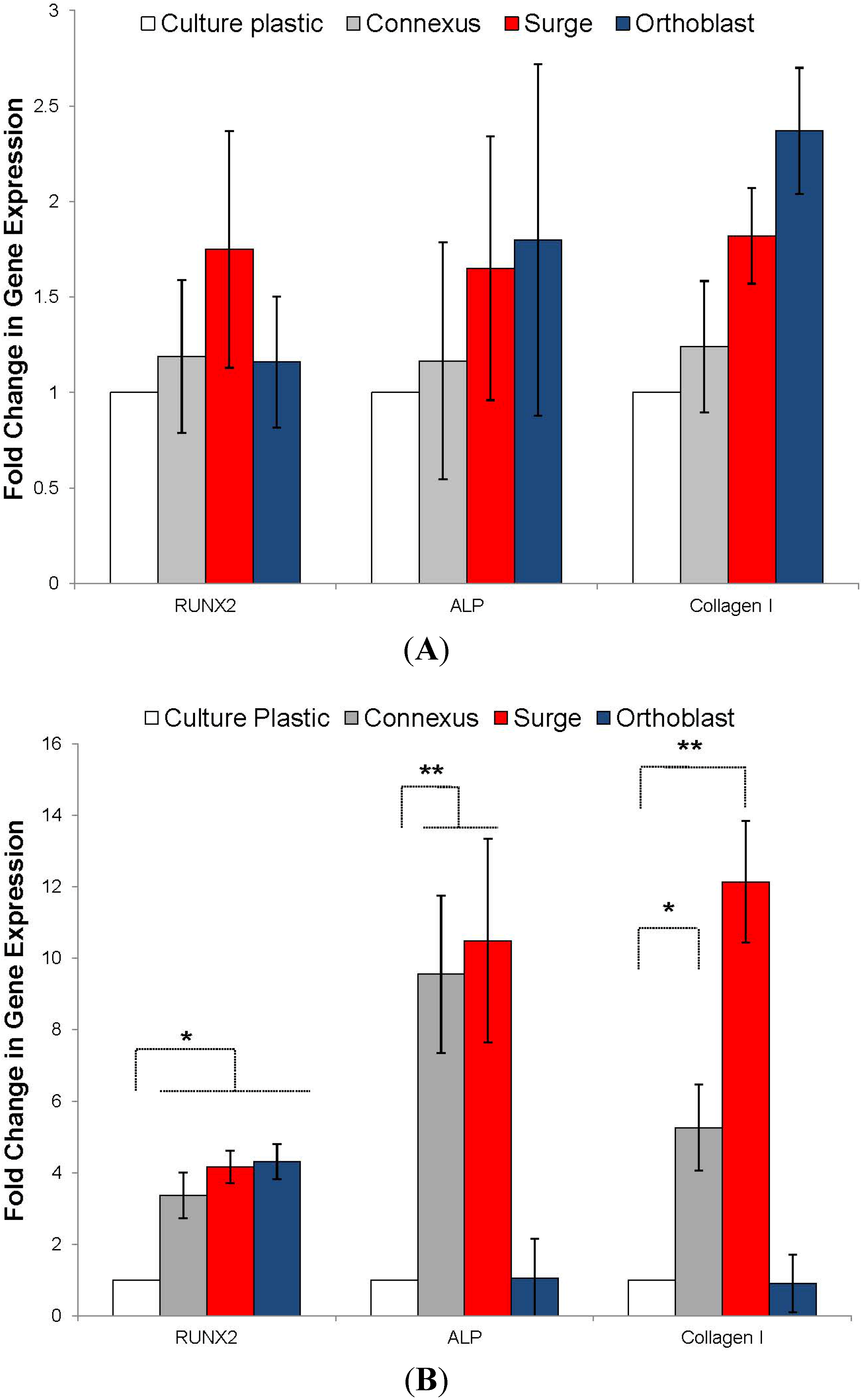
3.2. Osteogenic Differentiation
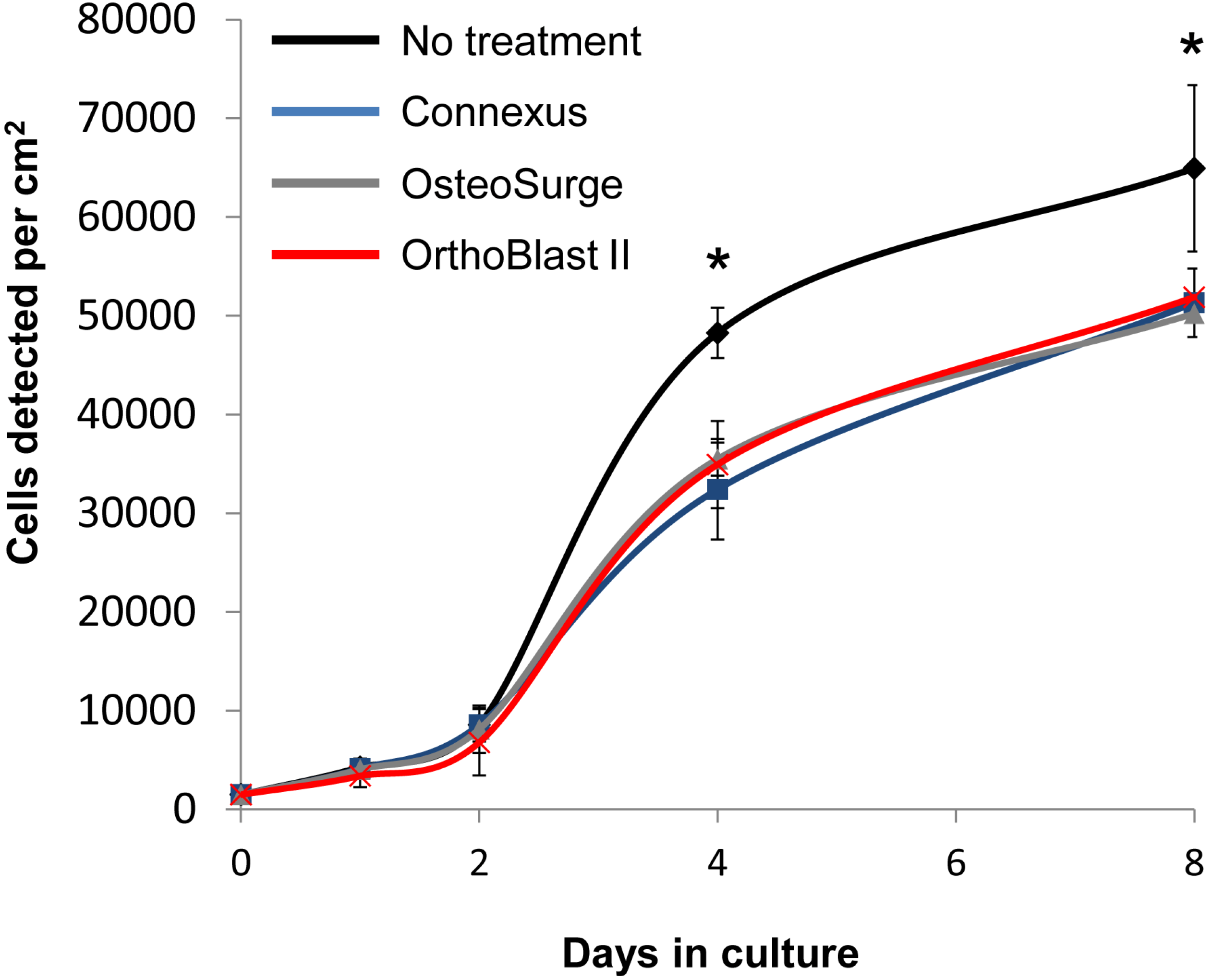
3.3. BMSC Expansion and Osteogenic Gene Expression with DBM and DBM-Poloxamer Putties
3.4. Resorption and pH
| Substrate | 1 min | 5 min | 30 min | 60 min | 24 h | |
|---|---|---|---|---|---|---|
| βTCP-Collagen-Bioactive glass | 8.47 (6.73) | 9.67 (7.33) | 9.68 (7.95) | 9.57 (9.16) | 10.09 (10.49) | Highly Basic |
| DBM putty | 7.66 (7.11) | 7.75 (7.34) | 7.72 (7.55) | 7.62 (7.52) | 7.92 (7.53) | Within Physiological Range |
| Cancellous bone chips | 7.61 (7.21) | 7.65 (7.17) | 7.59 (7.17) | 7.47 (7.17) | 7.75 (7.14) | |
| Collagen | 7.66 (7.33) | 7.62 (7.40) | 7.49 (7.30) | 7.47 (7.39) | 7.15 (7.35) | |
| Porous HA | 7.54 (7.23) | 7.56 (7.24) | 7.50 (7.33) | 7.42 (7.39) | 7.74 (7.36) | |
| βTCP-HA | 7.54 (7.20) | 7.73 (7.25) | 7.48 (7.20) | 7.39 (7.21) | 7.80 (7.22) | |
| HA-Collagen | 7.44 (6.94) | 7.47 (6.98) | 7.37 (7.06) | 7.32 (7.08) | 7.61 (6.85) | |
| βTCP-Collagen | 7.32 (7.01) | 7.38 (6.91) | 7.44 (6.84) | 7.29 (6.71) | 7.65 (6.62) | Slightly Acidic |
| DBM strip | 7.42 (6.70) | 7.34 (6.38) | 7.24 (6.39) | 7.14 (6.46) | 5.44 (6.27) | |
| DBM poloxamer | 6.23 (6.74) | 6.13 (6.07) | 5.61 (5.06) | 4.87 (4.66) | 4.95 (4.42) | Highly Acidic |
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Burstein, F.D. Bone substitutes. Cleft Palate Craniofac. J. 2000, 37, 1–4. [Google Scholar] [CrossRef]
- Shegarfi, H.; Reikeras, O. Review article: Bone transplantation and immune response. J. Orthop. Surg. 2009, 17, 206–211. [Google Scholar]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. North Am. 2007, 19, 513–521. [Google Scholar] [CrossRef]
- Aro, H.T.; Aho, A.J. Clinical use of bone allografts. Ann. Med. 1993, 25, 403–412. [Google Scholar] [CrossRef]
- Albrektsson, T. In vivo studies of bone grafts. The possibility of vascular anastomoses in healing bone. Acta Orthop. Scand. 1980, 51, 9–17. [Google Scholar] [CrossRef]
- De Boer, H.H. The history of bone grafts. Clin. Orthop. Relat. Res. 1988, 226, 292–298. [Google Scholar]
- Faour, O.; Dimitriou, R.; Cousins, C.A.; Giannoudis, P.V. The use of bone graft substitutes in large cancellous voids: Any specific needs? Injury 2011, 42, S87–S90. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Derubeis, A.R.; Cancedda, R. Bone marrow stromal cells (BMSCs) in bone engineering: Limitations and recent advances. Ann. Biomed. Eng. 2004, 32, 160–165. