The Seattle Heart Failure Model in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Patients and Methods
3. Results
3.1. Demographic and Clinical Data
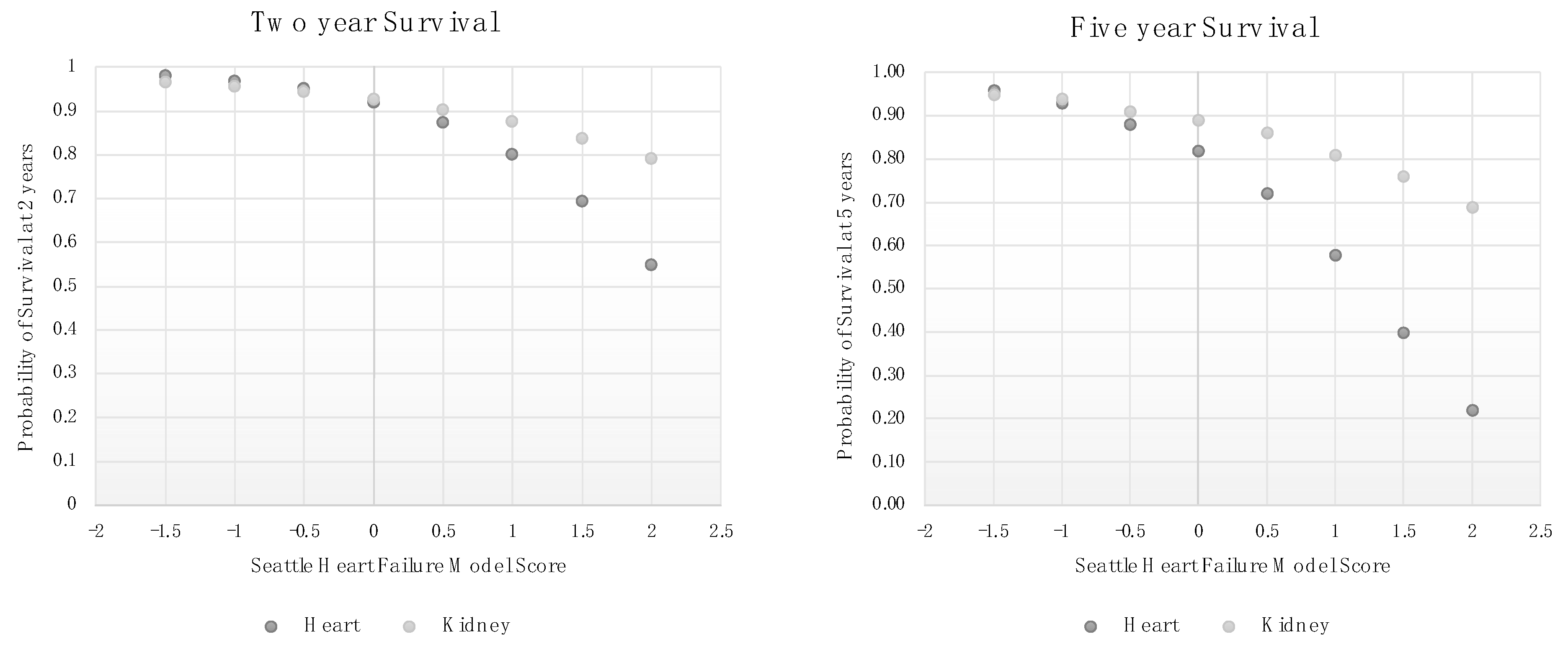
3.2. Association of the Seattle Heart Failure Model with Post-Transplant Mortality
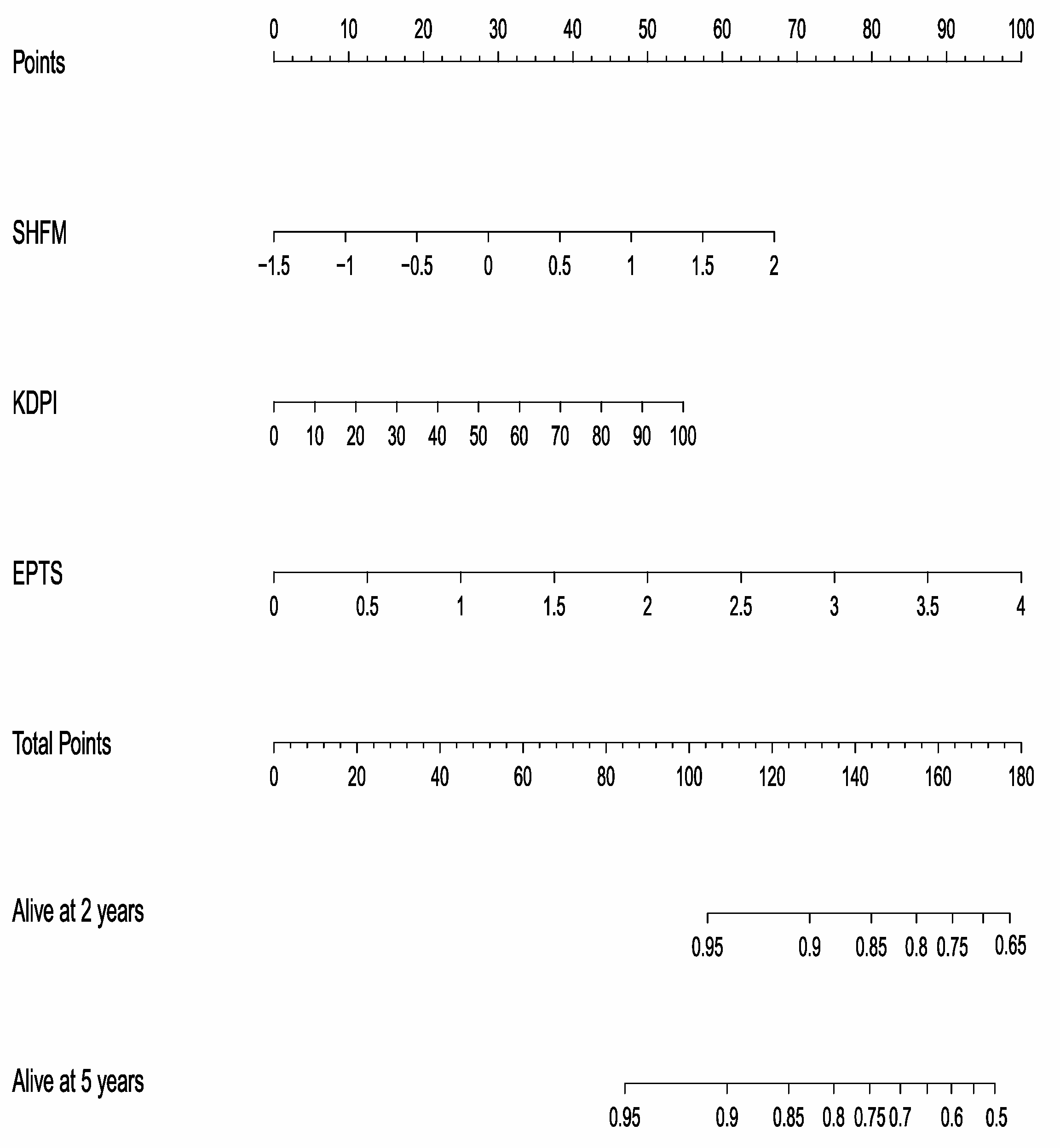
3.3. SHFM and Other Transplant-Specific Indexes
3.4. Low Ejection Fraction and Ventricular Hypertrophy Were Not Associated with Mortality
3.5. SHFM Scores Are Associated with Lower Mortality in Kidney Transplant Patients Than among Heart Failure Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Renal Data. 2018 USRDS annual report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2019, 73 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.A.; Niu, J.; Pan, J.S.; Erickson, K.F.; Mandayam, S.; Winkelmayer, W.C.; Navaneethan, S.D.; Ramanathan, V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996–2014). Am. J. Nephrol. 2018, 48, 472–481. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Kottgen, A.; Russell, S.D.; Loehr, L.R.; Crainiceanu, C.M.; Rosamond, W.D.; Chang, P.P.; Chambless, L.E.; Coresh, J. Reduced Kidney Function as a Risk Factor for Incident Heart Failure: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Soc. Nephrol. 2007, 18, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; de Filippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Schnitzler, M.A.; Abbott, K.C.; Li, L.; Burroughs, T.E.; Irish, W.; Brennan, D.C. De Novo Congestive Heart Failure After Kidney Transplantation: A Common Condition With Poor Prognostic Implications. Am. J. Kidney Dis. 2005, 46, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Corrà, U.; Agostoni, P.; Giordano, A.; Cattadori, G.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; et al. The metabolic exercise test data combined with Cardiac And Kidney Indexes (MECKI) score and prognosis in heart failure. A validation study. Int. J. Cardiol. 2016, 203, 1067–1072. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlström, U.; Edner, M.; Lund, L.H. Predicting survival in heart failure: Validation of the MAGGIC heart failure risk score in 51043 patients from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2014, 16, 173–179. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- May, H.T.; Horne, B.D.; Levy, W.C.; Kfoury, A.G.; Rasmusson, K.D.; Linker, D.T.; Mozaffarian, D.; Anderson, J.L.; Renlund, D.G. Validation of the Seattle Heart Failure Model in a community-based heart failure population and enhancement by adding B-type natriuretic peptide. Am. J. Cardiol. 2007, 100, 697–700. [Google Scholar] [CrossRef]
