Validation of a Three-Dimensional Weight-Bearing Measurement Protocol for Medial Open-Wedge High Tibial Osteotomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
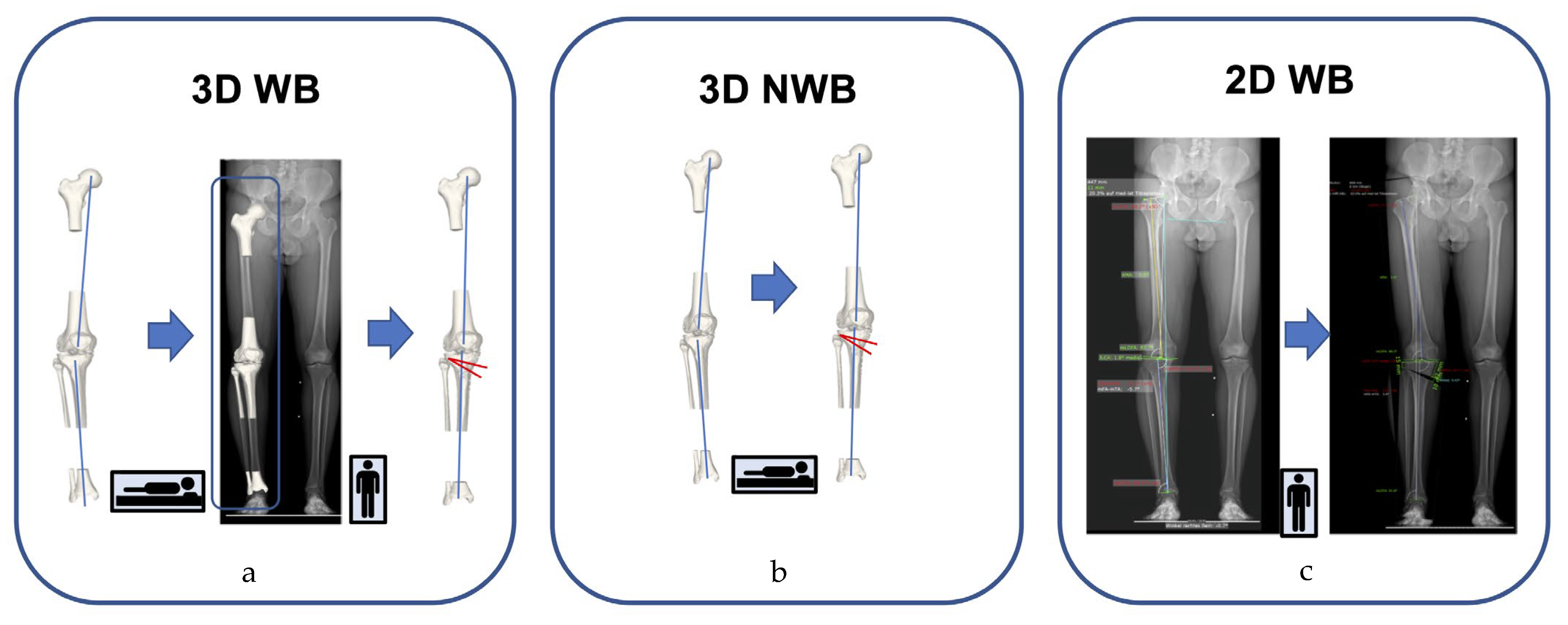
2.2. Overview of the Analyzed Leg Alignment Measurement Protocols
2.3. Validity Testing and Accuracy Assessment
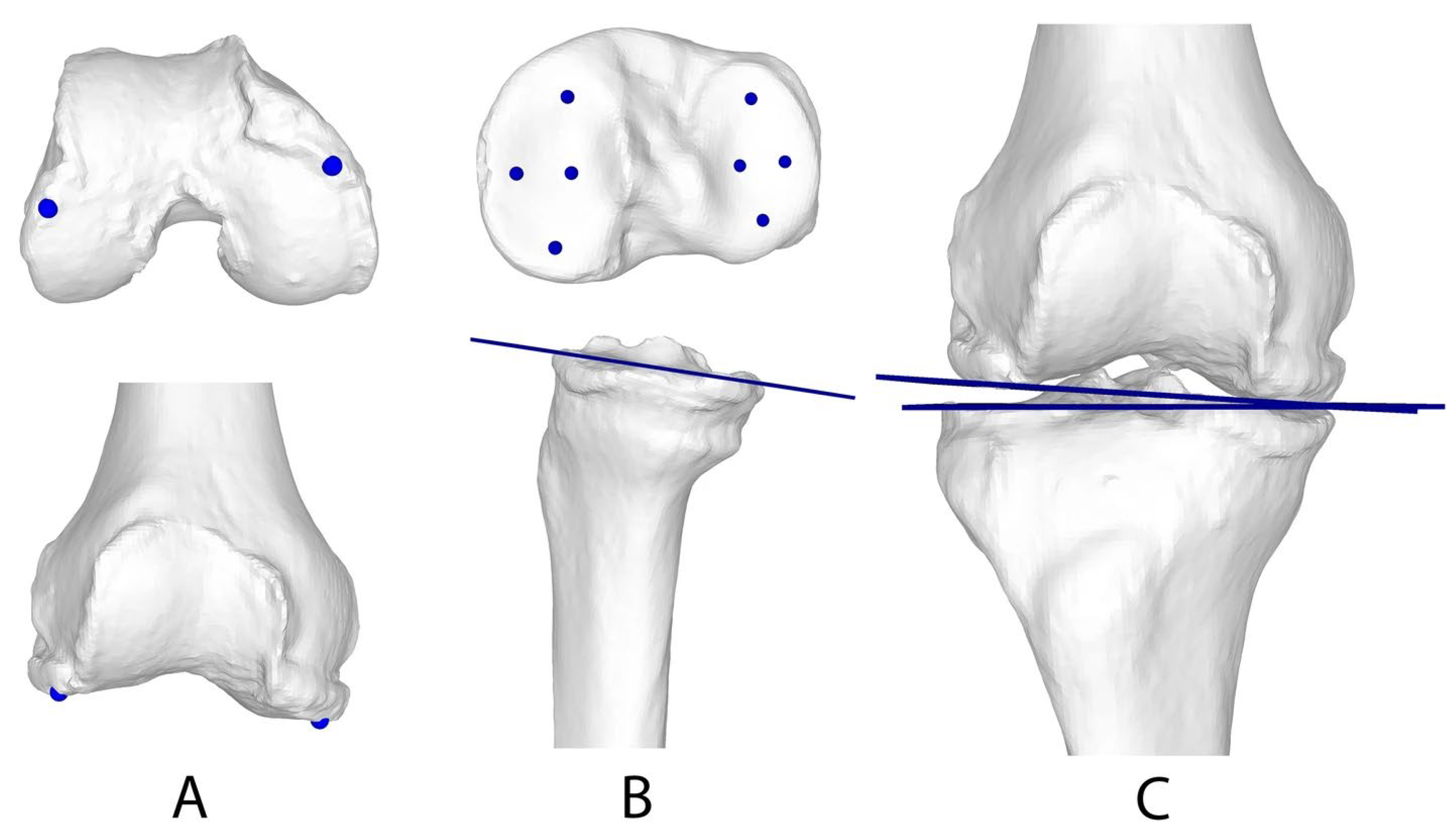
2.4. Generation of 3D Triangular Surface Models
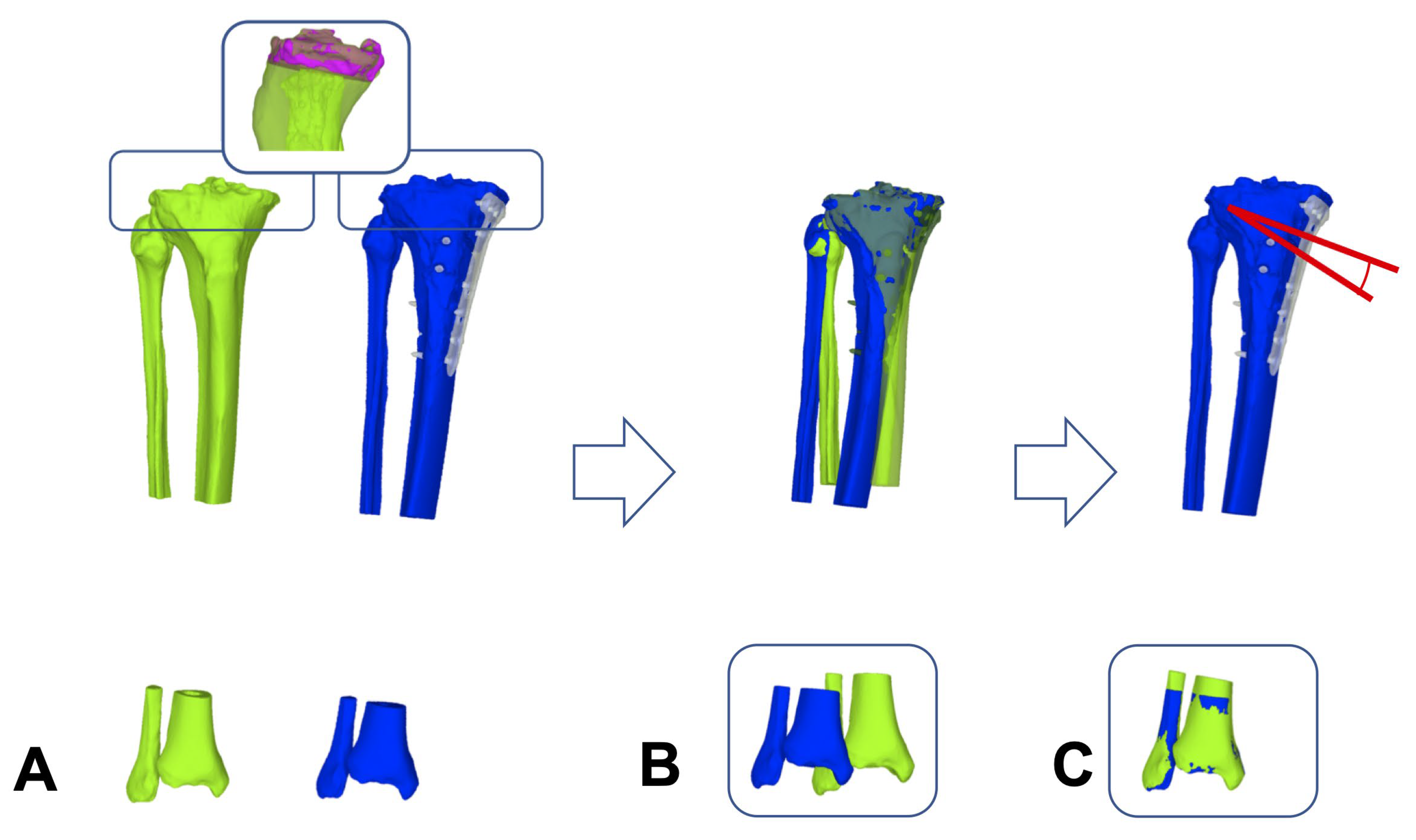
2.5. Registration Module for 3D WB Triangular Surface Models
2.6. 3D Measurement (WB and NWB)
2.7. 2D WB Measurements
2.8. Preoperative MRI Evaluation
2.9. Statistical Analysis
3. Results
3.1. Intermodal Agreement
3.2. Influence of the WB State and Modality (2D vs. 3D) on the Measurement Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujisawa, Y.; Masuhara, K.; Shiomi, S. The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop. Clin. N. Am. 1979, 10, 585–608. [Google Scholar] [CrossRef]
- Jung, W.H.; Takeuchi, R.; Chun, C.W.; Lee, J.S.; Ha, J.H.; Kim, J.H.; Jeong, J.H. Second-look arthroscopic assessment of cartilage regeneration after medial opening-wedge high tibial osteotomy. Arthroscopy 2014, 30, 72–79. [Google Scholar] [CrossRef]
- Rossi, R.; Bonasia, D.E.; Amendola, A. The role of high tibial osteotomy in the varus knee. J. Am. Acad. Orthop. Surg. 2011, 19, 590–599. [Google Scholar] [CrossRef]
