Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients
Abstract
:1. Introduction
2. Role of PCSK9 in Dyslipidemia and Atherosclerosis
3. PCSK9 Inhibitors Efficacy in Cardiovascular Diseases
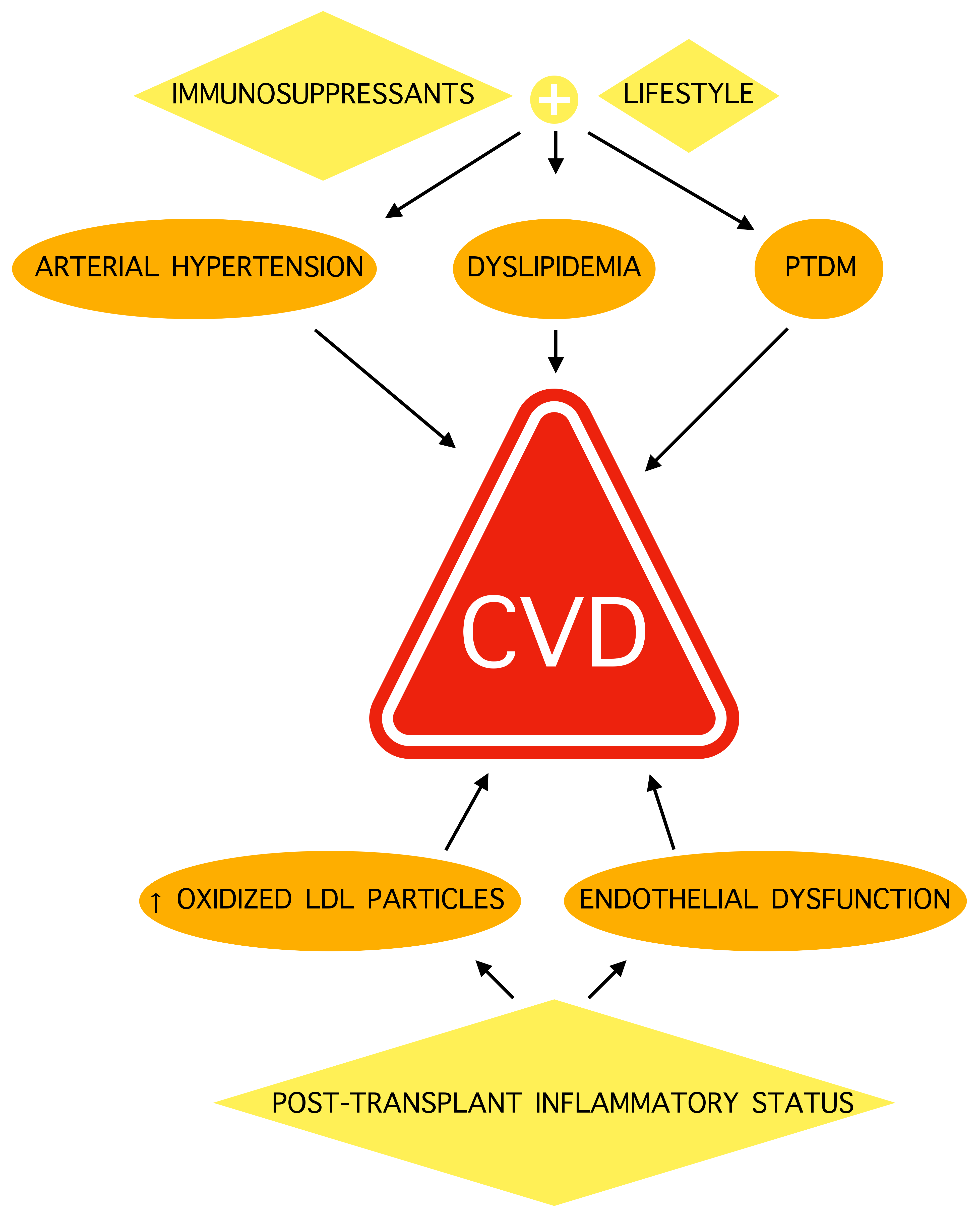
4. Atherogenic Dyslipidemia and Cardiovascular Diseases in Transplant Patients
5. PCSK9 Inhibitors Use in Transplant Patients
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Markell, M.S.; Armenti, V.; Danovitch, G.; Sumrani, N. Hyperlipidemia and glucose intolerance in the post-renal transplant patient. J. Am. Soc. Nephrol. 1994, 4, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Edwards, L.B.; Dipchand, A.I.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; Stehlik, J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report—2016; Focus Theme: Primary Diagnostic Indications for Transplant. J. Heart Lung Transplant. 2016, 35, 1158–1169. [Google Scholar] [CrossRef]
- Parekh, J.; Corley, D.A.; Feng, S. Diabetes, Hypertension and Hyperlipidemia: Prevalence Over Time and Impact on Long-Term Survival After Liver Transplantation. Am. J. Transplant. 2012, 12, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Peng, Y.; Maclean, J.R.; Weinhandl, E.D.; Kasiske, B.L. Predicting Coronary Heart Disease after Kidney Transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am. J. Transplant. 2010, 10, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Starling, R.; Mehra, M.; Kormos, R.; Bhat, G.; Barr, M.; Sigouin, C.; Kolesar, J.; Fitzsimmons, W. Multicenter Retrospective Analysis of Cardiovascular Risk Factors Affecting Long-term Outcome of De Novo Cardiac Transplant Recipients. J. Heart Lung Transplant. 2006, 25, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.; Nair, V.; Chih, S. Cardiac allograft vasculopathy: Insights on pathogenesis and therapy. Clin. Transplant. 2020, 34, e13794. [Google Scholar] [CrossRef]
- Yates, P.; Nicholson, M. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl. Immunol. 2006, 16, 148–157. [Google Scholar] [CrossRef]
- Claes, K.; Meier-Kriesche, H.-U.; Schold, J.D.; Vanrenterghem, Y.; Halloran, P.F.; Ekberg, H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: Evidence from the Symphony study. Nephrol. Dial. Transplant. 2011, 27, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kockx, M.; Kritharides, L. Hyperlipidaemia in immunosuppression. Curr. Opin. Lipidol. 2016, 27, 631–632. [Google Scholar] [CrossRef]
