CT Coronary Angiography: Technical Approach and Atherosclerotic Plaque Characterization
Abstract
:1. Introduction
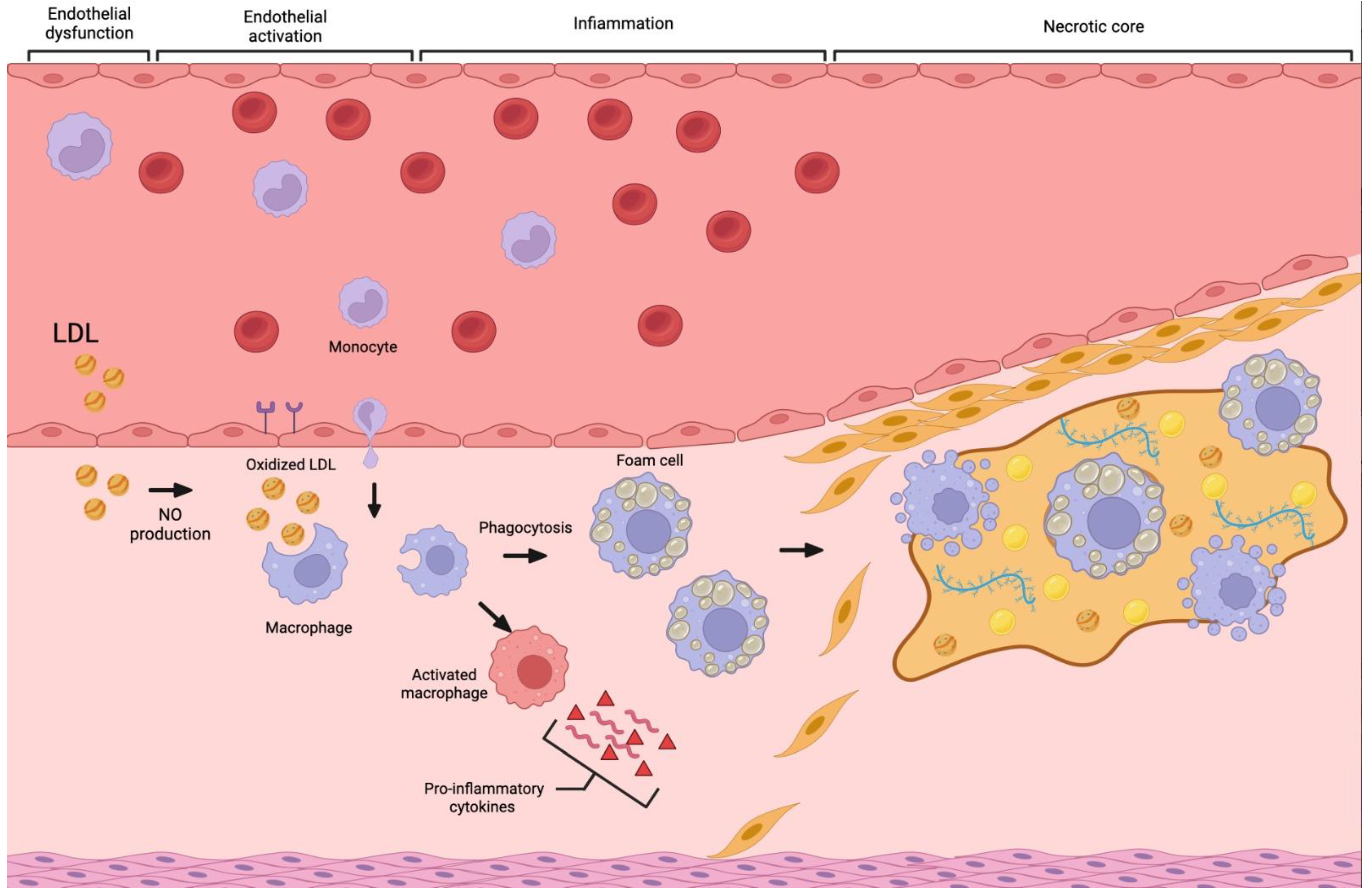
2. Atherosclerosis
3. CCTA Imaging Technique
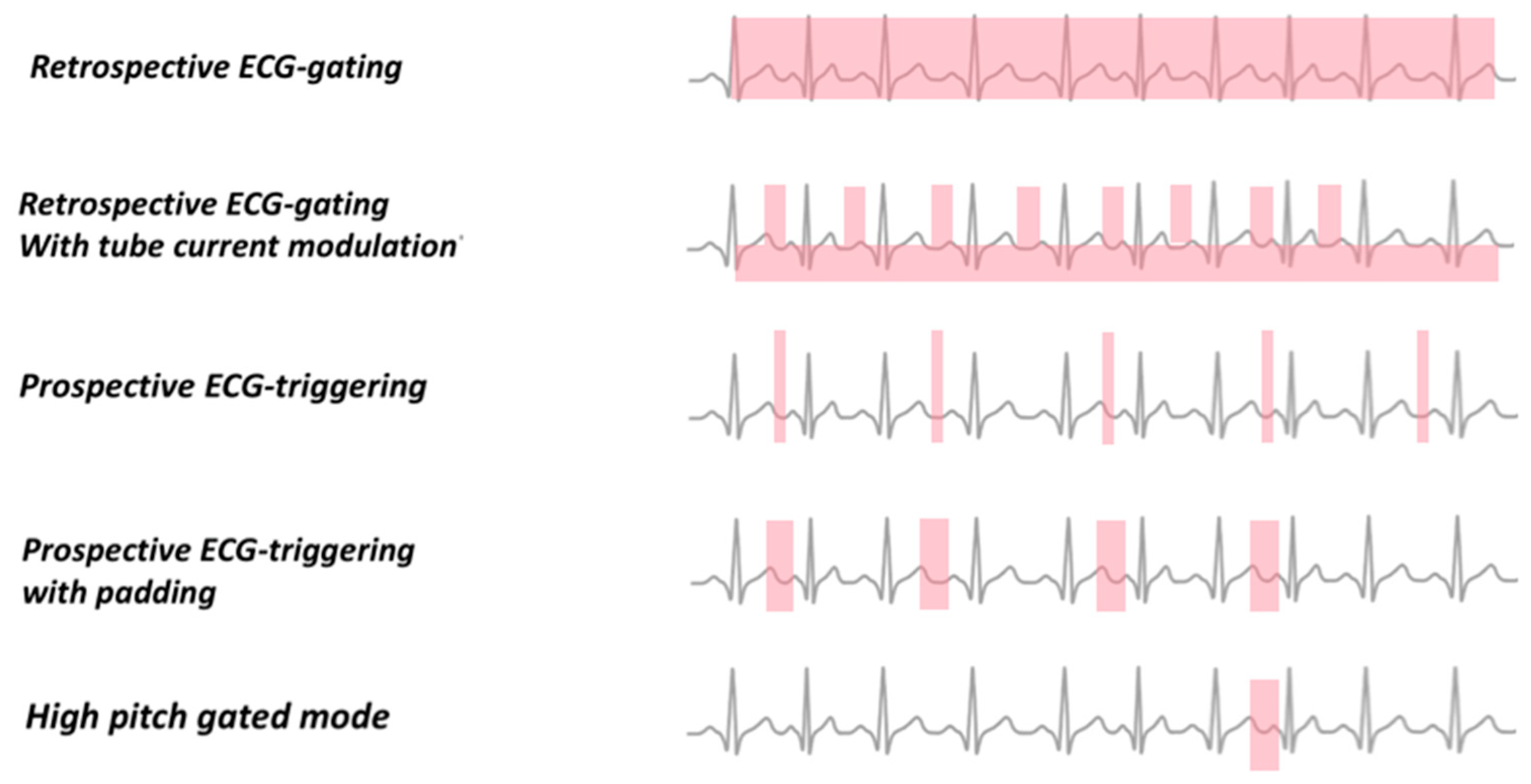
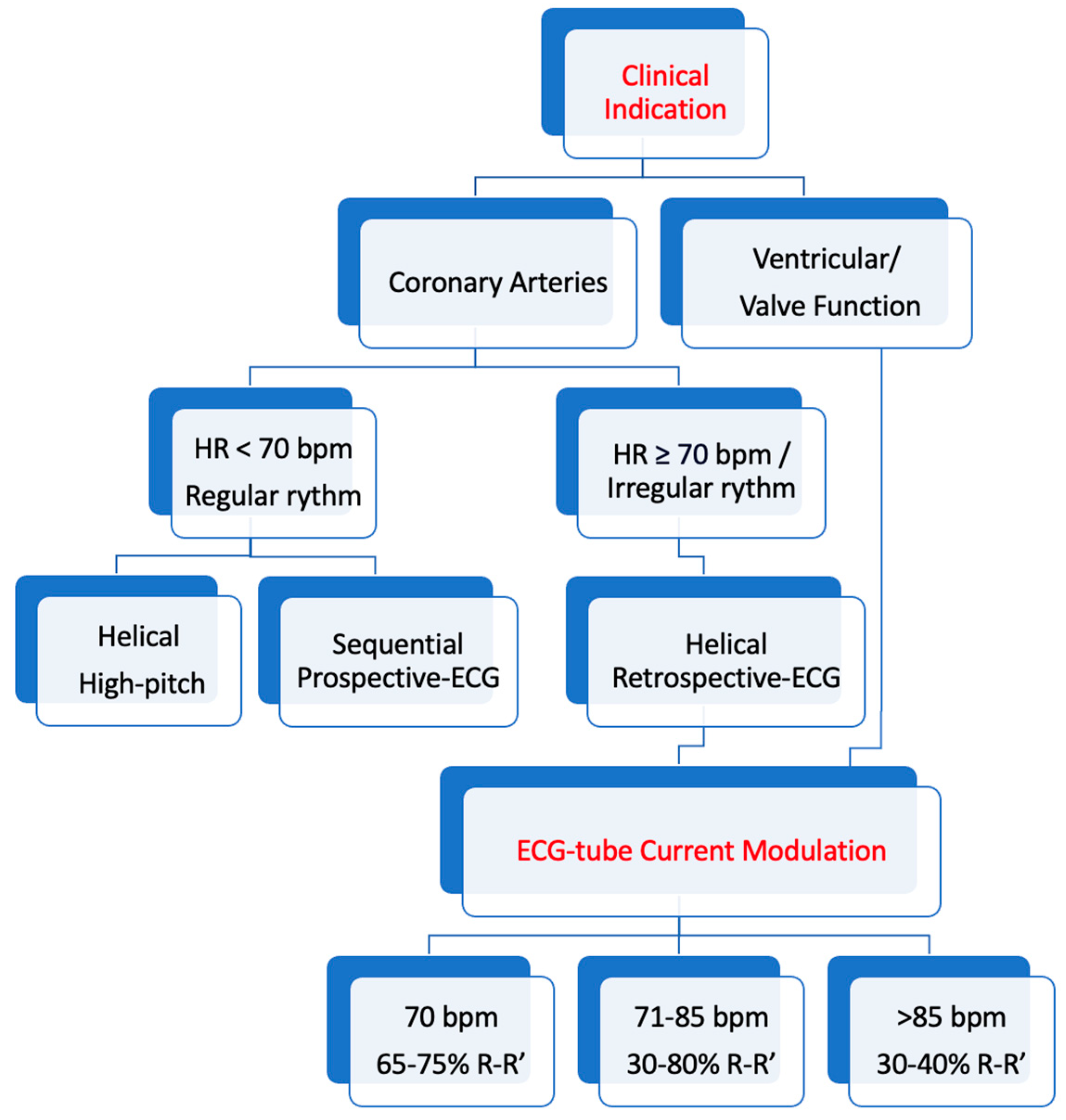
4. ECG Gating
4.1. Retrospective ECG Gating
4.2. Sequential Mode Prospective ECG Gating
4.3. Prospective ECG Gating with Spiral Data Acquisition and High Pitch
5. Coronary Artery Calcium Scoring
6. High-Risk Plaque Features at CCTA
6.1. Low Attenuation
6.2. Positive Remodeling
6.3. Spotty Calcifications
6.4. Napkin-Ring Sign
6.5. Pericoronary Fat
| First Author | Publication Year | Study Design | Patients (n) | Aim of the Study |
|---|---|---|---|---|
| Williams [60] | 2020 | Multicenter randomized controlled trial | 1769 | To explore whether the quantification of low-attenuation plaque identified by CCTA enhances the ability to predict fatal or nonfatal MI when compared to traditional cardiovascular risk scores, Agatston scoring and the severity of obstructive CAD in stable patients presenting with chest pain. |
| Williams [69] | 2019 | Multicenter randomized controlled trial | 4146 | To assess the prognostic implications of adverse coronary plaque characteristics with CCTA. |
| Puchner [79] | 2014 | Multicenter randomized controlled trial | 1000 | To assess whether the identification of high-risk plaque features detected by CCTA in the emergency department can enhance the diagnostic accuracy of ACS beyond the presence of significant CAD and clinical risk assessment in patients experiencing acute chest pain but without objective evidence of myocardial ischemia or MI. |
| Otsuka [80] | 2013 | Prospective study | 960 | To determine the predictive value of the napkin-ring sign detected by CCTA for future ACS events in patients with CAD. |
| Sun [91] | 2022 | Prospective study | 130 | To examine the correlation between pericoronary inflammation and plaque morphology and components with CCTA in individuals with non-ST elevation ACS |
6.6. Geometry of Coronary Plaques
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Kuller, L.H.; Arnold, A.M.; Psaty, B.M.; Robbins, J.A.; O’leary, D.H.; Tracy, R.P.; Burke, G.L.; Manolio, T.A.; Chaves, P.H.M. 10-Year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the cardiovascular health study. Arch. Intern. Med. 2006, 166, 71–78. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Chung, H.; Detrano, R.; Post, W.; Kronmal, R.A. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006, 113, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Dowsley, T.; Al-Mallah, M.; Ananthasubramaniam, K.; Dwivedi, G.; McArdle, B.; Chow, B.J.W. The Role of Noninvasive Imaging in Coronary Artery Disease Detection, Prognosis, and Clinical Decision Making. Can. J. Cardiol. 2013, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Barbato, E.; Capodanno, D.; Edvardsen, T.; Prescott, E.; Achenbach, S.; Bax, J.J.; Wijns, W.; Knuuti, J. Imaging in ESC clinical guidelines: Chronic coronary syndromes. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Friedrich, M.G.; Nagel, E.; Kramer, C.M.; Kaufmann, P.A.; Farkhooy, A.; Dilsizian, V.; Flachskampf, F.A. CV imaging: What was new in 2012? JACC Cardiovasc. Imaging 2013, 6, 714–734. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Maurovich-Horvat, P.; Ferencik, M.; Voros, S.; Merkely, B.; Hoffmann, U. Comprehensive plaque assessment by coronary CT angiography. Nat. Rev. Cardiol. 