Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review
Abstract
:1. History
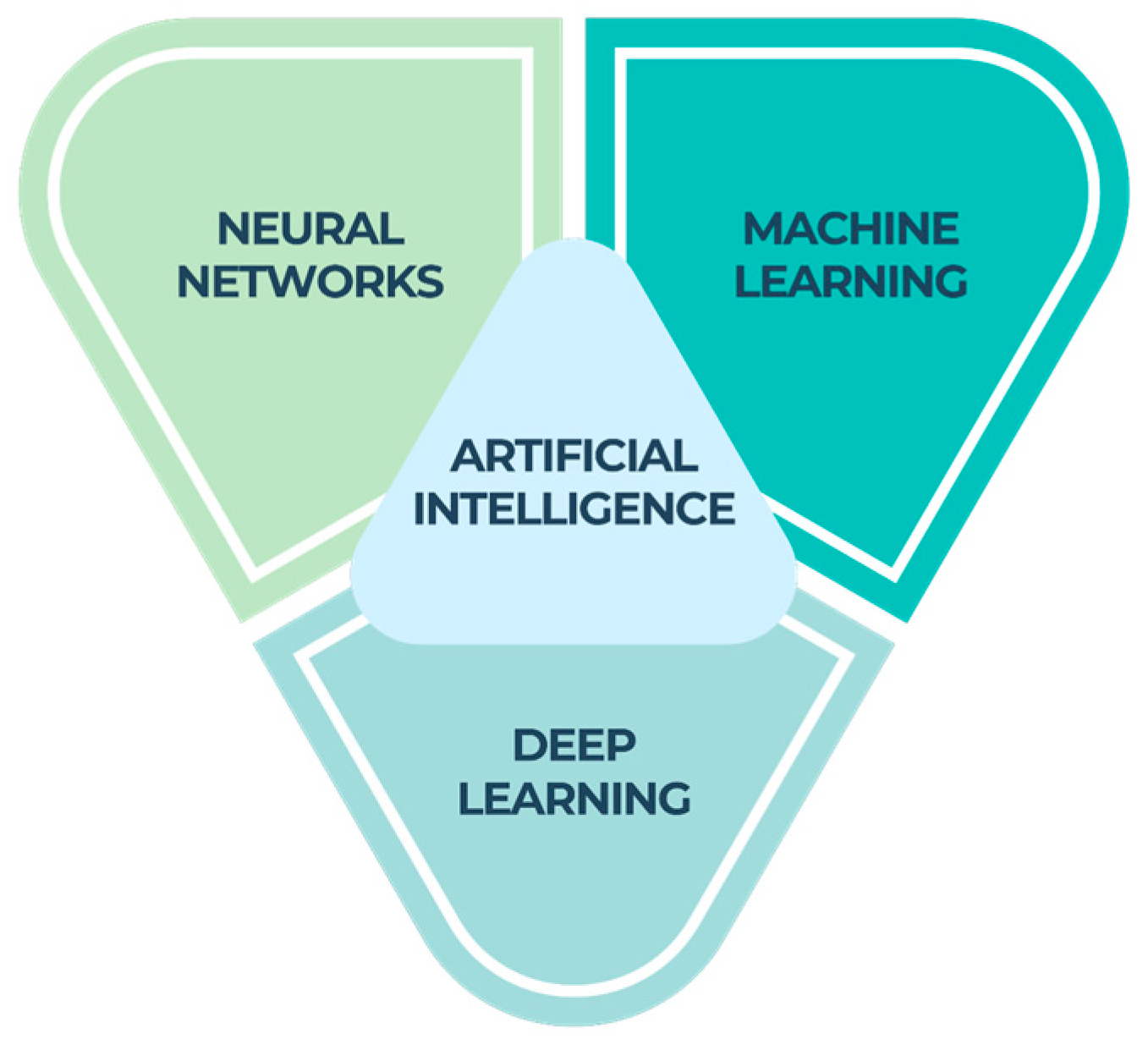
2. Types of AI
- ⇒
- Machine learning (ML), a component of artificial intelligence (AI), relies on algorithms to make predictions by analyzing a given dataset. The objective of machine learning is to enable machines to acquire knowledge from data in order to autonomously address problems without the need for human intervention [11].