- Whatling, G.M.; Biggs, P.R.; Elson, D.W.; Metcalfe, A.; Wilson, C.; Holt, C. High tibial osteotomy results in improved frontal plane knee moments, gait patterns and patient-reported outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.; Rahman, Q.A.; Zalzal, P. Navigation versus conventional high tibial osteotomy: Systematic review. Springerplus 2015, 4, 271. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Kobayashi, H.; Kusayama, Y.; Kumagai, K.; Saito, T. Comparative Study of Opening-Wedge High Tibial Osteotomy with and without a Combined Computed Tomography-Based and Image-Free Navigation System. Arthroscopy 2016, 32, 2072–2081. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, J.; Li, X.; Song, G.; Feng, H. Clinical outcome of simultaneous high tibial osteotomy and anterior cruciate ligament reconstruction for medial compartment osteoarthritis in young patients with anterior cruciate ligament-deficient knees: A systematic review. Arthroscopy 2015, 31, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.R.; Zabtia, N.; Weening, B.; Zalzal, P. Arthroscopic and computer-assisted high tibial osteotomy using standard total knee arthroplasty navigation software. Arthrosc. Tech. 2013, 2, e161–e166. [Google Scholar] [CrossRef]
- Akizuki, S.; Shibakawa, A.; Takizawa, T.; Yamazaki, I.; Horiuchi, H. The long-term outcome of high tibial osteotomy: A ten- to 20-year follow-up. J. Bone Jt. Surg. Br. 2008, 90, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Schallberger, A.; Jacobi, M.; Wahl, P.; Maestretti, G.; Jakob, R.P. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: A retrospective follow-up study over 13–21 years. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 122–127. [Google Scholar] [CrossRef]