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Zhang, Z. Bone regeneration by stem cell and tissue engineering in oral and maxillofacial region. Fron. Med. 2011, 5, 401–413. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Khan, W.S. Advances in the use of stem cells and tissue engineering applications in bone repair. Curr. Stem Cell Res. Ther. 2012, 7, 122–126. [Google Scholar] [CrossRef]
- Prockop, D.J. Further proof of the plasticity of adult stem cells and their role in tissue repair. J. Cell Biol. 2003, 160, 807–809. [Google Scholar] [CrossRef]
- Prockop, D.J. Adult stem cells gradually come of age. Nat. Biotechnol. 2002, 20, 791–792. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef]
- Gregory, C.A.; Prockop, D.J.; Spees, J.L. Non-hematopoietic bone marrow stem cells: Molecular control of expansion and differentiation. Exp. Cell Res. 2005, 306, 330–335. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Reyes, E.; Smolarz, A.J.; Munoz, J.; Spees, J.L.; Prockop, D.J. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann. N. Y. Acad. Sci. 2005, 1049, 97–106. [Google Scholar] [CrossRef]
- Gregory, C.A.; Prockop, D.J. Fundementals of Culture and Characterization of Mesenchymal Stem/Progenitor Cells from Bone Marrow Stroma. In Culture of Human Stem Cells; Ian Freshney, R., Stacey, G.N., Auerbach, J.M., Eds.; Wiley-Liss: Hoboken, NJ, USA; Volume 208, 2007. [Google Scholar] [CrossRef]
- Pittenger, M.F. Mesenchymal stem cells from adult bone marrow. Methods Mol. Biol. 2008, 449, 27–44. [Google Scholar]
- Pittenger, M.; Vanguri, P.; Simonetti, D.; Young, R. Adult mesenchymal stem cells: Potential for muscle and tendon regeneration and use in gene therapy. J. Musculoskelet. Neur. Interact. 2002, 2, 309–320. [Google Scholar]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Caplan, A.I.; Goldberg, V.M. Principles of tissue engineered regeneration of skeletal tissues. Clin. Orthop. Relat. Res. 1999, 367, S12–S16. [Google Scholar] [CrossRef]
- Zomorodian, E.; Eslaminejad, M.B. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Ichinose, S.; Shinomiyaet, K.; Muneta, T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 2004, 104, 2728–2735. [Google Scholar] [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. PNAS 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Hernigou, P.H.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Manicom, O.; Surgeon, O.; Mathieu, G.; Rouard, H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J. Bone Jt. Surg. 2005, 87, 896–902. [Google Scholar]