2014, 11, 390–402. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Rybicki, F.J.; Udelson, J.E.; Peacock, W.F.; Goldhaber, S.Z.; Isselbacher, E.M.; Kazerooni, E.; Kontos, M.C.; Litt, H.; Woodard, P.K. 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients with Chest Pain: A Joint Document of the American College of Radiology Appropriateness Criteria Comm. J. Am. Coll. Cardiol. 2016, 67, 853–879. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Benedetti, G.; Del Maschio, A.; De Cobelli, F.; Esposito, A. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: Impact of experience in the clinical practice. Radiol. Med. 2020, 125, 128–136. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Barbera, M.; Vignale, D.; Benedetti, G.; Spoladore, R.; Ancona, M.B.; Giannini, F.; Oppizzi, M.; Del Maschio, A.; et al. Cardiac Computed Tomography in Troponin-Positive Chest Pain: Sometimes the Answer Lies in the Late Iodine Enhancement or Extracellular Volume Fraction Map. JACC Cardiovasc. Imaging 2019, 12, 745–748. [Google Scholar] [CrossRef]
- Chen, J.; Wetzel, L.H.; Pope, K.L.; Meek, L.J.; Rosamond, T.; Walker, C.M. FFRCT: Current Status. Am. J. Roentgenol. 2021, 216, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.; Puglia, M.; Morisco, C.; Barbuto, L.; Rapacciuolo, A.; Santoro, M.; Spinelli, L.; Trimarco, B.; Cuocolo, A.; Imbriaco, M. A unique association of arrhythmogenic right ventricular dysplasia and acute myocarditis, as assessed by cardiac MRI: A case report. BMC Cardiovasc. Disord. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Henzler, T.; Porubsky, S.; Kayed, H.; Harder, N.; Krissak, U.R.; Meyer, M.; Sueselbeck, T.; Marx, A.; Michaely, H.; Schoepf, U.J.; et al. Attenuation-based characterization of coronary atherosclerotic plaque: Comparison of dual source and dual energy CT with single-source CT and histopathology. Eur. J. Radiol. 2011, 80, 54–59. [Google Scholar] [CrossRef]
- Cai, J.-M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of Human Carotid Atherosclerotic Lesions with In Vivo Multicontrast Magnetic Resonance Imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Vulnerable Plaque: The Pathology of Unstable Coronary Lesions. J. Interv. Cardiol. 2002, 15, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Ascione, R.; De Cobelli, F.; Esposito, A. Heart Diseases in Geriatric Patients. In Imaging in Geriatrics; Guglielmi, G., Maas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 109–135. [Google Scholar] [CrossRef]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef]
- Lewis, M.A.; Pascoal, A.; Keevil, S.F.; Lewis, C.A. Selecting a CT scanner for cardiac imaging: The heart of the matter. Br. J. Radiol. 2016, 89, 20160376. [Google Scholar] [CrossRef] [PubMed]
- Dell’aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Flohr, T.G.; Leng, S.; Yu, L.; Allmendinger, T.; Bruder, H.; Petersilka, M.; Eusemann, C.D.; Stierstorfer, K.; Schmidt, B.; McCollough, C.H. Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: Image reconstruction and assessment of image quality. Med. Phys. 2009, 36, 5641–5653. [Google Scholar] [CrossRef]