- Brinkman, J.M.; Lobenhoffer, P.; Agneskirchner, J.D.; Staubli, A.E.; Wymenga, A.B.; van Heerwaarden, R.J. Osteotomies around the knee: Patient selection, stability of fixation and bone healing in high tibial osteotomies. J. Bone Jt. Surg. Br. 2008, 90, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Medevielle, D.; Debeyre, J.; Goutallier, D. Proximal tibial osteotomy for osteoarthritis with varus deformity. A ten to thirteen-year follow-up study. J. Bone Jt. Surg. Am. 1987, 69, 332–354. [Google Scholar]
- Lutzner, J.; Gross, A.F.; Gunther, K.P.; Kirschner, S. Precision of navigated and conventional open-wedge high tibial osteotomy in a cadaver study. Eur. J. Med. Res. 2010, 15, 117–120. [Google Scholar] [CrossRef]
- Hasegawa, M.; Naito, Y.; Tone, S.; Sudo, A. High rates of outliers in computer-assisted high tibial osteotomy with excellent mid-term outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Jud, L.; Roth, T.; Furnstahl, P.; Vlachopoulos, L.; Sutter, R.; Fucentese, S.F. The impact of limb loading and the measurement modality (2D versus 3D) on the measurement of the limb loading dependent lower extremity parameters. BMC Musculoskelet. Disord. 