- ⇒
- Neural networks (NNs) are widely recognized as a prominent category of machine learning (ML) models. They have demonstrated superior performance compared with traditional ML techniques, particularly when dealing with intricate data formats such as photography or language. Neural networks refer to a collection of techniques that perform signal computation through the use of artificial neurons. The primary objective of neural networks is to develop computational models that simulate the everyday activities of the human brain [12].
- ⇒
- Deep learning is a fundamental element of machine learning that uses a multi-layered cognitive matrix within a deep neural network to examine and interpret incoming data. The objective of deep learning is to develop a neural network that autonomously recognizes patterns in order to enhance the process of feature detection [14].
3. Applications in Dentistry
3.1. AI in Diagnostics and Radiology
3.2. AI in Endodontics
3.3. AI in Periodontology
3.4. AI in Prosthodontics
3.5. AI in Orthodontics
4. Emerging Trends and Patterns
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| NNs | Neural networks |
| ML | Machine learning |
| CNNs | Convolutional neural networks |
| ANNs | Artificial neural networks |
| IOU | Intersection-over-union |
| NILT | Near-infrared light transillumination |
| CBCT | Cone Beam Computed Tomography |
| ALs | Apical lesions |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| VRF | Vertical root fractures |
| WL | Working length |
| RBL | Radiographic bone loss |
References
- Kriegeskorte, N.; Golan, T. Neural network models and deep learning. Curr. Biol. 2019, 29, R231–R236. [Google Scholar] [CrossRef]
- Pessoa, L. Understanding brain networks and brain organization. Phys. Life Rev. 2014, 11, 400–435. [Google Scholar] [CrossRef]
- Kalappanavar, A.; Sneha, S.; Annigeri, R.G. Artificial intelligence: A dentist’s perspective. J. Med. Radiol. Pathol. Surg. 2018, 5, 2–4. [Google Scholar] [CrossRef]
- Park, W.J.; Park, J.B. History and application of artificial neural networks in dentistry. Eur. J. Dent. 2018, 12, 594–601. [Google Scholar] [CrossRef]
- Righolt, A.J.; Jevdjevic, M.; Marcenes, W.; Listl, S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J. Dent. Res. 2018, 97, 501–507. [Google Scholar] [CrossRef]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef]
- Shan, T.; Tay, F.R.; Gu, L. Application of Artificial Intelligence in Dentistry. J. Dent. Res. 2021, 100, 232–244. [Google Scholar] [CrossRef]
- Schwendicke, F.; Singh, T.; Lee, J.H.; Gaudin, R.; Chaurasia, A.; Wiegand, T.; Uribe, S.; Krois, J. IADR e-oral health network and the ITU WHO focus group AI for Health. Artificial intelligence in dental research: Checklist for authors, reviewers, readers. J. Dent. 2021, 107, 103610. [Google Scholar] [CrossRef]
- Ma, J.; Schneider, L.; Lapuschkin, S.; Achtibat, R.; Duchrau, M.; Krois, J.; Schwendicke, F.; Samek, W. Towards Trustworthy AI in Dentistry. J. Dent. Res. 2022, 101, 1263–1268. [Google Scholar] [CrossRef]
- Pethani, F. Promises and perils of artificial intelligence in dentistry. Aust. Dent. J. 2021, 66, 124–135. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-Ehaideb, A.; Maganur, P.C.; Vishwanathaiah, S.; Patil, S.; Baeshen, H.A.; Sarode, S.C.; Bhandi, S. Developments, application, and performance of artificial intelligence in dentistry—A systematic review. J. Dent. Sci. 2021, 16, 508–522. [Google Scholar] [CrossRef]
- Machoy, M.E.; Szyszka-Sommerfeld, L.; Vegh, A.; Gedrange, T.; Woźniak, K. The ways of using machine learning in dentistry. Adv. Clin. Exp. Med. 2020, 29, 375–384. [Google Scholar] [CrossRef]