- Barreto, M.; Schoenhagen, P.; Nair, A.; Amatangelo, S.; Milite, M.; Obuchowski, N.A.; Lieber, M.L.; Halliburton, S.S. Potential of dual-energy computed tomography to characterize atherosclerotic plaque: Ex vivo assessment of human coronary arteries in comparison to histology. J. Cardiovasc. Comput. Tomogr. 2008, 2, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Dalager, M.G.; Bøttcher, M.; Andersen, G.; Thygesen, J.; Pedersen, E.M.; Dejbjerg, L.; Gøtzsche, O.; Bøtker, H.E. Impact of luminal density on plaque classification by CT coronary angiography. Int. J. Cardiovasc. Imaging 2011, 27, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Boehmer, K.; Pflederer, T.; Ropers, D.; Seltmann, M.; Lell, M.; Anders, K.; Kuettner, A.; Uder, M.; Daniel, W.G.; et al. Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J. Cardiovasc. Comput. Tomogr. 2010, 4, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Runza, G.; Belgrano, M.; Luccichenti, G.; Mollet, N.R.; Malagutti, P.; Silvestrini, M.; Midiri, M.; Cova, M.; Mucelli, R.P.; et al. Introduction to coronary imaging with 64-slice Computed Tomography. Radiol. Med. 2015, 110, 16–41. [Google Scholar]
- Desjardins, B.; Kazerooni, E.A. ECG-Gated Cardiac CT. Am. J. Roentgenol. 2004, 182, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.G.; De Cecco, C.N.; Schmidt, B.; Wang, R.; Schoepf, U.J.; Meinel, F.G. Computed Tomographic Assessment of Coronary Artery Disease. Radiol. Clin. N. Am. 2015, 53, 271–285. [Google Scholar] [CrossRef]
- Einstein, A.J.; Henzlova, M.J.; Rajagopalan, S. Estimating Risk of Cancer Associated with Radiation Exposure from 64-Slice Computed Tomography Coronary Angiography. JAMA 2007, 298, 317–323. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z. Coronary CT angiography with low radiation dose. Int. J. Cardiovasc. Imaging 2010, 26 (Suppl. 1), 17–25. [Google Scholar] [CrossRef]
- Jakobs, T.F.; Becker, C.R.; Ohnesorge, B.; Flohr, T.; Suess, C.; Schoepf, U.J.; Reiser, M.F. Multislice helical CT of the heart with retrospective ECG gating: Reduction of radiation exposure by ECG-controlled tube current modulation. Eur. Radiol. 2002, 12, 1081–1086. [Google Scholar] [CrossRef]
- Bischoff, B.; Hein, F.; Meyer, T.; Hadamitzky, M.; Martinoff, S.; Schömig, A.; Hausleiter, J. Trends in radiation protection in CT: Present and future status. J. Cardiovasc. Comput. Tomogr. 2009, 3, S65–S73. [Google Scholar] [CrossRef]
- Hausleiter, J.; Meyer, T.; Hadamitzky, M.; Huber, E.; Zankl, M.; Martinoff, S.; Kastrati, A.; Schomig, A. Radiation Dose Estimates from Cardiac Multislice Computed Tomography in Daily Practice. Circulation 2006, 113, 1305–1310. [Google Scholar] [CrossRef]
- Schroeder, S.; Achenbach, S.; Bengel, F.; Burgstahler, C.; Cademartiri, F.; de Feyter, P.; George, R.; Kaufmann, P.; Kopp, A.F.; Knuuti, J.; et al. Cardiac computed tomography: Indications, applications, limitations, and training requirements: Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur. Heart J. 2008, 29, 531–556. [Google Scholar] [CrossRef]
- Alkadhi, H.; Leschka, S. Radiation dose of cardiac computed tomography—What has been achieved and what needs to be done. Eur. Radiol. 2011, 21, 505–509. [Google Scholar] [CrossRef]
- Harder, A.D.; Willemink, M.; de Jong, P.; Schilham, A.; Rajiah, P.; Takx, R.; Leiner, T. New horizons in cardiac CT. Clin. Radiol. 2016, 71, 758–767. [Google Scholar] [CrossRef] [PubMed]
