BMI-Stratified Exploration of the ‘Obesity Paradox’: Heart Failure Perspectives from a Large German Insurance Database
Abstract
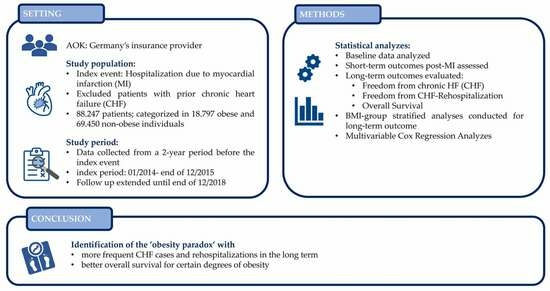
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Ethical Approval
2.4. Statistical Analyses
3. Results
3.1. Baseline
3.2. Outcome and Follow-Up
3.3. Long-Term Outcomes
3.4. Multivariable Analysis of Overall Survival
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Obesity. Our World in Data. 2017. Available online: https://ourworldindata.org/obesity#citation (accessed on 10 October 2023).
- Stamler, J. Epidemiologic findings on body mass and blood pressure in adults. Ann. Epidemiol. 1991, 1, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S. [Google Scholar]
- Koser, F.; Hobbach, A.J.; Abdellatif, M.; Herbst, V.; Türk, C.; Reinecke, H.; Krüger, M.; Sedej, S.; Linke, W.A. Acetylation and phosphorylation changes to cardiac proteins in experimental HFpEF due to metabolic risk reveal targets for treatment. Life Sci. 2022, 309, 120998. [Google Scholar] [CrossRef]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Rosner, B.; Monson, R.R.; Speizer, F.E.; Hennekens, C.H. A prospective study of obesity and risk of coronary heart disease in women. N. Engl. J. Med. 1990, 322, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lissner, L.; Odell, P.M.; D’Agostino, R.B.; Stokes, J., III; Kreger, B.E.; Belanger, A.J.; Brownell, K.D. Variability of body weight and health outcomes in the Framingham population. N. Engl. J. Med. 1991, 324, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Davos, C.H.; Doehner, W.; Rauchhaus, M.; Cicoira, M.; Francis, D.P.; Coats, A.J.; Clark, A.L.; Anker, S.D. Body mass and survival in patients with chronic heart failure without cachexia: The importance of obesity. J. Card. Fail. 2003, 9, 29–35. [Google Scholar] [CrossRef]
- Gustafsson, F.; Kragelund, C.B.; Torp-Pedersen, C.; Seibaek, M.; Burchardt, H.; Akkan, D.; Thune, J.J.; Kober, L. Effect of obesity and being overweight on long-term mortality in congestive heart failure: Influence of left ventricular systolic function. Eur. Heart J. 2005, 26, 58–64. [Google Scholar] [CrossRef]
- Curtis, J.P.; Selter, J.G.; Wang, Y.; Rathore, S.S.; Jovin, I.S.; Jadbabaie, F.; Kosiborod, M.; Portnay, E.L.; Sokol, S.I.; Bader, F.; et al. The obesity paradox: Body mass index and outcomes in patients with heart failure. Arch. Intern. Med. 2005, 165, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Pocock, S.J.; Wang, D.; Finn, P.V.; Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; Yusuf, S.; Swedberg, K.; Michelson, E.L.; et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 116, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Sharma, A.; Alpert, M.A.; De Schutter, A.; Lopez-Jimenez, F.; Milani, R.V.; Ventura, H.O. Update on Obesity and Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2016, 58, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J. Obesity Paradox in Aging: From Prevalence to Pathophysiology. Prog. Cardiovasc. Dis. 2018, 61, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, J.R.; Heidenreich, P.A. Obesity and survival in patients with heart failure and preserved systolic function: A U-shaped relationship. Am. Heart J. 2010, 159, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Gallardo, J.S.; Romeo, F.J.; Bhatia, K.; Correa, A.; Mechanick, J.I.; Contreras, J.P. Severe Obesity and Heart Failure. Am. J. Cardiol. 2022, 177, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Köppe, J.; Engelbertz, C.; Kühnemund, L.; Fischer, A.J.; Lange, S.A.; Dröge, P.; Ruhnke, T.; Günster, C.; Malyar, N.; et al. Sex-related differences in treatment and outcome of chronic limb-threatening ischaemia: A real-world cohort. Eur. Heart J. 2022, 43, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef]