2020, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Carrillo, F.; Wieczorek, M.; Ceschi, G.; Esfandiari, H.; Sutter, R.; Vlachopoulos, L.; Wein, W.; Fucentese, S.F.; Furnstahl, P. Three-dimensional preoperative planning in the weight-bearing state: Validation and clinical evaluation. Insights Imaging 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Sigrist, B.; Wieczorek, M.; Schilling, N.; Hodel, S.; Walker, J.; Somm, M.; Wein, W.; Sutter, R.; Vlachopoulos, L.; et al. An automated optimization pipeline for clinical-grade computer-assisted planning of high tibial osteotomies under consideration of weight-bearing. Comput. Assist. Surg. 2023, 28, 2211728. [Google Scholar] [CrossRef]
- Fürnstahl, P.; Schweizer, A.; Graf, M.; Vlachopoulos, L.; Fucentese, S.F.; Wirth, S. Surgical Treatment of Long-Bone Deformities: 3D Preoperative Planning and Patient-Specific Instrumentation. In Computational Radiology for Orthopaedic Interventions; Springer: New York, NY, USA, 2016; pp. 123–149. [Google Scholar]
- Schneider, P.; Eberly, D. Geometric Tools for Computer Graphics; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Tschannen, M.; Vlachopoulos, L.; Gerber, C.; Szekely, G.; Furnstahl, P. Regression forest-based automatic estimation of the articular margin plane for shoulder prosthesis planning. Med. Image Anal. 2016, 31, 88–97. [Google Scholar] [CrossRef]
- Paley, D.; Pfeil, J. Principles of deformity correction around the knee. Orthopade 2000, 29, 18–38. [Google Scholar] [CrossRef]