- von Ballmoos, M.W.; Haring, B.; Juillerat, P.; Alkadhi, H. Meta-analysis: Diagnostic Performance of Low-Radiation-Dose Coronary Computed Tomography Angiography. Ann. Intern. Med. 2011, 154, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.; Unterberg-Buchwald, C.; Staab, W.; Sohns, J.M.; Hosseini, A.S.A.; Schwarz, A. Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: Meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am. Heart J. 2013, 165, 154–163.e3. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Masukawa, A.; Tanaka, I.; Fukui, R.; Suzuki, K.; Ueno, E.; Kodera, K.; Nakano, K.; Shen, Y. Prospective Electrocardiogram-Gated Axial 64-Detector Computed Tomographic Angiography vs Retrospective Gated Helical Technique to Assess Coronary Artery Bypass Graft Anastomosis: Comparison of Image Quality and Patient Radiation Dose. Circ. J. 2010, 74, 735–740. [Google Scholar] [CrossRef] [PubMed]
- LaBounty, T.M.; Leipsic, J.; Min, J.K.; Heilbron, B.; Mancini, G.B.J.; Lin, F.Y.; Earls, J.P. Effect of Padding Duration on Radiation Dose and Image Interpretation in Prospectively ECG-Triggered Coronary CT Angiography. Am. J. Roentgenol. 2012, 194, 933–937. [Google Scholar] [CrossRef]
- Gordic, S.; Desbiolles, L.; Sedlmair, M.; Manka, R.; Plass, A.; Schmidt, B.; Husarik, D.B.; Maisano, F.; Wildermuth, S.; Alkadhi, H.; et al. Optimizing radiation dose by using advanced modelled iterative reconstruction in high-pitch coronary CT angiography. Eur. Radiol. 2016, 26, 459–468. [Google Scholar] [CrossRef]
- Achenbach, S.; Marwan, M.; Schepis, T.; Pflederer, T.; Bruder, H.; Allmendinger, T.; Petersilka, M.; Anders, K.; Lell, M.; Kuettner, A.; et al. High-pitch spiral acquisition: A new scan mode for coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2009, 3, 117–121. [Google Scholar] [CrossRef]
- Hassan, A.; Nazir, S.A.; Alkadhi, H. Technical challenges of coronary CT angiography: Today and tomorrow. Eur. J. Radiol. 2011, 79, 161–171. [Google Scholar] [CrossRef]
- Leschka, S.; Stolzmann, P.; Desbiolles, L.; Baumueller, S.; Goetti, R.; Schertler, T.; Scheffel, H.; Plass, A.; Falk, V.; Feuchtner, G.; et al. Diagnostic accuracy of high-pitch dual-source CT for the assessment of coronary stenoses: First experience. Eur. Radiol. 2009, 19, 2896–2903. [Google Scholar] [CrossRef]
- Schuhbaeck, A.; Achenbach, S.; Layritz, C.; Eisentopf, J.; Hecker, F.; Pflederer, T.; Gauss, S.; Rixe, J.; Kalender, W.; Daniel, W.G.; et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur. Radiol. 2013, 23, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Clayton, B.; Roobottom, C.; Morgan-Hughes, G. CT coronary angiography in atrial fibrillation: A comparison of radiation dose and diagnostic confidence with retrospective gating vs prospective gating with systolic acquisition. Br. J. Radiol. 2015, 88, 20150533. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Lyu, J.; Yang, Y.; Yuan, W.; Song, Z. Low kV and low concentration contrast agent with iterative reconstruction of computed tomography (CT) coronary angiography: A preliminary study. Med. Sci. Monit. 2017, 23, 5005–5010. [Google Scholar] [CrossRef]
