How Age Affects Graves’ Orbitopathy—A Tertiary Center Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Clinical Assessment
2.3. Statistical Evaluation
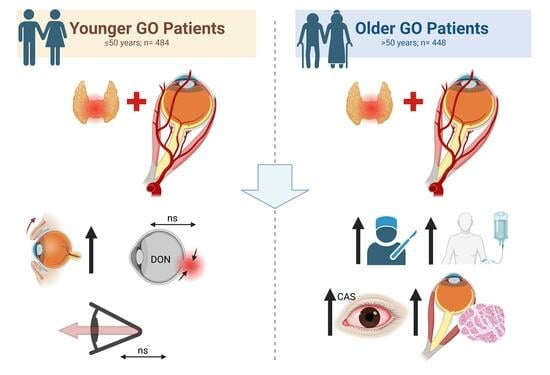
3. Results
3.1. Study Population
3.2. Clinical Examination
3.3. Motility Disorder
4. Discussion
4.1. Study Population
4.2. Clinical Presentation
4.3. Severity of GO Patients over a Ten-Year Period
4.4. Dysthyroid Optic Neuropathy
4.5. Motility Disorder
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartley, G.B.; Fatourechi, V.; Kadrmas, E.F.; Jacobsen, S.J.; Ilstrup, D.M.; Garrity, J.A.; Gorman, C.A. The incidence of Graves’ ophthalmopathy in Olmsted County, Minnesota. Am. J. Ophthalmol. 1995, 120, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Douglas, R.S. The pathophysiology of thyroid eye disease. J. Neuroophthalmol. 2014, 34, 177–185. [Google Scholar] [CrossRef]
- Bahn, R.S. Current Insights into the Pathogenesis of Graves’ Ophthalmopathy. Horm. Metab. Res. 2015, 47, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Krieger, C.C.; Place, R.F.; Bevilacqua, C.; Marcus-Samuels, B.; Abel, B.S.; Skarulis, M.C.; Kahaly, G.J.; Neumann, S.; Gershengorn, M.C. TSH/IGF-1 Receptor Cross Talk in Graves’ Ophthalmopathy Pathogenesis. J. Clin. Endocrinol. Metab. 2016, 101, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.P.; Saeed, M.E.M.; Ponto, K.A.; Elflein, H.M.; Lee, A.C.H.; Fang, S.; Zhou, H.; Frommer, L.; Langericht, J.; Efferth, T.; et al. A Multicenter, Single-Blind, Case-Control, Immunohistochemical Study of Orbital Tissue in Thyroid Eye Disease. Thyroid 2022, 32, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Gerding, M.N.; Terwee, C.B.; Dekker, F.W.; Koornneef, L.; Prummel, M.F.; Wiersinga, W.M. Quality of life in patients with Graves’ ophthalmopathy is markedly decreased: Measurement by the medical outcomes study instrument. Thyroid 1997, 7, 885–889. [Google Scholar] [CrossRef]
- Burch, H.B.; Wartofsky, L. Graves’ ophthalmopathy: Current concepts regarding pathogenesis and management. Endocr. Rev. 1993, 14, 747–793. [Google Scholar] [CrossRef]
- Dunne, J.W.; Edis, R.H. Optic nerve involvement in Graves’ ophthalmopathy: A case report and review. Aust. N. Z. J. Med. 1985, 15, 258–261. [Google Scholar] [CrossRef]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M.; on behalf of the European Group on Graves. Orbitopathy (EUGOGO) The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef]
- Eckstein, A.; Esser, J.; Oeverhaus, M.; Saeed, P.; Jellema, H.M. Surgical Treatment of Diplopia in Graves Orbitopathy Patients. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S75–S84. [Google Scholar] [CrossRef]
- Oeverhaus, M.; Stohr, M.; Moller, L.; Fuhrer, D.; Eckstein, A. Graves’ Orbitopathy: Current Concepts for Medical Treatment. Klin. Monatsblatter Augenheilkd. 2021, 238, 24–32. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marino, M.; Vaidya, B.; Wiersinga, W.M.; Dagger, E. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Westermark, K.; Dahlberg, P.A.; Jansson, R.; Enoksson, P. Ophthalmopathy and thyroid stimulation. Lancet 1989, 2, 691. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.; Zarkovic, M.; Bartalena, L.; Donati, S.; Perros, P.; Okosieme, O.; Morris, D.; Fichter, N.; Lareida, J.; von Arx, G.; et al. Predictive score for the development or progression of Graves’ orbitopathy in patients with newly diagnosed Graves’ hyperthyroidism. Eur. J. Endocrinol. 2018, 178, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.K.; Plicht, M.; Lax, H.; Neuhauser, M.; Mann, K.; Lederbogen, S.; Heckmann, C.; Esser, J.; Morgenthaler, N.G. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J. Clin. Endocrinol. Metab. 2006, 91, 3464–3470. [Google Scholar] [CrossRef]