- Putra, R.H.; Doi, C.; Yoda, N.; Astuti, E.R.; Sasaki, K. Current applications and development of artificial intelligence for digital dental radiography. Dentomaxillofac. Radiol. 2022, 51, 20210197. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Krois, J.; Schwendicke, F. Demystifying artificial intelligence and deep learning in dentistry. Braz. Oral Res. 2021, 35, 094. [Google Scholar] [CrossRef]
- Dave, M.; Patel, N. Artificial intelligence in healthcare and education. Br. Dent. J. 2023, 234, 761–764. [Google Scholar] [CrossRef]
- Vaishya, R.; Javaid, M.; Khan, I.H.; Haleem, A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. 2020, 14, 337–339. [Google Scholar] [CrossRef]
- Mörch, C.M.; Atsu, S.; Cai, W.; Li, X.; Madathil, S.A.; Liu, X.; Mai, V.; Tamimi, F.; Dilhac, M.A.; Ducret, M. Artificial Intelligence and Ethics in Dentistry: A Scoping Review. J. Dent. Res. 2021, 100, 1452–1460. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, K.; Lyu, P.; Li, H.; Zhang, L.; Wu, J.; Lee, C.H. A deep learning approach to automatic teeth detection and numbering based on object detection in dental periapical films. Sci. Rep. 2019, 9, 384. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.H.; Jeong, S.N.; Choi, S.H. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dent. 2018, 77, 106–111. [Google Scholar] [CrossRef]
- Casalegno, F.; Newton, T.; Daher, R.; Abdelaziz, M.; Lodi-Rizzini, A.; Schürmann, F.; Krejci, I.; Markram, H. Caries detection with near-infrared transillumination using deep learning. J. Dent. Res. 2019, 98, 1227–1233. [Google Scholar] [CrossRef]
- Talpur, S.; Azim, F.; Rashid, M.; Syed, S.A.; Talpur, B.A.; Khan, S.J. Uses of Different Machine Learning Algorithms for Diagnosis of Dental Caries. J. Healthc. Eng. 2022, 2022, 5032435. [Google Scholar] [CrossRef]
- Hung, M.; Voss, M.W.; Rosales, M.N.; Li, W.; Su, W.; Xu, J.; Bounsanga, J.; Ruiz-Negrón, B.; Lauren, E.; Licari, F.W. Application of machine learning for diagnostic prediction of root caries. Gerodontology 2019, 36, 395–404. [Google Scholar] [CrossRef]
- Schwendicke, F.; Elhennawy, K.; Paris, S.; Friebertshauser, P.; Krois, J. Deep learning for caries lesion detection in nearinfrared light transillumination images: A pilot study. J. Dent. 2020, 92, 103260. [Google Scholar] [CrossRef]
- Hiraiwa, T.; Ariji, Y.; Fukuda, M.; Kise, Y.; Nakata, K.; Katsumata, A.; Fujita, H.; Ariji, E. A deep-learning artificial intelligence system for assessment of root morphology of the mandibular first molar on panoramic radiography. Dentomaxillofac. Radiol. 2019, 48, 20180218. [Google Scholar] [CrossRef]
- Ekert, T.; Krois, J.; Meinhold, L.; Elhennawy, K.; Emara, R.; Golla, T.; Schwendicke, F. Deep learning for the radiographic detection of apical lesions. J. Endod. 2019, 45, 917–922. [Google Scholar] [CrossRef]
- Murata, M.; Ariji, Y.; Ohashi, Y.; Kawai, T.; Fukuda, M.; Funakoshi, T.; Kise, Y.; Nozawa, M.; Katsumata, A.; Fujita, H.; et al. Deep-learning classification using convolutional neural network for evaluation of maxillary sinusitis on panoramic radiography. Oral Radiol. 2019, 35, 301–307. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.J.; Sunwoo, L.; Choi, D.; Nam, C.M.; Cho, J.; Kim, J.; Bae, Y.J.; Yoo, R.E.; Choi, B.S.; et al. Deep Learning in diagnosis of maxillary sinusitis using conventional radiography. Investig. Radiol. 2019, 54, 7–15. [Google Scholar] [CrossRef]
- Lee, J.S.; Adhikari, S.; Liu, L.; Jeong, H.G.; Kim, H.; Yoon, S.J. Osteoporosis detection in panoramic radiographs using a deep convolutional neural network-based computer-assisted diagnosis system: A preliminary study. Dentomaxillofac. Radiol. 2019, 48, 20170344. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.K.; Ryu, J.J.; Shin, S.W.; Choi, J. Evaluation of transfer learning with deep convolutional neural networks for screening osteoporosis in dental panoramic radiographs. J. Clin. Med. 2020, 9, 392. [Google Scholar] [CrossRef]