- Benz, D.C.; Ersözlü, S.; Mojon, F.L.A.; Messerli, M.; Mitulla, A.K.; Ciancone, D.; Kenkel, D.; Schaab, J.A.; Gebhard, C.; Pazhenkottil, A.P.; et al. Radiation dose reduction with deep-learning image reconstruction for coronary computed tomography angiography. Eur. Radiol. 2022, 32, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Matsumoto, N.; Yoda, S.; Amano, Y.; Okumura, Y. Coronary artery calcium score: Current status of clinical application and how to handle the results. J. Cardiol. 2022, 79, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Clouse, M.E.; Sabir, A.; Yam, C.-S.; Yoshimura, N.; Lin, S.; Welty, F.; Martinez-Clark, P.; Raptopoulos, V. Measuring Noncalcified Coronary Atherosclerotic Plaque Using Voxel Analysis with MDCT Angiography: A Pilot Clinical Study. Am. J. Roentgenol. 2008, 190, 1553–1560. [Google Scholar] [CrossRef]
- Dey, D.; Schepis, T.; Marwan, M.; Slomka, P.J.; Berman, D.S.; Achenbach, S. Automated Three-dimensional Quantification of Noncalcified Coronary Plaque from Coronary CT Angiography: Comparison with Intravascular US. Radiology 2010, 257, 516–522. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results from the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef]
- Deseive, S.; Straub, R.; Kupke, M.; Broersen, A.; Kitslaar, P.; Massberg, S.; Hadamitzky, M.; Hausleiter, J. Quantification of coronary low-attenuation plaque volume for long-term prediction of cardiac events and reclassification of patients. J. Cardiovasc. Comput. Tomogr. 2018, 12, 118–124. [Google Scholar] [CrossRef]
- Yamaura, H.; Otsuka, K.; Ishikawa, H.; Shirasawa, K.; Fukuda, D.; Kasayuki, N. Determinants of Non-calcified Low-Attenuation Coronary Plaque Burden in Patients without Known Coronary Artery Disease: A Coronary CT Angiography Study. Front. Cardiovasc. Med. 2022, 9, 824470. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory Enlargement of Human Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Varnava, A.M.; Mills, P.G.; Davies, M.J. Relationship Between Coronary Artery Remodeling and Plaque Vulnerability. Circulation 2002, 105, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Ropers, D.; Hoffmann, U.; MacNeill, B.; Baum, U.; Pohle, K.; Brady, T.J.; Pomerantsev, E.; Ludwig, J.; Flachskampf, F.A.; et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J. Am. Coll. Cardiol. 2004, 43, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Gauss, S.; Achenbach, S.; Pflederer, T.; Schuhback, A.; Daniel, W.G.; Marwan, M. Assessment of coronary artery remodelling by dual-source CT: A head-to-head comparison with intravascular ultrasound. Heart 2011, 97, 991–997. [Google Scholar] [CrossRef]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice Computed Tomographic Characteristics of Coronary Lesions in Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sarai, M.; Narula, J.; Ozaki, Y. Coronary CT angiography and high-risk plaque morphology. Cardiovasc. Interv. Ther. 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Karanasos, A.; Ligthart, J.M.R.; Witberg, K.T.; Regar, E. Calcified Nodules. JACC Cardiovasc. Imaging 2012, 5, 1071–1072. [Google Scholar] [CrossRef]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.; Smialek, J.; Virmani, R. Coronary Risk Factors and Plaque Morphology in Men with Coronary Disease Who Died Suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef]
- Farb, A.; Tang, A.L.; Burke, A.P.; Sessums, L.; Liang, Y.; Virmani, R. Sudden Coronary Death. Circulation 1995, 92, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Virmani, R.; Younis, H.; Burke, A.P.; Kamm, R.D.; Lee, R.T. The Impact of Calcification on the Biomechanical Stability of Atherosclerotic Plaques. Circulation 2001, 103, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.; Gyöngyösi, M.; Benedek, I. Multislice Computed Tomographic Coronary Angiography for Quantitative Assessment of Culprit Lesions in Acute Coronary Syndromes. Can. J. Cardiol. 2013, 29, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Saremi, F.; Achenbach, S. Coronary Plaque Characterization Using CT. Am. J. Roentgenol. 2015, 204, W249–W260. [Google Scholar] [CrossRef]