- Outerbridge, R.E. The etiology of chondromalacia patellae. 1961. Clin. Orthop. Relat. Res. 2001, 389, 5–8. [Google Scholar] [CrossRef]
- Jones, L.D.; Mellon, S.J.; Kruger, N.; Monk, A.P.; Price, A.J.; Beard, D.J. Medial meniscal extrusion: A validation study comparing different methods of assessment. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J. Design and Analysis of Clinical Experiments; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Keller, G.; Hagen, F.; Neubauer, L.; Rachunek, K.; Springer, F.; Kraus, M.S. Ultra-low dose CT for scaphoid fracture detection-a simulational approach to quantify the capability of radiation exposure reduction without diagnostic limitation. Quant. Imaging Med. Surg. 2022, 12, 4622–4632. [Google Scholar] [CrossRef] [PubMed]
- Colyn, W.; Vanbecelaere, L.; Bruckers, L.; Scheys, L.; Bellemans, J. The effect of weight-bearing positions on coronal lower limb alignment: A systematic review. Knee 2023, 43, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Fucentese, S.F.; Meier, P.; Jud, L.; Köchli, G.L.; Aichmair, A.; Vlachopoulos, L.; Fürnstahl, P. Accuracy of 3D-planned patient specific instrumentation in high tibial open wedge valgisation osteotomy. J. Exp. Orthop. 2020, 7, 7. [Google Scholar] [CrossRef]
- Ogino, T.; Kumagai, K.; Yamada, S.; Akamatsu, T.; Nejima, S.; Sotozawa, M.; Inaba, Y. Relationship between the bony correction angle and mechanical axis change and their differences between closed and open wedge high tibial osteotomy. BMC Musculoskelet. Disord. 2020, 21, 675. [Google Scholar] [CrossRef]