- Stohr, M.; Oeverhaus, M.; Lytton, S.D.; Horstmann, M.; Zwanziger, D.; Moller, L.; Stark, A.; Fuhrer-Sakel, D.; Bechrakis, N.; Berchner-Pfannschmidt, U.; et al. Predicting the Course of Graves’ Orbitopathy Using Serially Measured TSH-Receptor Autoantibodies by Automated Binding Immunoassays and the Functional Bioassay. Horm. Metab. Res. 2021, 53, 435–443. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Bartalena, L.; Hegedus, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid. J. 2018, 7, 167–186. [Google Scholar] [CrossRef]
- Kendler, D.L.; Lippa, J.; Rootman, J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch. Ophthalmol. 1993, 111, 197–201. [Google Scholar] [CrossRef]
- Perros, P.; Crombie, A.L.; Matthews, J.N.; Kendall-Taylor, P. Age and gender influence the severity of thyroid-associated ophthalmopathy: A study of 101 patients attending a combined thyroid-eye clinic. Clin. Endocrinol. 1993, 38, 367–372. [Google Scholar] [CrossRef]
- Lin, M.C.; Hsu, F.M.; Bee, Y.S.; Ger, L.P. Age influences the severity of Graves’ ophthalmopathy. Kaohsiung J. Med. Sci. 2008, 24, 283–288. [Google Scholar] [CrossRef]
- Abraham-Nordling, M.; Bystrom, K.; Torring, O.; Lantz, M.; Berg, G.; Calissendorff, J.; Nystrom, H.F.; Jansson, S.; Jorneskog, G.; Karlsson, F.A.; et al. Incidence of hyperthyroidism in Sweden. Eur. J. Endocrinol. 2011, 165, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Berman, D.C.; Bulow Pedersen, I.; Andersen, S.; Carle, A. Incidence and clinical presentation of moderate to severe graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J. Clin. Endocrinol. Metab. 2012, 97, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Hegedus, L.; Bartalena, L.; Marcocci, C.; Kahaly, G.J.; Baldeschi, L.; Salvi, M.; Lazarus, J.H.; Eckstein, A.; Pitz, S.; et al. Graves’ orbitopathy as a rare disease in Europe: A European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J. Rare Dis. 2017, 12, 72. [Google Scholar] [CrossRef]
- Vestergaard, P. Smoking and thyroid disorders—A meta-analysis. Eur. J. Endocrinol. 2002, 146, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Manji, N.; Carr-Smith, J.D.; Boelaert, K.; Allahabadia, A.; Armitage, M.; Chatterjee, V.K.; Lazarus, J.H.; Pearce, S.H.; Vaidya, B.; Gough, S.C.; et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J. Clin. Endocrinol. Metab. 2006, 91, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, G.J.; Katz, G.; Zloto, O.; Leiba, H.; Hadas, B.; Huna-Baron, R. Age differences in clinical manifestation and prognosis of thyroid eye disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Leiba, H.; Landau, K.; Zloto, O.; Huna-Baron, R. Clinical profile of 80-year-old and older thyroid eye disease patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Oeverhaus, M.; Winkler, L.; Stahr, K.; Daser, A.; Bechrakis, N.; Stohr, M.; Chen, Y.; Eckstein, A. Influence of biological sex, age and smoking on Graves’ orbitopathy—A ten-year tertiary referral center analysis. Front. Endocrinol. 2023, 14, 1160172. [Google Scholar] [CrossRef]
- Burch, H.B.; Perros, P.; Bednarczuk, T.; Cooper, D.S.; Dolman, P.J.; Leung, A.M.; Mombaerts, I.; Salvi, M.; Stan, M.N. Management of Thyroid Eye Disease: A Consensus Statement by the American Thyroid Association and the European Thyroid Association. Thyroid 2022, 32, 1439–1470. [Google Scholar] [CrossRef]
- Haase, W. Measurements of the maximal excursion-ability of the globe (author’s transl). Albrecht Graefes Arch. Klin. Exp. Ophthalmol. Albrecht Graefe’s Arch. Clin. Exp. Ophthalmol. 1976, 198, 291–296. [Google Scholar] [CrossRef]