- Burke, A.P.; Weber, D.K.; Kolodgie, F.D.; Farb, A.; Taylor, A.J.; Virmani, R. Pathophysiology of Calcium Deposition in Coronary Arteries. Herz 2001, 26, 239–244. [Google Scholar] [CrossRef]
- van Velzen, J.E.; de Graaf, F.R.; de Graaf, M.A.; Schuijf, J.D.; Kroft, L.J.; de Roos, A.; Reiber, J.H.C.; Bax, J.J.; Jukema, J.W.; Boersma, E.; et al. Comprehensive assessment of spotty calcifications on computed tomography angiography: Comparison to plaque characteristics on intravascular ultrasound with radiofrequency backscatter analysis. J. Nucl. Cardiol. 2011, 18, 893–903. [Google Scholar] [CrossRef]
- Yahagi, K.; Joner, M.; Virmani, R. The Mystery of Spotty Calcification. Circ. Cardiovasc. Imaging 2016, 9, e004252. [Google Scholar] [CrossRef]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-Risk Plaque Detected on Coronary CT Angiography Predicts Acute Coronary Syndromes Independent of Significant Stenosis in Acute Chest Pain. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef]
- Otsuka, K.; Fukuda, S.; Tanaka, A.; Nakanishi, K.; Taguchi, H.; Yoshikawa, J.; Shimada, K.; Yoshiyama, M. Napkin-Ring Sign on Coronary CT Angiography for the Prediction of Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2013, 6, 448–457. [Google Scholar] [CrossRef]
- Achenbach, S. Imaging the Vulnerable Plaque on Coronary CTA. JACC Cardiovasc. Imaging 2020, 13, 1418–1421. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Fuster, V. The Myth of the “Vulnerable Plaque”. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Feuchtner, G.; Kerber, J.; Burghard, P.; Dichtl, W.; Friedrich, G.; Bonaros, N.; Plank, F. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: A long-term follow-up study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef]
- Verhagen, S.N.; Visseren, F.L.J. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis 2011, 214, 3–10. [Google Scholar] [CrossRef]
- Margaritis, M.; Antonopoulos, A.S.; Digby, J.; Lee, R.; Reilly, S.; Coutinho, P.; Shirodaria, C.; Sayeed, R.; Petrou, M.; De Silva, R.; et al. Interactions between Vascular Wall and Perivascular Adipose Tissue Reveal Novel Roles for Adiponectin in the Regulation of Endothelial Nitric Oxide Synthase Function in Human Vessels. Circulation 2013, 127, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared with Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Sun, J.T.; Sheng, X.C.; Feng, Q.; Yin, Y.; Li, Z.; Ding, S.; Pu, J. Pericoronary Fat Attenuation Index Is Associated with Vulnerable Plaque Components and Local Immune-Inflammatory Activation in Patients with Non-ST Elevation Acute Coronary Syndrome. J. Am. Heart Assoc. 2022, 11, e022879. [Google Scholar] [CrossRef]
- Oikonomou, E.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Simantiris, S.; Oikonomou, E.; Siasos, G.; Tsioufis, K.; Tousoulis, D. Pericoronary fat attenuation index-a new imaging biomarker and its diagnostic and prognostic utility: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e526–e536. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques from Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef]