- Page, R.L.; Miller, G.G.; Lindenfeld, J. Drug therapy in the heart transplant recipient: Part IV: Drug-drug interactions. Circulation 2005, 111, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warden, B.A.; Duell, P.B. Management of dyslipidemia in adult solid organ transplant recipients. J. Clin. Lipidol. 2019, 13, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Iannuzzo, G.; Gentile, M.; Bresciani, A.; Mallardo, V.; Di Lorenzo, A.; Merone, P.; Cuomo, G.; Pacileo, M.; Sarullo, F.; Venturini, E.; et al. Inhibitors of Protein Convertase Subtilisin/Kexin 9 (PCSK9) and Acute Coronary Syndrome (ACS): The State-of-the-Art. J. Clin. Med. 2021, 10, 1510. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Paciullo, F.; Momi, S.; Gresele, P. PCSK9 in Haemostasis and Thrombosis: Possible Pleiotropic Effects of PCSK9 Inhibitors in Cardiovascular Prevention. Thromb. Haemost. 2019, 119, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef]
- Warden, B.A.; Kaufman, T.; Minnier, J.; Duell, P.B.; Fazio, S.; Shapiro, M.D. Use of PCSK9 Inhibitors in Solid Organ Transplantation Recipients. JACC Case Rep. 2020, 2, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Simha, V.; Qin, S.; Shah, P.; Smith, B.H.; Kremers, W.K.; Kushwaha, S.; Wang, L.; Pereira, N.L. Sirolimus Therapy Is Associated with Elevation in Circulating PCSK9 Levels in Cardiac Transplant Patients. J. Cardiovasc. Transl. Res. 2016, 10, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Akamine, Y.; Kagaya, H.; Saito, M.; Inoue, T.; Numakura, K.; Habuchi, T.; Satoh, S.; Miura, M. Changes in PCSK9 and LDL cholesterol concentrations by everolimus treatment and their effects on polymorphisms in PCSK9 and mTORC1. Pharmacol. Rep. 2020, 72, 622–630. [Google Scholar] [CrossRef]
- Chiang, L.W.; Grenier, J.M.; Ettwiller, L.; Jenkins, L.P.; Ficenec, D.; Martin, J.; Jin, F.; DiStefano, P.S.; Wood, A. An orchestrated gene expression component of neuronal programmed cell death revealed by cDNA array analysis. Proc. Natl. Acad. Sci. USA 2001, 98, 2814–2819. [Google Scholar] [CrossRef] [Green Version]
- Piper, D.E.; Jackson, S.; Liu, Q.; Romanow, W.G.; Shetterly, S.; Thibault, S.T.; Shan, B.; Walker, N.P. The Crystal Structure of PCSK9: A Regulator of Plasma LDL-Cholesterol. Structure 2007, 15, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar] [CrossRef]
- Abifadel, M.; Guerin, M.; Benjannet, S.; Rabès, J.-P.; Le Goff, W.; Julia, Z.; Hamelin, J.; Carreau, V.; Varret, M.; Bruckert, E.; et al. Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis 2012, 223, 394–400. [Google Scholar] [CrossRef]
- Abboud, S.; Karhunen, P.J.; Lütjohann, D.; Goebeler, S.; Luoto, T.; Friedrichs, S.; Lehtimaki, T.; Pandolfo, M.; Laaksonen, R. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Gene Is a Risk Factor of Large-Vessel Atherosclerosis Stroke. PLoS ONE 2007, 2, e1043. [Google Scholar] [CrossRef]