- den Dekker, W.K.; Slot, M.C.; Kho, M.M.L.; Galema, T.W.; van de Wetering, J.; Boersma, E.; Roodnat, J.I. Predictors of postoperative cardiovascular complications up to 3 months after kidney transplantation. Neth. Hear. J. 2020, 28, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Aalten, J.; Hoogeveen, E.K.; Roodnat, J.I.; Weimar, W.; Borm, G.F.; de Fijter, J.W.; Hoitsma, A.J. Associations between pre-kidney-transplant risk factors and post-transplant cardiovascular events and death. Transpl. Int. 2008, 21, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Abichandani, R.; Kausz, A.T.; Pereira, B.J.G. Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int. 2002, 62, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, F.; Arik, M.K.; Erbiş, H.; Akbaş, A.; Yilmaz, V.T.; Barutcu, A.; Osmanoğlu, I.A.; Kocak, H. Left ventricle ejection fraction may predict mortality in renal transplant patients. Ren. Fail. 2016, 38, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Dohler, B. Improved Long-Term Outcomes After Renal Transplantation Associated with Blood Pressure Control. Am. J. Transplant. 2005, 5, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Dahle, D.O.; Jenssen, T.; Holdaas, H.; Leivestad, T.; Vårdal, M.; Mjøen, G.; Reisaeter, A.V.; Toft, I.; Hartmann, A. Uric acid has a J-shaped association with cardiovascular and all-cause mortality in kidney transplant recipients. Clin. Transplant. 2014, 28, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular risk in renal transplant recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef]
- Estimated Post Transplant Survival Calculator. Organ Procure Transplant Network. 2020. Available online: https://optn.transplant.hrsa.gov/resources/allocation-calculators/epts-calculator (accessed on 1 December 2022).
- Foley, R.N.; Curtis, B.M.; Randell, E.W.; Parfrey, P.S. Left Ventricular Hypertrophy in New Hemodialysis Patients without Symptomatic Cardiac Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 805–813. [Google Scholar] [CrossRef]