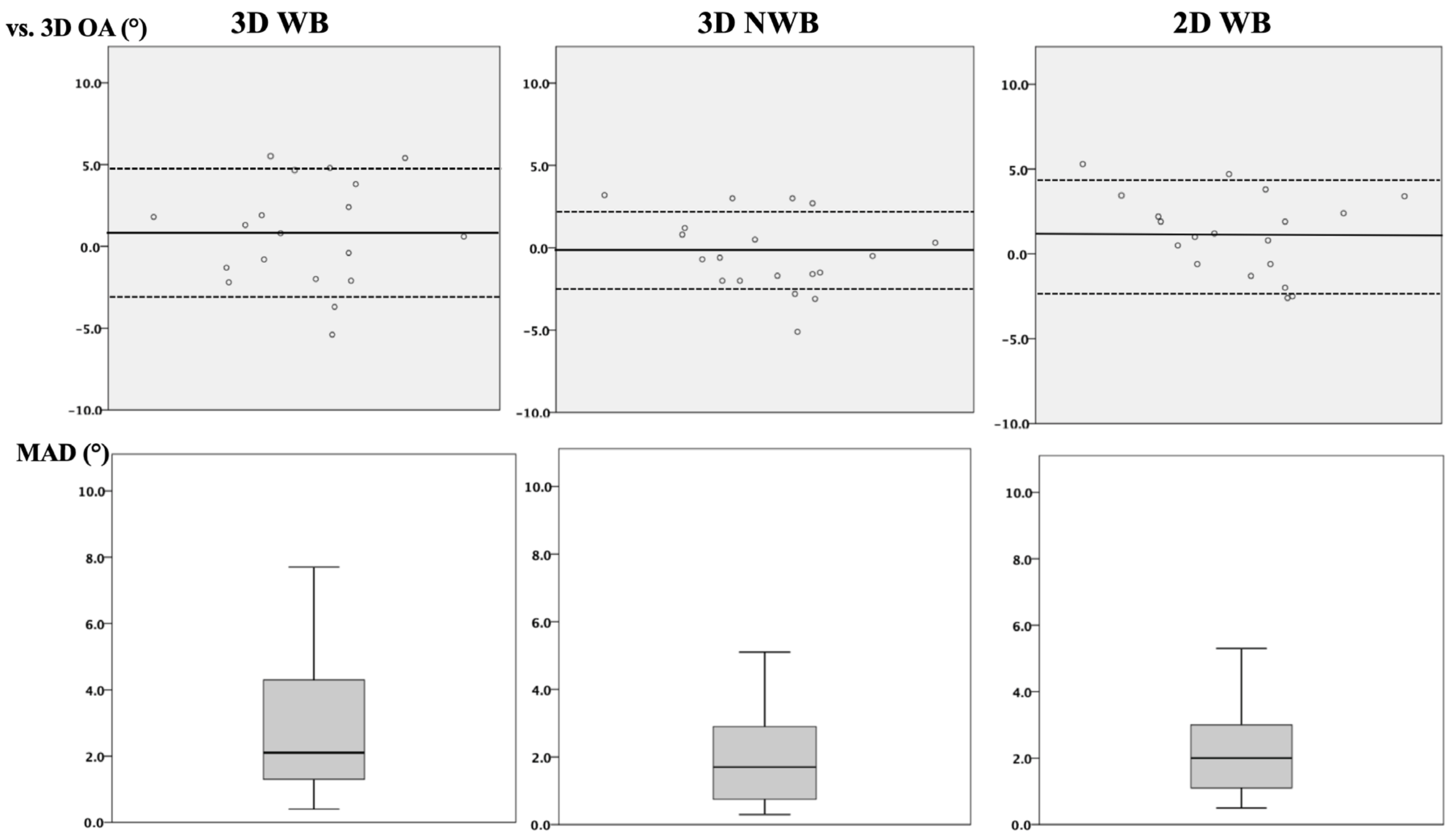
| 3D-WB vs. 3D OA | 3D-NWB vs. 3D OA | 2D-WB vs. 3D OA | p-Value | |
|---|---|---|---|---|
| ICC (95% CI) | 0.71 (0.29–0.89) + | 0.84 (0.60– 0.94) * | 0.74 (0.32–0.90) x | + = 0.005; * < 0.001; x = 0.002 |
| MAD (°) | 2.7 ± 1.8 | 1.9 ± 1.3 | 2.2 ± 1.3 | (n.s.) |
| 3D-WB | 3D NWB | 2D WB | 3D Opening Angle | p-Value | |
|---|---|---|---|---|---|
| HKA (°) | |||||
| Preoperative | 6.6 ± 4.4 | 6.0 ± 2.6 | 7.0 ± 3.3 | - | (n.s.) |
| Postoperative | −2.1 ± 2.3 * | −1.2 ± 1.9 * | −2.0 ± 1.6 | - | * 0.014 |
| Achieved surgical correction | 8.7 ± 4.9 | 7.2 ± 3.2 | 9.1 ± 3.6 | 7.6 ± 2.9 | (n.s.) |
| JLCA (°) | |||||
| Preoperative | 3.9 ± 2.2 * | 3.3 ± 2.1 | 2.6 ± 2.3 * | - | p = 0.011 |
| Postoperative | 3.4 ± 2.0 | 3.5 ± 2.0 | 1.8 ± 1.3 | - | (n.s.) |
| 3D WB vs. 3D NWB | 3D WB vs. 2D-WB | 3D NWB vs. 2D WB | |
|---|---|---|---|
| HKA preoperative | |||
| ICC (95% CI) | 0.88 (0.70–0.95) (p < 0.001) | 0.91 (0.79–0.97) (p < 0.001) | 0.89 (0.64–0.96) (p < 0.001) |
| MAD: Mean ± SD | 1.9 ± 1.5 | 1.5 ± 1.6 | 1.5 ± 1.2 |
| p-value (Friedman’s) | p = 0.12 | p = 0.12 | p = 0.12 |
| JLCA preoperative | |||
| ICC (95% CI) | 0.94 (0.70–0.98) (p < 0.001) | 0.62 (0.06–0.85) (p = 0.01) | 0.57 (−0.08–0.83) (p = 0.039) |
| MAD: Mean ± SD | 0.9 ± 0.6 | 2.1 ± 2.5 | 2.0 ± 1.5 |
| p-value (Friedman’s) | p = 0.155 | p = 0.011 | p = 0.991 |
| HKA postoperative | |||