- Melendez, Q.M.; Krishnaji, S.T.; Wooten, C.J.; Lopez, D. Hypercholesterolemia: The role of PCSK9. Arch. Biochem. Biophys. 2017, 625–626, 39–53. [Google Scholar] [CrossRef]
- Ouguerram, K.; Chetiveaux, M.; Zair, Y.; Costet, P.; Abifadel, M.; Varret, M.; Boileau, C.; Magot, T.; Krempf, M. Apolipoprotein B100 Metabolism in Autosomal-Dominant Hypercholesterolemia Related to Mutations in PCSK9. Arter. Thromb. Vasc. Biol. 2004, 24, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Costet, P.; Cariou, B.; Lambert, G.; Lalanne, F.; Lardeux, B.; Jarnoux, A.-L.; Grefhorst, A.; Staels, B.; Krempf, M. Hepatic PCSK9 Expression Is Regulated by Nutritional Status via Insulin and Sterol Regulatory Element-binding Protein 1c. J. Biol. Chem. 2006, 281, 6211–6218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavori, H.; Giunzioni, I.; Predazzi, I.M.; Plubell, D.; Shivinsky, A.; Miles, J.; DeVay, R.M.; Liang, H.; Rashid, S.; Linton, M.F.; et al. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc. Res. 2016, 110, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le May, C.; Kourimate, S.; Langhi, C.; Chétiveaux, M.; Jarry, A.; Comera, C.; Collet, X.; Kuipers, F.; Krempf, M.; Cariou, B.; et al. Proprotein Convertase Subtilisin Kexin Type 9 Null Mice Are Protected from Postprandial Triglyceridemia. Arter. Thromb. Vasc. Biol. 2009, 29, 684–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roubtsova, A.; Munkonda, M.N.; Awan, Z.; Marcinkiewicz, J.; Chamberland, A.; Lazure, C.; Cianflone, K.; Seidah, N.G.; Prat, A. Circulating Proprotein Convertase Subtilisin/Kexin 9 (PCSK9) Regulates VLDLR Protein and Triglyceride Accumulation in Visceral Adipose Tissue. Arter. Thromb. Vasc. Biol. 2011, 31, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Hansson, G.K. Inflammation and Immunity in Diseases of the Arterial Tree. Circ. Res. 2015, 116, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Giunzioni, I.; Tavori, H.; Covarrubias, R.; Major, A.S.; Ding, L.; Zhang, Y.; DeVay, R.M.; Hong, L.; Fan, D.; Predazzi, I.M.; et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 2015, 238, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.U.; Kee, P.; Danila, D.; Teng, B.-B.; Gim, E.; Shim, D.-W.; Hwang, I.; Shin, O.S.; Yu, J.-W. A Critical Role of PCSK9 in Mediating IL-17-Producing T Cell Responses in Hyperlipidemia. Immune Netw. 2019, 19, e41. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and Along the Mouse Aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef] [PubMed]