- Gradman, A.H.; Alfayoumi, F. From Left Ventricular Hypertrophy to Congestive Heart Failure: Management of Hypertensive Heart Disease. Prog. Cardiovasc. Dis. 2006, 48, 326–341. [Google Scholar] [CrossRef]
- Dekker, M.J.; Marcelli, D.; Canaud, B.; Konings, C.J.; Leunissen, K.M.; Levin, N.W.; Raimann, J.G.; van der Sande, F.M.; Usvyat, L.A.; Kotanko, P.; et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: Results from the international MONDO initiative. Eur. J. Clin. Nutr. 2016, 70, 779–784. [Google Scholar] [CrossRef]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, J.J.; Samana, C.J.; Kasiske, B.L.; Israni, A.K.; Stewart, D.; Cherikh, W.; Formica, R.N. The kidney allocation system. Surg. Clin. N. Am. 2013, 93, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.B.; Ahn, C.; Axelrod, D.A.M.; Foster, B.J.M.; Kasiske, B.L.; Kher, V.M.; Kumar, D.M.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Benedetto, F.A.; Mallamaci, F.; Tripepi, G.; Giacone, G.; Stancanelli, B.; Cataliotti, A.; Malatino, L.S. Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004, 65, 1492–1498. [Google Scholar] [CrossRef]
- Hewing, B.; Dehn, A.M.; Staeck, O.; Knebel, F.; Spethmann, S.; Stangl, K.; Baumann, G.; Dreger, H.; Budde, K.; Halleck, F. Improved Left Ventricular Structure and Function after Successful Kidney Transplantation. Kidney Blood Press. Res. 2016, 41, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Zapolski, T.; Furmaga, J.; Wysokiński, A.P.; Wysocka, A.; Rudzki, S.; Jaroszyński, A. The atrial uremic cardiomyopathy regression in patients after kidney transplantation—The prospective echocardiographic study. BMC Nephrol. 2019, 20, 152. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Kent, G.M.; Harnett, J.D.; Murray, D.C.; Barre, P.E. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J. Am. Soc. Nephrol. 2000, 11, 912–916. [Google Scholar] [CrossRef]
- Heywood, T.; Fonarow, G. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the adhere database. J. Card. Fail. 2007, 13, 422–430. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Perkovic, V.; Johnson, D.W.; Nigwekar, S.U.; Craig, J.C.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2015, CD005019. [Google Scholar]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Johnson, D.W.; Perkovic, V.; Nigwekar, S.U.; Hegbrant, J.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst. Rev. 2013, 9, CD004289. [Google Scholar] [CrossRef]