- Funakoshi, T.; Shibata, T.; Inamoto, K.; Shibata, N.; Ariji, Y.; Fukuda, M.; Nakata, K.; Ariji, E. Cone-beam computed tomography classification of the mandibular second molar root morphology and its relationship to panoramic radiographic appearance. Oral Radiol. 2021, 37, 494–501. [Google Scholar] [CrossRef]
- Lahoud, P.; EzEldeen, M.; Beznik, T.; Willems, H.; Leite, A.; Van Gerven, A.; Jacobs, R. Artificial intelligence for fast and accurate 3-dimensional tooth segmentation on cone-beam computed tomography. J. Endod. 2021, 47, 827–835. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, H.; Setzer, F.C.; Shi, K.J.; Mupparapu, M.; Li, J. Anatomically constrained deep learning for automating dental CBCT segmentation and lesion detection. IEEE Trans. Autom. Sci. Eng. 2021, 18, 603–614. [Google Scholar] [CrossRef]
- Fukuda, M.; Inamoto, K.; Shibata, N.; Ariji, Y.; Yanashita, Y.; Kutsuna, S.; Nakata, K.; Katsumata, A.; Fujita, H.; Ariji, E. Evaluation of an artificial intelligence system for detecting vertical root fracture on panoramic radiography. Oral Radiol. 2019, 36, 337–343. [Google Scholar] [CrossRef]
- Kositbowornchai, S.; Plermkamon, S.; Tangkosol, T. Performance of an artificial neural network for vertical root fracture detection: An ex vivo study. Dent. Traumatol. 2013, 29, 151–155. [Google Scholar] [CrossRef]
- Ramezanzade, S.; Laurentiu, T.; Bakhshandah, A.; Ibragimov, B.; Kvist, T.; Bjørndal, L. The efficiency of artificial intelligence methods for finding radiographic features in different endodontic treatments–a systematic review. Acta Odontol. Scand. 2023, 81, 422–435. [Google Scholar]
- Khanagar, S.B.; Alfadley, A.; Alfouzan, K.; Awawdeh, M.; Alaqla, A.; Jamleh, A. Developments and Performance of Artificial Intelligence Models Designed for Application in Endodontics: A Systematic Review. Diagnostics 2023, 13, 414. [Google Scholar] [CrossRef]
- Karobari, M.I.; Adil, A.H.; Basheer, S.N.; Murugesan, S.; Savadamoorthi, K.S.; Mustafa, M.; Abdulwahed, A.; Almokhatieb, A.A. Evaluation of the Diagnostic and Prognostic Accuracy of Artificial Intelligence in Endodontic Dentistry: A Comprehensive Review of Literature. Comput. Math. Methods Med. 2023, 2023, 7049360. [Google Scholar] [CrossRef]
- Setzer, F.C.; Shi, K.J.; Zhang, Z.; Yan, H.; Yoon, H.; Mupparapu, M.; Li, J. Artifi cial intelligence for the computer-aided detection of periapical lesions in cone-beam computed tomographic images. J. Endod. 2020, 46, 987–993. [Google Scholar] [CrossRef]
- Orhan, K.; Bayrakdar, I.S.; Ezhov, M.; Kravtsov, A.; Özyürek, T.A. Evaluation of artifi cial intelligence for detecting periapical pathosis on cone-beam computed tomography scans. Int. Endod. J. 2020, 53, 680–689. [Google Scholar] [CrossRef]
- Buyuk, C.; Arican Alpay, B.; Er, F. Detection of the separated root canal instrument on panoramic radiograph: A comparison of LSTM and CNN deep learning methods. Dentomaxillofac. Radiol. 2023, 52, 20220209. [Google Scholar] [CrossRef]
- Bindal, P.; Bindal, U.; Kazemipoor, M.; Jha, S.K. Hybrid machine learning approaches in viability assessment of dental pulp stem cells treated with platelet-rich concentrates on different periods. Appl. Med. Inform. 2019, 41, 93–101. [Google Scholar]
- Mohan, S.P.; Ramalingam, M. Dental pulp stem cells in neuroregeneration. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. S1), S60–S66. [Google Scholar] [CrossRef]