- Mourits, M.P.; Koornneef, L.; Wiersinga, W.M.; Prummel, M.F.; Berghout, A.; van der Gaag, R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: A novel approach. Br. J. Ophthalmol. 1989, 73, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.P.; Prummel, M.F.; Wiersinga, W.M.; Koornneef, L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin. Endocrinol. 1997, 47, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nordyke, R.A.; Gilbert, F.I., Jr.; Harada, A.S. Graves’ disease. Influence of age on clinical findings. Arch. Intern. Med. 1988, 148, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Torring, O.; Tallstedt, L.; Wallin, G.; Lundell, G.; Ljunggren, J.G.; Taube, A.; Saaf, M.; Hamberger, B. Graves’ hyperthyroidism: Treatment with antithyroid drugs, surgery, or radioiodine—A prospective, randomized study. Thyroid Study Group. J. Clin. Endocrinol. Metab. 1996, 81, 2986–2993. [Google Scholar] [CrossRef]
- Ma, C.; Xie, J.; Wang, H.; Li, J.; Chen, S. Radioiodine therapy versus antithyroid medications for Graves’ disease. Cochrane Database Syst. Rev. 2016, 2, CD010094. [Google Scholar] [CrossRef]
- Bartley, G.B.; Fatourechi, V.; Kadrmas, E.F.; Jacobsen, S.J.; Ilstrup, D.M.; Garrity, J.A.; Gorman, C.A. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am. J. Ophthalmol. 1996, 121, 284–290. [Google Scholar] [CrossRef]
- Cockerham, K.P.; Hidayat, A.A.; Brown, H.G.; Cockerham, G.C.; Graner, S.R. Clinicopathologic evaluation of the Mueller muscle in thyroid-associated orbitopathy. Ophthalmic Plast. Reconstr. Surg. 2002, 18, 11–17. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Fang, S.; Huang, Y.; Li, Y.; Zhong, S.; Wang, Y.; Zhang, S.; Zhou, H.; Sun, J.; et al. Age-related difference in extraocular muscles and its relation to clinical manifestations in an ethnically homogenous group of patients with Graves’ orbitopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 583–589. [Google Scholar] [CrossRef]
- Schuh, A.; Ayvaz, G.; Baldeschi, L.; Baretic, M.; Bechtold, D.; Boschi, A.; Brix, T.H.; Burlacu, M.C.; Ciric, J.; Covelli, D.; et al. Presentation of Graves’ orbitopathy within European Group on Graves’ Orbitopathy (EUGOGO) centres from 2012 to 2019 (PREGO III). Br. J. Ophthalmol. 2023. [Google Scholar] [CrossRef]
- Perros, P.; Crombie, A.L.; Kendall-Taylor, P. Natural history of thyroid associated ophthalmopathy. Clin. Endocrinol. 1995, 42, 45–50. [Google Scholar] [CrossRef]
- Perros, P.; Zarkovic, M.; Azzolini, C.; Ayvaz, G.; Baldeschi, L.; Bartalena, L.; Boschi, A.; Bournaud, C.; Brix, T.H.; Covelli, D.; et al. PREGO (presentation of Graves’ orbitopathy) study: Changes in referral patterns to European Group On Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br. J. Ophthalmol. 2015, 99, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Neigel, J.M.; Rootman, J.; Belkin, R.I.; Nugent, R.A.; Drance, S.M.; Beattie, C.W.; Spinelli, J.A. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 1988, 95, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Saeed, P.; Tavakoli Rad, S.; Bisschop, P. Dysthyroid Optic Neuropathy. Ophthalmic Plast. Reconstr. Surg. 2018, 34 (Suppl. S1), S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Trobe, J.D.; Glaser, J.S.; Laflamme, P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch. Ophthalmol. 1978, 96, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chin, Y.H.; Ng, C.H.; Nistala, K.R.Y.; Ow, Z.G.W.; Sundar, G.; Yang, S.P.; Khoo, C.M. Risk Factors of Thyroid Eye Disease. Endocr. Pract. 2021, 27, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.; Kelly, S.P.; Harrison, R.A.; Edwards, R. Cigarette smoking and thyroid eye disease: A systematic review. Eye 2007, 21, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.Y.; Yoon, J.S. Risk factors associated with the severity of thyroid-associated orbitopathy in Korean patients. Korean J. Ophthalmol. 2010, 24, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Stahr, K.; Daser, A.; Oeverhaus, M.; Hussain, T.; Lang, S.; Eckstein, A.; Mattheis, S. Proposing a surgical algorithm for graduated orbital decompression in patients with Graves’ orbitopathy. Eur. Arch. Otorhinolaryngol. 2022, 279, 2401–2407. [Google Scholar] [CrossRef]