- Han, D.; Lin, A.; Kuronuma, K.; Tzolos, E.; Kwan, A.C.; Klein, E.; Andreini, D.; Bax, J.J.; Cademartiri, F.; Chinnaiyan, K.; et al. Association of Plaque Location and Vessel Geometry Determined by Coronary Computed Tomographic Angiography with Future Acute Coronary Syndrome-Causing Culprit Lesions. JAMA Cardiol. 2022, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, R.; Pelgrim, G.J.; Ebersberger, U.; Rowe, G.W.; Oudkerk, M.; Schoepf, U.J. Dual-Energy CT of the Heart. Am. J. Roentgenol. 2012, 199, S54–S63. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef] [PubMed]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; West, N.E.; Goddard, M.; Rudd, J.H.; Bennett, M.R. Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: A prospective study with tissue validation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 230–237. [Google Scholar] [CrossRef]
- Tanami, Y.; Ikeda, E.; Jinzaki, M.; Satoh, K.; Nishiwaki, Y.; Yamada, M.; Okada, Y.; Kuribayashi, S. Computed tomographic attenuation value of coronary atherosclerotic plaques with different tube voltage: An ex vivo study. J. Comput. Assist. Tomogr. 2010, 34, 58–63. [Google Scholar] [CrossRef]
- Pack, J.D.; Xu, M.; Wang, G.; Baskaran, L.; Min, J.; De Man, B. Cardiac CT blooming artifacts: Clinical significance, root causes and potential solutions. Vis. Comput. Ind. Biomed. Art 2022, 5, 29. [Google Scholar] [CrossRef]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef]
- Holmes, T.W.; Liu, L.P.; Shapira, N.; McVeigh, E.; Litt, H.I.; Pourmorteza, A.; Noel, P.B. Mixed coronary plaque characterization with the first clinical dual-source photon-counting CT scanner: A phantom study. In Proceedings of the 7th International Conference on Image Formation in X-ray Computed Tomography, Baltimore, MD, USA, 12–16 June 2022; Volume 12304. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Richards, T.; Nikpanah, M.; Sahbaee, P.; Shanbhag, S.M.; Bandettini, W.P.; Saboury, B.; Malayeri, A.A.; Pritchard, W.F.; et al. Evaluation of Coronary Plaques and Stents with Conventional and Photon-counting CT: Benefits of High-Resolution Photon-counting CT. Radiol. Cardiothorac. Imaging 2021, 3, e210102. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L.; et al. In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT. Radiology 2021, 300, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T. A New Era in Atherosclerotic Plaque Characterization with Photon-counting CT. Radiology 2021, 300, 108–109. [Google Scholar] [CrossRef]
- Song, Q.; Chen, M.; Shang, J.; Hu, Z.; Cai, H. Analysis of Predictive Model of Coronary Vulnerable Plaque under Hemodynamic Numerical Simulation. J. Heal. Eng. 2022, 2022, 3434910. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Antunovic, L.; Chiti, A.; Kirienko, M. Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Ashrafinia, S.; Dalaie, P.; Sadaghiani, M.S.; Schindler, T.H.; Pomper, M.G.; Rahmim, A. Radiomics Analysis of Clinical Myocardial Perfusion Stress SPECT Images to Identify Coronary Artery Calcification. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (Suppl. 1), S17–S18. [Google Scholar] [CrossRef]
- Ponsiglione, A.; Stanzione, A.; Cuocolo, R.; Ascione, R.; Gambardella, M.; De Giorgi, M.; Nappi, C.; Cuocolo, A.; Imbriaco, M. Cardiac CT and MRI radiomics: Systematic review of the literature and radiomics quality score assessment. Eur. Radiol. 2022, 32, 2629–2638. [Google Scholar] [CrossRef]