- Sivari, E.; Senirkentli, G.B.; Bostanci, E.; Guzel, M.S.; Acici, K.; Asuroglu, T. Deep Learning in Diagnosis of Dental Anomalies and Diseases: A Systematic Review. Diagnostics 2023, 13, 2512. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, T.; Kamio, N.; Shiota, S.; Shibata, Y.; Suzuki, N.; Yoneda, M.; Hirofuji, T.; Yamashita, Y. Supervised machine learningbased classification of oral malodor based on the microbiota in saliva samples. Artif. Intell. Med. 2014, 60, 97–101. [Google Scholar] [CrossRef]
- Lee, C.T.; Kabir, T.; Nelson, J.; Sheng, S.; Meng, H.W.; Van Dyke, T.E.; Walji, M.F.; Jiang, X.; Shams, S. Use of the deep learning approach to measure alveolar bone level. J. Clin. Periodontol. 2022, 49, 260–269. [Google Scholar] [CrossRef]
- Chang, H.J.; Lee, S.J.; Yong, T.H.; Shin, N.Y.; Jang, B.G.; Kim, J.E.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; et al. Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Sci. Rep. 2020, 10, 7531. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhou, Y.; Chen, Q.; She, Y.; Gao, F.; Xu, Y.; Chen, J.; Gao, X. An Interpretable Computer-Aided Diagnosis Method for Periodontitis from Panoramic Radiographs. Front. Physiol. 2021, 12, 655556. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.S.; Song, I.S.; Jung, K.H. DeNTNet: Deep Neural Transfer Network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019, 9, 17615. [Google Scholar] [CrossRef]
- Heo, M.S.; Kim, J.E.; Hwang, J.J.; Han, S.S.; Kim, J.S.; Yi, W.J.; Park, I.W. Artificial intelligence in oral and maxillofacial radiology: What is currently possible? Dentomaxillofac. Radiol. 2021, 50, 20200375. [Google Scholar] [CrossRef]
- Krois, J.; Ekert, T.; Meinhold, L.; Golla, T.; Kharbot, B.; Wittemeier, A.; Dörfer, C.; Schwendicke, F. Deep Learning for the Radiographic Detection of Periodontal Bone Loss. Sci. Rep. 2019, 9, 8495. [Google Scholar] [CrossRef]
- Aliaga, I.J.; Vera, V.; De Paz, J.F.; García, A.E.; Mohamad, M.S. Modelling the longevity of dental restorations by means of a CBR system. BioMed Res. Int. 2015, 2015, 540306. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, A.B.; Gallucci, G.O.; Att, W.; Özcan, M.; Krishnamurthy, V.R. Artificial intelligence models for tooth-supported fixed and removable prosthodontics: A systematic review. J. Prosthet. Dent. 2023, 129, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and precision of 5 intraoral scanners in the impressions of single and multiple implants: A comparative in vitro study. BMC Oral Health 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Albdour, E.A.; Shaheen, E.; Vranckx, M.; Mangano, F.G.; Politis, C.; Jacobs, R. A novel in vivo method to evaluate trueness of digital impressions. BMC Oral Health 2018, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Mangano, C.; Margiani, B.; Admakin, O. Combining Intraoral and Face Scans for the Design and Fabrication of Computer-Assisted Design/Computer-Assisted Manufacturing (CAD/CAM) Polyether-Ether-Ketone (PEEK) Implant-Supported Bars for Maxillary Overdentures. Scanning 2019, 2019, 4274715. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Veronesi, G. Digital versus Analog Procedures for the Prosthetic Restoration of Single Implants: A Randomized Controlled Trial with 1 Year of Follow-Up. BioMed Res. Int. 2018, 2018, 5325032. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Gallucci, G.; Wismeijer, D.; Zitzmann, N. Augmented and virtual reality in dental medicine: A systematic review. Comput. Biol. Med. 2019, 108, 93–100. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Matthisson, L.; Ohla, H.; Joda, T. Digital undergraduate education in dentistry: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 3269. [Google Scholar] [CrossRef]