- Paridaens, D.; Hans, K.; van Buitenen, S.; Mourits, M.P. The incidence of diplopia following coronal and translid orbital decompression in Graves’ orbitopathy. Eye 1998, 12 Pt 5, 800–805. [Google Scholar] [CrossRef]
- Oeverhaus, M.; Copei, A.; Mattheis, S.; Ringelstein, A.; Tiemessen, M.; Esser, J.; Eckstein, A.; Stahr, K. Influence of orbital morphology on proptosis reduction and ocular motility after decompression surgery in patients with Graves’ orbitopathy. PLoS ONE 2019, 14, e0218701. [Google Scholar] [CrossRef]
- Jellema, H.M.; Eckstein, A.; Oeverhaus, M.; Lacraru, I.; Saeed, P. Incidence of A pattern strabismus after inferior rectus recession in patients with Graves’ orbitopathy: A retrospective multicentre study. Acta Ophthalmol. 2022, 101, E106–E112. [Google Scholar] [CrossRef] [PubMed]
- Oeverhaus, M.; Fischer, M.; Hirche, H.; Schluter, A.; Esser, J.; Eckstein, A.K. Tendon Elongation with Bovine Pericardium in Patients with Severe Esotropia after Decompression in Graves’ Orbitopathy-efficacy and Long-term Stability. Strabismus 2018, 26, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M. Squint surgery in TED—Hints and fints, or why Graves’ patients are difficult patients. Orbit 2009, 28, 245–250. [Google Scholar] [PubMed]





| All (n = 932) | >50 (n = 448) | ≤50 (n = 484) | p | |
|---|---|---|---|---|
| Age at onset | 49.6 ± 13 | 60.4 ± 8 | 39.2 ± 8 | <0.0001 a |
| Thyroid disease | ||||
| Graves’ disease | 88% | 89% | 87% | 0.06 b |
| Hypothyroidism | 7% | 8% | 8% | 0.81 b |
| Eutyhroidism | 4% | 4% | 4% | 0.62 b |
| Thyroid treatment | ||||
| ATD | 27% | 23% | 27% | 0.102 b |
| Thyroidectomy | 39% | 40% | 37% | 0.4583 b |
| Primary RAI | 26% | 34% | 22% | 0.0001 b |
| GO status at baseline | ||||
| Mild | 41% | 33% | 53% | 0.0001 b |
| Moderate-to-severe | 55% | 64% | 44% | 0.0001 b |
| Sight-threatening | 3% | 3% | 3% | 1.000 b |
| Asymmetric GO | 22% | 24% | 21% | 0.2717 b |
| Treatment period | 13.3 ± 19 | 15.1 ± 20 | 11.7 ± 17 | 0.008 a |
| Smoking status | ||||
| Non-smoker | 36% | 36% | 37% | 0.8650 b |
| Smoker | 50% | 47% | 53% | 0.1201 b |
| Past smoker | 13% | 17% | 10% | 0.0157 b |
| Cigarettes per day | 13.9 ± 9 | 13.8 ± 9 | 13.9 ± 9 | 0.95 a |
| Treatments | ||||
| Steroids | 49% | 58% | 42% | 0.0001 b |
| Orbital irradiation | 29% | 39% | 20% | 0.0001 b |
| Lid surgery | 16% | 20% | 12% | 0.0015 b |
| Eye muscle surgery | 19% | 26% | 13% | 0.0001 b |
| No. of procedures | 1.53 ± 0.8 | 0.41 ± 0.8 | 0.2 ± 0.5 | <0.0001 a. |
| Orbital decompression | 21% | 21% | 20% | 0.7465 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oeverhaus, M.; Sander, J.; Smetana, N.; Bechrakis, N.E.; Inga, N.; Al-Ghazzawi, K.; Chen, Y.; Eckstein, A. How Age Affects Graves’ Orbitopathy—A Tertiary Center Study. J. Clin. Med. 2024, 13, 290. https://doi.org/10.3390/jcm13010290
Oeverhaus M, Sander J, Smetana N, Bechrakis NE, Inga N, Al-Ghazzawi K, Chen Y, Eckstein A. How Age Affects Graves’ Orbitopathy—A Tertiary Center Study. Journal of Clinical Medicine. 2024; 13(1):290. https://doi.org/10.3390/jcm13010290
Chicago/Turabian StyleOeverhaus, Michael, Julius Sander, Nicolai Smetana, Nikolaos E. Bechrakis, Neumann Inga, Karim Al-Ghazzawi, Ying Chen, and Anja Eckstein. 2024. "How Age Affects Graves’ Orbitopathy—A Tertiary Center Study" Journal of Clinical Medicine 13, no. 1: 290. https://doi.org/10.3390/jcm13010290