- Krause, U.; Harris, S.; Green, A.; Ylostalo, J.; Zeitouni, S.; Lee, N.; Gregory, C.A. Pharmaceutical modulation of canonical Wnt signaling in multipotent stromal cells for improved osteoinductive therapy. PNAS 2010, 107, 4147–4152. [Google Scholar]
- Zeitouni, S.; Krause, U.; Clough, B.H.; Halderman, H.; Falster, A.; Blalock, D.T.; Chaput, C.D.; Sampson, H.W.; Gregory, C.A. Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci. Transl. Med. 2012, 4, 132–155. [Google Scholar]
- Demirbag, B.; Huri, P.Y.; Kose, G.T.; Buyuksungur, A.; Hasirci, V. Advanced cell therapies with and without scaffolds. Biotechnol. J. 2011, 6, 1437–1453. [Google Scholar] [CrossRef]
- Nakamura, A.; Akahane, M.; Shigematsu, H.; Tadokoro, M.; Morita, Y.; Ohgushic, H.; Dohi, Y.; Imamura, T.; Tanakaa, Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone 2010, 46, 418–424. [Google Scholar] [CrossRef]
- Bruder, S.P.; Kurth, A.A.; Shea, M.; Hayes, C.; Jaiswal, N.; Kadiyala, S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J. Orthop. Res. 1998, 16, 155–162. [Google Scholar] [CrossRef]
- Bruder, S.P.; Kraus, K.H.; Goldberg, V.M.; Kadiyala, S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone Jt. Surg. 1998, 80, 985–996. [Google Scholar]
- Bruder, S.P.; Jaiswal, N.; Ricalton, N.S.; Mosca, J.; Krauset, K.H.; Kadiyala, S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin. Orthop. Relat. Res. 1998, 355, S247–S256. [Google Scholar]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Jt. Surg. 2011, 93, 2227–2236. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar]
- Graham, S.M.; Leonidou, A.; Aslam-Pervez, N.; Hamza, A.; Panteliadis, P.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Biological therapy of bone defects: The immunology of bone allo-transplantation. Expert Opin. Biol. Ther. 2010, 10, 885–901. [Google Scholar] [CrossRef]
- Rihn, J.A.; Kirkpatrick, K.; Albert, T.J. Graft options in posterolateral and posterior interbody lumbar fusion. Spine 2010, 35, 1629–1639. [Google Scholar] [CrossRef]
- Gan, Y.; Dai, K.; Zhang, P.; Tang, T.; Zhu, Z.; Lu, J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar] [CrossRef]
- Boden, S.D. Biology of lumbar spine fusion and use of bone graft substitutes: Present, future, and next generation. Tissue Eng. 2000, 6, 383–399. [Google Scholar] [CrossRef]