- Nishimoto, S.; Sotsuka, Y.; Kawai, K.; Ishise, H.; Kakibuchi, M. Personal computer-based cephalometric landmark detection with deep learning, using cephalograms on the Internet. J. Craniofac. Surg. 2019, 30, 91–95. [Google Scholar] [CrossRef]
- Kunz, F.; Stellzig-Eisenhauer, A.; Zeman, F.; Boldt, J. Artificial intelligence in orthodontics. J. Orofac. Orthop. 2019, 81, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Durka-Zajac, M.; Mitus-Kenig, M.; Derwich, M.; Marcinkowska-Mitus, A.; Loboda, M. Radiological indicators of bone age assessment in cephalometric images: Review. Pol. J. Radiol. 2016, 81, 347–353. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.A., Jr.; Franchi, L. The cervical vertebral maturation method: A user’s guide. Angle Orthod. 2018, 88, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Cho, S.R.; Kim, M.J.; Kim, W.H.; Kim, J.W.; Choi, J. Automated skeletal classification with lateral cephalometry based on artificial intelligence. J. Dent. Res. 2020, 99, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Soheilifar, S.; Ataei, H.; Mollabashi, V.; Amini, P.; Bakhshaei, A.; Naghdi, N. Extraction versus non-extraction orthodontic treatment: Soft tissue profile changes in borderline class I patients. Dent. Med. Probl. 2020, 57, 275–283. [Google Scholar]
- Xie, X.; Wang, L.; Wang, A. Artificial neural network modeling for deciding if extractions are necessary prior to orthodontic treatment. Angle Orthod. 2010, 80, 262–266. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, T.W. New approach for the diagnosis of extractions with neural network machine learning. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 127–133. [Google Scholar] [CrossRef]
- Joda, T.; Yeung, A.W.K.; Hung, K.; Zitzmann, N.U.; Bornstein, M.M. Disruptive Innovation in Dentistry: What It Is and What Could Be Next. J. Dent. Res. 2021, 100, 448–453. [Google Scholar] [CrossRef]
- Hung, K.; Montalvao, C.; Tanaka, R.; Kawai, T.; Bornstein, M.M. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20190107. [Google Scholar] [CrossRef]
- Hung, K.; Yeung, A.W.K.; Tanaka, R.; Bornstein, M.M. Current Applications, Opportunities, and Limitations of AI for 3D Imaging in Dental Research and Practice. Int. J. Environ. Res. Public Health 2020, 17, 4424. [Google Scholar] [CrossRef]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, A.B.; Özcan, M.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in restorative dentistry: A systematic review. J. Prosthet. Dent. 2022, 128, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhou, Z.; Zhou, Z.; Wu, X.; Li, Y.; Wang, S.; Liao, W.; Ying, S.; Zhao, Z. Artificial intelligence for caries and periapical periodontitis detection. J. Dent. 2022, 122, 104107. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.; Nagendrababu, V. Artificial Intelligence in Endodontics: Current Applications and Future Directions. J. Endod. 2021, 47, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Kierce, E.A.; Kolts, R.J. Improving Periodontal Disease Management With Artificial Intelligence. Compend Contin. Educ. Dent. 2023, 44, 1–4. [Google Scholar]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Pirayesh, Z.; Haiat, A.; Zahedrozegar, S.; Mahmoudinia, E.; Rohban, M.H.; Krois, J.; Lee, J.H.; Schwendicke, F. Deep learning in periodontology and oral implantology: A scoping review. J. Periodontal Res. 2022, 57, 942–951. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, B.A.; Galluci, G.O.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in implant dentistry: A systematic review. J. Prosthet. Dent. 2023, 129, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Singi, S.R.; Sathe, S.; Reche, A.R.; Sibal, A.; Mantri, N. Extended Arm of Precision in Prosthodontics: Artificial Intelligence. Cureus 2022, 14, 30962. [Google Scholar] [CrossRef]