| ICC (95% CI) | 0.78 (0.40–0.91) (p < 0.001) | 0.84 (0.57–0.94) (p < 0.001) | 0.87 (0.43–0.96) (p < 0.001) |
| MAD: Mean ± SD | 1.5 ± 1.2 | 1.1 ± 1.0 | 0.9 ± 0.8 |
| p-value (Friedman’s) | p = 0.014 | p = 1.00 | p = 0.069 |
| JLCA postoperative | |||
| ICC (95% CI) | 0.96 (0.96–0.99) (p < 0.001) | 0.64 (0.12–0.86) (p = 0.013) | 0.53 (−0.13–0.82) (p = 0.048) |
| MAD: Mean ± SD | 0.4 ± 0.3 | 2.0 ± 1.3 | 2.0 ± 1.4 |
| p-value (Friedman’s) | p = 0.241 | p = 0.241 | p = 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodel, S.; Hasler, J.; Roth, T.A.; Flury, A.; Sutter, C.; Fucentese, S.F.; Fürnstahl, P.; Vlachopoulos, L. Validation of a Three-Dimensional Weight-Bearing Measurement Protocol for Medial Open-Wedge High Tibial Osteotomy. J. Clin. Med. 2024, 13, 1280. https://doi.org/10.3390/jcm13051280
Hodel S, Hasler J, Roth TA, Flury A, Sutter C, Fucentese SF, Fürnstahl P, Vlachopoulos L. Validation of a Three-Dimensional Weight-Bearing Measurement Protocol for Medial Open-Wedge High Tibial Osteotomy. Journal of Clinical Medicine. 2024; 13(5):1280. https://doi.org/10.3390/jcm13051280
Chicago/Turabian StyleHodel, Sandro, Julian Hasler, Tabitha Arn Roth, Andreas Flury, Cyrill Sutter, Sandro F. Fucentese, Philipp Fürnstahl, and Lazaros Vlachopoulos. 2024. "Validation of a Three-Dimensional Weight-Bearing Measurement Protocol for Medial Open-Wedge High Tibial Osteotomy" Journal of Clinical Medicine 13, no. 5: 1280. https://doi.org/10.3390/jcm13051280