- Carraro, G.; Albertin, G.; Forneris, M.; Nussdorfer, G.G. Similar sequence-free amplification of human glyceraldehyde-3-phosphate dehydrogenase for real time RT-PCR applications. Mol. Cell. Probes 2005, 19, 181–186. [Google Scholar] [CrossRef]
- Hoshiba, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions. J. Biol. Chem. 2009, 284, 31164–31173. [Google Scholar] [CrossRef]
- Pattyn, F.; Speleman, F.; de Paepe, A.; Vandesompele, J. RTPrimerDB: The real-time PCR primer and probe database. Nucleic Acids Res. 2003, 31, 122–123. [Google Scholar] [CrossRef]
- Schaap-Oz87iemlak, A.M.; Raymakers, R.A.; Bergevoet, S.M.; Gilissen, C.; Jansen, B.J.H.; Adema, G.J.; Kögler, G.; le Sage, C.; Agami, R.; van der Reijden, B.A.; Jansen, J.H. MicroRNA hsa-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells Dev. 2010, 19, 877–885. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−∆∆CT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Krause, U.; Seckinger, A.; Gregory, C.A. Assays of osteogenic differentiation by cultured human mesenchymal stem cells. Methods Mol. Biol. 2011, 698, 215–230. [Google Scholar] [CrossRef]
- Sampath, T.K.; Reddi, A.H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. PNAS 1981, 78, 7599–7603. [Google Scholar] [CrossRef]
- Reddi, A.H.; Huggins, C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. PNAS 1972, 69, 1601–1605. [Google Scholar] [CrossRef]
- Russell, J.L.; Block, J.E. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: Impact of processing techniques and study methodology. Orthopedics 1999, 22, 524–533. [Google Scholar]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen as a biomaterial. Ann. Rev. Biophys. Bioeng. 1974, 3, 231–253. [Google Scholar] [CrossRef]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415–418. [Google Scholar]
- Valimaki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar]
- Thomas, M.V.; Puleo, D.A.; Al-Sabbagh, M. Bioactive glass three decades on. J. Long Term Eff. Med. Implant. 2005, 15, 585–597. [Google Scholar] [CrossRef]
- Tilocca, A.; Cormack, A.N. Modeling the water-bioglass interface by ab initio molecular dynamics simulations. ACS Appl. Mater. Interfaces 2009, 1, 1324–1333. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef]
- Byrne, E.M.; Farrell, E.; McMahon, L.A.; Haugh, M.G.; O’Brien, F.J.; Campbell, V.A.; Prendergast, P.J.; O’Connell, B.C. Gene expression by marrow stromal cells in a porous collagen-glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J. Mater. Sci. 2008, 19, 3455–3463. [Google Scholar]