| All | Survived | Died | p-Value | ||
|---|---|---|---|---|---|
| N | N = 360 | n = 317 | n = 43 | ||
| Recipient | Age in years at transplant | 52.3 (12.7) | 51.4 (12.7) | 59.1 (11.4) | <0.001 |
| Male Gender, % | 64.4 | 65.9 | 53.5 | 0.1 | |
| Ethnicity, % | |||||
| African American | 68.6 | 68.5 | 69.8 | 0.06 | |
| White | 23.6 | 24.6 | 16.3 | ||
| Hispanic | 5.8 | 5.7 | 7.0 | ||
| Asian | 2.0 | 1.3 | 7.0 | ||
| Body mass index at transplant | 28.2 (5.3) | 28.1 (5.3) | 28.3 (5.4) | 0.9 | |
| EPTS, raw score | 2.0 (0.7) | 2.0 (0.7) | 2.4 (0.6) | <0.001 | |
| Ischemic disease, % | 18.1 | 17.4 | 23.3 | 0.3 | |
| Left ventricular hypertrophy, % | 69.4 | 69.2 | 71.4 | 0.6 | |
| Mildly reduced EF, % | 4.4 | 4.1 | 6.9 | 0.6 | |
| Reduced EF, % | 2.7 | 2.8 | 2.3 | 0.6 | |
| Smoking history, % | 42.9 | 43.0 | 41.9 | 0.9 | |
| Months of dialysis, median (IQR) * | 72 (47, 90) | 71 (46, 90) | 77 (52, 89) | 0.4 | |
| Hypertension, % | 83.9 | 83.6 | 86.1 | 0.7 | |
| Diabetes, % | 38.1 | 36.0 | 53.5 | 0.02 | |
| Donor | Kidney Donor Profile Index | 54 (23) | 53 (23) | 62 (25) | 0.009 |
| Hypothermic perfusion, % | 42.0 | 40.3 | 54.8 | 0.07 | |
| Donation after cardiac death, % | 40.8 | 41.3 | 37.2 | 0.6 |
| HR (95% CI) | p-Value | |
|---|---|---|
| SHFM | 1.76 (1.10, 2.83) | 0.02 |
| Age, per year | 1.05 (1.02, 1.08) | <0.001 |
| Male sex | 0.62 (0.34, 1.12) | 0.1 |
| NYHA (ref: NYHA class 1) | ||
| Class 2 | 1.13 (0.35, 3.57) | 0.8 |
| Class 3 | 2.04 (0.28, 14.9) | 0.4 |
| Ischemic | 1.38 (0.68, 2.79) | 0.4 |
| LV ejection fraction, per 10% | 1.02 (0.71, 1.47) | 0.9 |
| Systolic BP, per 10 mm Hg | 1.03 (0.90, 1.16) | 0.7 |
| Sodium, per 1 mEq/L | 0.90 (0.84, 0.96) | 0.003 |
| Hemoglobin | 0.94 (0.79, 1.11) | 0.5 |
| % Lymphocytes | 0.96 (0.93, 1.00) | 0.04 |
| Uric acid | 1.01 (0.87, 1.17) | 0.9 |
| Cholesterol, per 20 mg/dL | 0.83 (0.70, 0.99) | 0.03 |
| Diuretics | 1.00 (0.99, 1.01) | 0.7 |
| Allopurinol | 1.23 (0.38, 4.00) | 0.7 |
| Beta blocker | 1.05 (0.56, 1.97) | 0.9 |
| ARB | 0.83 (0.33, 2.10) | 0.7 |
| ACE | 1.01 (0.50, 2.05) | 0.9 |
| All | Survived | Died | p-Value | |
|---|---|---|---|---|
| N | N = 360 | n = 317 | n = 43 | |