- Liu, K.; Daviglus, M.L.; Loria, C.M.; Colangelo, L.A.; Spring, B.; Moller, A.C.; Lloyd-Jones, D.M. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: The Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 2012, 125, 996–1004. [Google Scholar] [CrossRef]
- Hansel, B.; Roussel, R.; Elbez, Y.; Marre, M.; Krempf, M.; Ikeda, Y.; Eagle, K.A.; Elisaf, M.; Bhatt, D.L.; Steg, P.G.; et al. Cardiovascular risk in relation to body mass index and use of evidence-based preventive medications in patients with or at risk of atherothrombosis. Eur. Heart J. 2015, 36, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uber, P.A.; Park, M.H.; Scott, R.L.; Ventura, H.O.; Harris, B.C.; Frohlich, E.D. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef]




| Non-Obese (BMI < 30 kg/m2) | Obese (BMI ≥ 30 kg/m2) | Total | p-Value | |
|---|---|---|---|---|
| Number of patients—a.n. | 69,450 | 18,797 | 88,247 | |
| Female sex—a.n. (%) | 23,203 (33.41) | 7642 (40.66) | 30,845 (34.95) | <0.001 |
| Age median—years (IQR) | 70.69 (21.40) | 68.69 (18.94) | 70.22 (20.82) | <0.001 |
| Cardiovascular risk factors—a.n. (%) | ||||
| Arterial hypertension (AHT) | 60,116 (86.56) | 18,050 (96.03) | 78,166 (88.58) | <0.001 |
| Diabetes mellitus (DM) | 23,506 (33.85) | 11,523 (61.30) | 35,029 (39.69) | <0.001 |
| Dyslipidemia | 50,341 (72.49) | 15,532 (82.63) | 65,873 (74.65) | <0.001 |
| Nicotine abuses | 17,019 (24.51) | 4977 (26.48) | 21,996 (24.93) | <0.001 |
| Comorbidities and other risk factors—a.n. (%) | ||||
| Previous myocardial infarction (MI) | 21,005 (30.24) | 7150 (38.04) | 28,155 (31.94) | <0.001 |
| Previous stroke | 6705 (9.65) | 2144 (11.41) | 8849 (10.03) | <0.001 |
| Malignancies | 12,080 (17.39) | 3291 (12.17) | 15,371 | 0.714 |
| Atrial flutter/fibrillation (AFL/AF) | 13,599 (19.58) | 4137 (22.01) | 17,736 (20.1) | <0.001 |
| Peripheral artery disease (PAD) | 6867 (9.89) | 2287 (12.17) | 9154 (10.37) | <0.001 |
| Cerebrovascular disease (CeVD) | 8269 (11.91) | 2832 (15.07) | 11,101 (12.58) | <0.001 |
| Chronic kidney disease (CKD) | 17,332 (24.96) | 6276 (33.39) | 23,608 (26.75) | <0.001 |
| Ischemic cardiomyopthy (ICM) | 4880 (7.02) | 1298 (6.91) | 6178 (7.0) | 0.563 |
| Dilated cardiomyopthy (DCM) | 796 (1.15) | 224 (1.19) | 1020 (1.16) | 0.604 |
| Hypertrophic obstructive cardiomyopthy (HOCM) | 153 (0.22) | 42 (0.22) | 195 (0.22) | 0.935 |
| Hypertrophic cardiomyopthy (HCM) | 191 (0.28) | 64 (0.34) | 255 (0.29) | 0.138 |
| Iron deficiency | 777 (1.12) | 238 (1.27) | 1015 (1.15) | 0.093 |
| Obstructive sleep apnea syndrome (OSAS) | 2271 (3.27) | 2105 (11.2) | 4376 (4.96) | <0.001 |
| Left bundle branch block (LBBB) | 1685 (2.42) | 2552 (2.94) | 2237 (2.53) | <0.001 |
| Left heart failure (LHF) | 23,086 (33.24) | 6415 (34.13) | 29,501 (33.43) | 0.022 |
| Right heart failure (RHF) | 4516 (6.5) | 1375 (7.31) | 5891 (6.68) | <0.001 |
| Interventions associated with cardiovascular comorbidities—a.n. (%) | ||||
| Implanted cardiac device | 2237 (3.22) | 672 (3.58) | 2909 (3.3) | 0.016 |
| Previous Percutaneous coronary intervention (PCI) | 1788 (2.57) | 885 (4.71) | 2673 (3.03) | <0.001 |
| Previous Coronary artery bypass grafting (CABG) | 3262 (4.7) | 1363 (7.25) | 4625 (5.24) | <0.001 |
| Previous valve replacement | 313 (0.45) | 134 (0.71) | 447 (0.51) | <0.001 |
| Occurrence and severity grading of HF symptoms—a.n. (%) | ||||