- Dadsetan, M.; Hefferan, T.E.; Szatkowski, J.P.; Mishra, P.K.; Macura, S.I.; Lu, L.; Yaszemski, M.J. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials 2008, 29, 2193–2202. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol 2012, 13, 27–38. [Google Scholar] [CrossRef]
- Huggins, C.; Wiseman, S.; Reddi, A.H. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J. Exp. Med. 1970, 132, 1250–1258. [Google Scholar] [CrossRef]
- Lindholm, T.S.; Nilsson, O.S.; Lindholm, T.C. Extraskeletal and intraskeletal new bone formation induced by demineralized bone matrix combined with bone marrow cells. Clin. Orthop. Relat. Res. 1982, 171, 251–255. [Google Scholar]
- McLaughlin, R.E.; Reger, S.I.; Bolander, M.; Eschenroeder, H.C. Enhancement of bone ingrowth by the use of bone matrix as a biologic cement. Clin. Orthop. Relat. Res. 1984, 183, 255–261. [Google Scholar]
- Lee, K.J.; Roper, J.G.; Wang, J.C. Demineralized bone matrix and spinal arthrodesis. Spine 2005, 5, 217–223. [Google Scholar] [CrossRef]
- Pacaccio, D.J.; Stern, S.F. Demineralized bone matrix: Basic science and clinical applications. Clin. Podiatric. Med. Surg. 2005, 22, 599–606. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murphy, M.B.; Suzuki, R.K.; Sand, T.T.; Chaput, C.D.; Gregory, C.A. Short Term Culture of Human Mesenchymal Stem Cells with Commercial Osteoconductive Carriers Provides Unique Insights into Biocompatibility. J. Clin. Med. 2013, 2, 49-66. https://doi.org/10.3390/jcm2030049
Murphy MB, Suzuki RK, Sand TT, Chaput CD, Gregory CA. Short Term Culture of Human Mesenchymal Stem Cells with Commercial Osteoconductive Carriers Provides Unique Insights into Biocompatibility. Journal of Clinical Medicine. 2013; 2(3):49-66. https://doi.org/10.3390/jcm2030049
Chicago/Turabian StyleMurphy, Matthew B., Richard K. Suzuki, Theodore T. Sand, Christopher D. Chaput, and Carl A. Gregory. 2013. "Short Term Culture of Human Mesenchymal Stem Cells with Commercial Osteoconductive Carriers Provides Unique Insights into Biocompatibility" Journal of Clinical Medicine 2, no. 3: 49-66. https://doi.org/10.3390/jcm2030049
APA StyleMurphy, M. B., Suzuki, R. K., Sand, T. T., Chaput, C. D., & Gregory, C. A. (2013). Short Term Culture of Human Mesenchymal Stem Cells with Commercial Osteoconductive Carriers Provides Unique Insights into Biocompatibility. Journal of Clinical Medicine, 2(3), 49-66. https://doi.org/10.3390/jcm2030049