| SHFM score | −0.01 (0.60) | −0.04 (0.59) | 0.16 (0.66) | 0.04 |
| Age in years at evaluation | 51.4 (12.7) | 50.5 (12.6) | 57.9 (11.6) | <0.001 |
| Male Gender, % | 64.4 | 65.9 | 53.5 | 0.1 |
| NYHA class, % | 0.9 | |||
| Class 1 | 91.1 | 91.2 | 90.7 | |
| Class 2 | 7.0 | 6.9 | 7.0 | |
| Class 3 | 1.9 | 1.9 | 2.3 | |
| Ischemic disease, % | 18.1 | 17.4 | 23.3 | 0.3 |
| LV ejection fraction | 60 (8) | 60(8) | 60 (8) | 0.9 |
| Systolic blood pressure, mm Hg | 134 (24) | 134 (23) | 137 (26) | 0.5 |
| Serum sodium, mEq/L | 139 (3) | 140 (3) | 138 (3) | 0.008 |
| Hemoglobin, g/dL | 11.4 (1.7) | 11.4 (1.7) | 11.2 (1.9) | 0.6 |
| % Lymphocytes | 24 (9) | 25 (10) | 22 (7) | 0.01 |
| Serum uric acid, mg/dL | 5.7 (1.9) | 5.7 (1.9) | 5.8 (1.8) | 0.8 |
| Serum cholesterol, mg/dL | 163 (42) | 165 (43) | 149 (30) | 0.004 |
| Diuretic use, % | 20.6 | 20.5 | 20.9 | 0.9 |
| Allopurinol use, % | 6.1 | 6.0 | 7.0 | 0.8 |
| Beta blocker use, % | 64.4 | 64.4 | 65.1 | 0.9 |
| ARB/ACE use, % | 36.9 | 37.2 | 34.9 | 0.7 |
| Statin use, % | 41.4 | 40.7 | 46.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Gutierrez, A.; McGill, R.L.; Juengel, B.; Bachul, P.J.; Danz, D.N.; Josephson, M.; Chung, B.B.; Nguyen, A.; Fung, J.J.; Barth, R.N.; et al. The Seattle Heart Failure Model in Kidney Transplant Recipients. J. Clin. Med. 2023, 12, 7614. https://doi.org/10.3390/jcm12247614
Perez-Gutierrez A, McGill RL, Juengel B, Bachul PJ, Danz DN, Josephson M, Chung BB, Nguyen A, Fung JJ, Barth RN, et al. The Seattle Heart Failure Model in Kidney Transplant Recipients. Journal of Clinical Medicine. 2023; 12(24):7614. https://doi.org/10.3390/jcm12247614
Chicago/Turabian StylePerez-Gutierrez, Angelica, Rita L. McGill, Braden Juengel, Piotr J. Bachul, David N. Danz, Michelle Josephson, Ben B. Chung, Ann Nguyen, John J. Fung, Rolf N. Barth, and et al. 2023. "The Seattle Heart Failure Model in Kidney Transplant Recipients" Journal of Clinical Medicine 12, no. 24: 7614. https://doi.org/10.3390/jcm12247614
APA StylePerez-Gutierrez, A., McGill, R. L., Juengel, B., Bachul, P. J., Danz, D. N., Josephson, M., Chung, B. B., Nguyen, A., Fung, J. J., Barth, R. N., & Becker, Y. T. (2023). The Seattle Heart Failure Model in Kidney Transplant Recipients. Journal of Clinical Medicine, 12(24), 7614. https://doi.org/10.3390/jcm12247614