- Monill-González, A.; Rovira-Calatayud, L.; d’Oliveira, N.G.; Ustrell-Torrent, J.M. Artificial intelligence in orthodontics: Where are we now? A scoping review. Orthod. Craniofac. Res. 2021, 24, 6–15. [Google Scholar] [CrossRef]
- Vishwanathaiah, S.; Fageeh, H.N.; Khanagar, S.B.; Maganur, P.C. Artificial Intelligence Its Uses and Application in Pediatric Dentistry: A Review. Biomedicines 2023, 11, 788. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Preininger, A. AI in Health: State of the Art, Challenges, and Future Directions. Yearb. Med. Inform. 2019, 28, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Pascadopoli, M.; Zampetti, P.; Nardi, M.G.; Pellegrini, M.; Scribante, A. Smartphone Applications in Dentistry: A Scoping Review. Dent. J. 2023, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Ostaș, D.; Almășan, O.; Ileșan, R.R.; Andrei, V.; Thieringer, F.M.; Hedeșiu, M.; Rotar, H. Point-of-Care Virtual Surgical Planning and 3D Printing in Oral and Cranio-Maxillofacial Surgery: A Narrative Review. J. Clin. Med. 2022, 11, 6625. [Google Scholar] [CrossRef] [PubMed]
- Schulam, P.; Saria, S. Reliable decision support using counterfactual models. Adv. Neural Inf. Process Syst. 2017, 30, 1697–1708. [Google Scholar]
- Dzobo, K.; Adotey, S.; Thomford, N.E.; Dzobo, W. Integrating Artificial and Human Intelligence: A Partnership for Responsible Innovation in Biomedical Engineering and Medicine. OMICS 2020, 24, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.H.; Jeong, S.N. Diagnosis of cystic lesions using panoramic and cone beam computed tomographic images based on deep learning neural network. Oral Dis. 2020, 26, 152–158. [Google Scholar] [CrossRef]
- Yang, S.; Lee, H.; Jang, B.; Kim, K.D.; Kim, J.; Kim, H.; Park, W. Development and Validation of a Visually Explainable Deep Learning Model for Classification of C-shaped Canals of the Mandibular Second Molars in Periapical and Panoramic Dental Radiographs. J. Endod. 2022, 48, 914–921. [Google Scholar] [CrossRef]
- Kurt Bayrakdar, S.; Orhan, K.; Bayrakdar, I.S.; Bilgir, E.; Ezhov, M.; Gusarev, M.; Shumilov, E. A deep learning approach for dental implant planning in cone-beam computed tomography images. BMC Med. Imaging 2021, 21, 86. [Google Scholar] [CrossRef]
- Mine, Y.; Iwamoto, Y.; Okazaki, S.; Nakamura, K.; Takeda, S.; Peng, T.; Mitsuhata, C.; Kakimoto, N.; Kozai, K.; Murayama, T. Detecting the presence of supernumerary teeth during the early mixed dentition stage using deep learning algorithms: A pilot study. Int. J. Paediatr. Dent. 2022, 32, 678–685. [Google Scholar] [CrossRef]
- Tandon, D.; Rajawat, J. Present and future of artificial intelligence in dentistry. J. Oral Biol. Craniofac. Res. 2020, 10, 391–396. [Google Scholar] [CrossRef] [PubMed]

| Authors | Goals of Research | Outcomes |
|---|---|---|
| Chen et al. [18] | Artificial intelligence-based algorithm for detecting proximal dental caries | The accuracy of proximal caries diagnoses may be raised with the help of this neural network. |
| Lee et al. [19] | Artificial intelligence deep learning technique for finding caries | Dental caries identification on periapical radiographs was significantly improved with the use of this DCNN (deep convolutional neural network)-based system algorithm. |
| Casalegno et al. [20] | Dental lesion detection and localization in near-infrared transillumination (TI) pictures using an artificial intelligence-based algorithm | This CNN-based algorithm performed impressively in terms of speed and accuracy in detecting caries. |