- De Cecco, C.N.; van Assen, M. Can Radiomics Help in the Identification of Vulnerable Coronary Plaque? Radiology 2023, 307, 223342. [Google Scholar] [CrossRef]
- Kolossváry, M.; Karády, J.; Kikuchi, Y.; Ivanov, A.; Schlett, C.L.; Lu, M.T.; Foldyna, B.; Merkely, B.; Aerts, H.J.; Hoffmann, U.; et al. Radiomics versus visual and histogram-based assessment to identify atheromatous lesions at coronary CT angiography: An ex vivo study. Radiology 2019, 293, 89–96. [Google Scholar] [CrossRef]
- Li, X.-N.; Yin, W.-H.; Sun, Y.; Kang, H.; Luo, J.; Chen, K.; Hou, Z.-H.; Gao, Y.; Ren, X.-S.; Yu, Y.-T.; et al. Identification of pathology-confirmed vulnerable atherosclerotic lesions by coronary computed tomography angiography using radiomics analysis. Eur. Radiol. 2022, 32, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, T.; Wang, Y.N.; Schoepf, U.J.; Bidwell, S.L.; Qiao, H.; Feng, Y.; Xu, C.; Xu, H.; Xie, G.; et al. A Coronary CT Angiography Radiomics Model to Identify Vulnerable Plaque and Predict Cardiovascular Events. Radiology 2023, 307, 221693. [Google Scholar] [CrossRef] [PubMed]
- Gudigar, A.; Nayak, S.; Samanth, J.; Raghavendra, U.; A J, A.; Barua, P.D.; Hasan, N.; Ciaccio, E.J.; Tan, R.-S.; Acharya, U.R. Recent trends in artificial intelligence-assisted coronary atherosclerotic plaque characterization. Int. J. Environ. Res. Public Health 2021, 18, 10003. [Google Scholar] [CrossRef] [PubMed]

| Revolution Apex (GE) | IQon Spectral CT (Philips) | Somatom Definition Flash (Siemens) | Somatom Definition Drive (Siemens) | Somatom Definition Force (Siemens) | Naeotom Alpha (Siemens) | Aquilion One (Canon) | |
|---|---|---|---|---|---|---|---|
| Detector type | Gemstone scintillator | Spectral Detector –NanoPanel Prism | 2× Multislice Stellar detector | 2× Multi-slice StellarInfnity | 2× Multislice StellarInfinity | 2× QuantaMax | 2× Pure Vision |
| Detector rows | 256 | 64 rows, 256 slice | 128 (2 × 64) | 128 (2 × 64) | 384 (2 × 192) | 288 (2 × 144) or 240 (2 × 120) | 640 |
| Gantry aperture, cm | 80 | 70 | 78 | 78 | 78 | 82 | 78 |
| z-axis, mm | 160 | 40 | 38.4 | 38.4 | 57.6 | 60 | 160 |
| Rotation time, sec | 0.23 | 0.27 | 0.28 | 0.28 | 0.25 | 0.25 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Aversana, S.; Ascione, R.; Vitale, R.A.; Cavaliere, F.; Porcaro, P.; Basile, L.; Napolitano, G.; Boccalatte, M.; Sibilio, G.; Esposito, G.; et al. CT Coronary Angiography: Technical Approach and Atherosclerotic Plaque Characterization. J. Clin. Med. 2023, 12, 7615. https://doi.org/10.3390/jcm12247615
Dell’Aversana S, Ascione R, Vitale RA, Cavaliere F, Porcaro P, Basile L, Napolitano G, Boccalatte M, Sibilio G, Esposito G, et al. CT Coronary Angiography: Technical Approach and Atherosclerotic Plaque Characterization. Journal of Clinical Medicine. 2023; 12(24):7615. https://doi.org/10.3390/jcm12247615
Chicago/Turabian StyleDell’Aversana, Serena, Raffaele Ascione, Raffaella Antonia Vitale, Fabrizia Cavaliere, Piercarmine Porcaro, Luigi Basile, Giovanni Napolitano, Marco Boccalatte, Gerolamo Sibilio, Giovanni Esposito, and et al. 2023. "CT Coronary Angiography: Technical Approach and Atherosclerotic Plaque Characterization" Journal of Clinical Medicine 12, no. 24: 7615. https://doi.org/10.3390/jcm12247615