| Dyspnea | 5702 (8.21) | 2513 (13.37) | 8215 (9.31) | <0.001 |
| Oedema | 3270 (4.71) | 1814 (9.65) | 5084 (5.76) | <0.001 |
| New York Heart Association (NYHA) I | 2052 (2.95) | 532 (2.83) | 2584 (2.93) | 0.369 |
| NYHA II | 5431 (7.82) | 1491 (7.93) | 6922 (7.84) | 0.612 |
| NYHA III | 7768 (11.19) | 2293 (12.2) | 10,061 (11.40) | <0.001 |
| NYHA IV | 9121 (13.13) | 2508 (13.34) | 11,629 (13.18) | 0.452 |
| Pharmacological therapy—a.n. (%) | ||||
| Platelet activation inhibition (PAI) | 8135 (11.71) | 3005 (15.99) | 11,140 (12.62) | <0.001 |
| Oral anticoagulation (OAC) | 2057 (2.96) | 860 (4.58) | 2917 (3.31) | <0.001 |
| PAI+OAC | 225 (0.32) | 105 (0.56) | 330 (0.37) | <0.001 |
| Angiotensin receptor inhibitors/angiotensin receptor blocker (ACEi/ARB) | 25,411 (36.59) | 9912 (52.73) | 35,323 (40.03) | <0.001 |
| Statine | 11,287 (16.25) | 4709 (25.05) | 15,996 (18.12) | <0.001 |
| Betablockers | 18,488 (26.62) | 7102 (37.78) | 25,590 (29.0) | <0.001 |
| Diuretics | 3007 (4.33) | 1456 (7.75) | 4463 (5.06) | <0.001 |
| Ivabradine | 171 (0.25) | 91 (0.48) | 262 (0.3) | <0.001 |
| Mineralocorticoid receptor antagonist (MRA) | 603 (0.87) | 308 (1.64) | 911 (1.03) | <0.001 |
| Sodium glucose cotransporter-2 inhibitor (SGLT-2i) | 68 (0.1) | 101 (0.54) | 169 (0.19) | <0.001 |
| Digitalis | 803 (1.16) | 200 (1.06) | 1003 (1.14) | 0.29 |
| Drug classes of HF therapy (None) | 36,289 (52.25) | 6427 (34.19) | 42,716 (48.41) | <0.001 |
| Drug classes of HF therapy (One) | 11,744 (19.91) | 4947 (26.32) | 16,691 (18.91) | |
| Drug classes of HF therapy (two) | 20,047 (28.87) | 6661 (35.44) | 26,708 (30.27) | |
| Drug classes of HF therapy (three) | 1336 (1.92) | 735 (3.91) | 2071 (2.35) | |
| Drug classes of HF therapy (four) | 33 (0.05) | 26 (0.14) | 59 (0.07) | |
| Non-Obese (BMI < 30 kg/m2) | Obese (BMI ≥ 30 kg/m2) | Total | p-Value | |
|---|---|---|---|---|
| Number of patients | 69,450 | 18,797 | 88,247 | |
| Acute renal failure—a.n. (%) | 4133 (5.95) | 1288 (6.85) | 5421 (6.14) | <0.001 |
| Renal replacement therapy—a.n. (%) | 1727 (2.49) | 620 (3.3) | 2347 (2.66) | <0.001 |
| Shock—a.n. (%) | 5646 (8.13) | 1304 (6.94) | 6950 (7.88) | <0.001 |
| In-hospital resuscitation—a.n. (%) | 4901 (7.06) | 1246 (6.63) | 6147 (6.97) | 0.041 |
| Shock/resuscitation/LV support—a.n. (%) | 8617 (12.41) | 2094 (11.14) | 10,711 (12.14) | <0.001 |
| Death within case chain—a.n. (%) | 7326 (10.55) | 1673 (8.9) | 8999 (10.2) | <0.001 |
| Overall mortality—a.n. (%) | 6430 (9.26) | 1422 (7.57) | 7852 (8.9) | <0.001 |
| Length of stay median—days (IQR) | 7 (6) | 7 (6) | 7 (6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobbach, A.J.; Feld, J.; Linke, W.A.; Sindermann, J.R.; Dröge, P.; Ruhnke, T.; Günster, C.; Reinecke, H. BMI-Stratified Exploration of the ‘Obesity Paradox’: Heart Failure Perspectives from a Large German Insurance Database. J. Clin. Med. 2024, 13, 2086. https://doi.org/10.3390/jcm13072086
Hobbach AJ, Feld J, Linke WA, Sindermann JR, Dröge P, Ruhnke T, Günster C, Reinecke H. BMI-Stratified Exploration of the ‘Obesity Paradox’: Heart Failure Perspectives from a Large German Insurance Database. Journal of Clinical Medicine. 2024; 13(7):2086. https://doi.org/10.3390/jcm13072086
Chicago/Turabian StyleHobbach, Anastasia J., Jannik Feld, Wolfgang A. Linke, Jürgen R. Sindermann, Patrik Dröge, Thomas Ruhnke, Christian Günster, and Holger Reinecke. 2024. "BMI-Stratified Exploration of the ‘Obesity Paradox’: Heart Failure Perspectives from a Large German Insurance Database" Journal of Clinical Medicine 13, no. 7: 2086. https://doi.org/10.3390/jcm13072086