| Talpur et al. [21] | Proximal caries diagnosis using an artificial intelligence model | Proximal caries diagnosis may benefit from the use of this neural network. |
| Hung et al. [22] | Root caries analysis with AI | The results of this model are satisfactory enough to permit its use in clinical settings. |
| Schwendicke et al. [23] | Caries lesion detection in near-infrared light transillumination (NILT) pictures using convolutional neural networks (CNNs) | The model’s selective power to identify caries lesions was found to be acceptable. |
| Hiraiwa et al. [24] | Artificial intelligence for the identification of first molar root shapes in the mandible | The deep learning system performed exceptionally well in determining whether or not the distal roots of mandibular first molars included a single or an additional root. |
| Ekert et al. [25] | An artificial intelligence system based on CNNs for the diagnosis of apical lesions (ALs) | The AI system based on a deep CNN was able to identify apical lesions. |
| Murata et al. [26] | Diagnostic artificial intelligence for maxillary sinusitis | Diagnostic accuracy was improved through the AI-based deep learning method. |
| Kim et al. [27] | Maxillary sinusitis diagnosis using CNNs | In both datasets, the average area under the curve achieved by the AI-based (CNNs) was statistically superior to that achieved by the radiologist. |
| Lee et al. [28] | Classification of osteoporosis characteristics using deep neural networks | This DCNN approach could be useful and reliable for automating the screening of patients for osteoporosis. |
| Lee et al. [29] | The objective of this study was to assess the discriminatory capabilities of deep convolutional neural networks (CNNs) when used with different transfer learning techniques. The focus is on classifying distinct characteristics of osteoporosis in digital panoramic radiographs (DPRs). | The findings indicate that the utilization of suitable deep convolutional neural network (CNN) structures in conjunction with transfer learning methods has successfully addressed the challenge posed by a limited training dataset of images. Moreover, the results show that dual-energy X-ray absorptiometry (DXA)-based projection radiography (DPR) images have promise for the pre-screening of osteoporosis. |
| Authors | Area of Dentistry | Year of Publication |
|---|---|---|
| Hung K et al. [70] | Oral diagnosis | 2022 |
| Ezhov et al. [71] | Oral diagnosis | 2021 |
| Revilla-León et al. [72] | Restorative dentistry | 2022 |
| Li et al. [73] | Restorative dentistry | 2022 |
| Aminoshariae et al. [74] | Endodontics | 2021 |
| Karobari et al. [37] | Endodontics | 2023 |
| Kierce et al. [75] | Periodontal disease | 2023 |
| Mohammad-Rahimi et al. [76] | Periodontal disease | 2022 |
| Singi et al. [77] | Prosthetic dentistry | 2022 |
| Revilla-León et al. [78] | Implantology | 2023 |
| Monill-González et al. [79] | Orthodontics | 2021 |
| Vishwanathaiah et al. [80] | Pedodontics | 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surlari, Z.; Budală, D.G.; Lupu, C.I.; Stelea, C.G.; Butnaru, O.M.; Luchian, I. Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review. J. Clin. Med. 2023, 12, 7378. https://doi.org/10.3390/jcm12237378
Surlari Z, Budală DG, Lupu CI, Stelea CG, Butnaru OM, Luchian I. Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review. Journal of Clinical Medicine. 2023; 12(23):7378. https://doi.org/10.3390/jcm12237378
Chicago/Turabian StyleSurlari, Zinovia, Dana Gabriela Budală, Costin Iulian Lupu, Carmen Gabriela Stelea, Oana Maria Butnaru, and Ionut Luchian. 2023. "Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review" Journal of Clinical Medicine 12, no. 23: 7378. https://doi.org/10.3390/jcm12237378