- Saenko, E.L.; Yakhyaev, A.V.; Mikhailenko, I.; Strickland, D.K.; Sarafanov, A.G. Role of the Low Density Lipoprotein-related Protein Receptor in Mediation of Factor VIII Catabolism. J. Biol. Chem. 1999, 274, 37685–37692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, N.; Girelli, D.; Lunghi, B.; Pinotti, M.; Marchetti, G.; Malerba, G.; Pignatti, P.F.; Corrocher, R.; Olivieri, O.; Bernardi, F. Polymorphisms at LDLR locus may be associated with coronary artery disease through modulation of coagulation factor VIII activity and independently from lipid profile. Blood 2010, 116, 5688–5697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhu, C.-G.; Xu, R.-X.; Li, S.; Guo, Y.-L.; Sun, J.; Li, J.-J. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J. Clin. Lipidol. 2014, 8, 494–500. [Google Scholar] [CrossRef]
- Ridker, P.M.; Tardif, J.-C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.P.; Kim, A.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N. Engl. J. Med. 2017, 376, 1517–1526. [Google Scholar] [CrossRef]
- Murphy, S.A.; Pedersen, T.R.; Gaciong, Z.A.; Ceska, R.; Ezhov, M.V.; Connolly, D.L.; Jukema, J.W.; Toth, K.; Tikkanen, M.J.; Im, K.; et al. Effect of the PCSK9 Inhibitor Evolocumab on Total Cardiovascular Events in Patients With Cardiovascular Disease: A Prespecified Analysis from the FOURIER Trial. JAMA Cardiol. 2019, 4, 613–619. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Pineda, A.L.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.; Ginsberg, H.; Amarenco, P.; Catapano, A.L.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Gentile, M.; Simeon, V.; Iannuzzo, G.; Mattiello, A.; di Taranto, M.D.; Panico, S.; Rubba, P. Lipoprotein (a) is an independent predictor of cardiovascular events in Mediterranean women (Progetto Atena). Eur. J. Prev. Cardiol. 2019, 27, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-term kidney transplant graft survival—Making progress when most needed. Am. J. Transplant. 2020, 21, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Albeldawi, M.; Aggarwal, A.; Madhwal, S.; Cywinski, J.; Lopez, R.; Eghtesad, B.; Zein, N.N. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transplant. 2011, 18, 370–375. [Google Scholar] [CrossRef]
- Tsai, H.-I.; Liu, F.-C.; Lee, C.-W.; Kuo, C.-F.; See, L.-C.; Chung, T.-T.; Yu, H.-P. Cardiovascular disease risk in patients receiving organ transplantation: A national cohort study. Transpl. Int. 2017, 30, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Hüsing, A.; Kabar, I.; Schmidt, H. Lipids in liver transplant recipients. World J. Gastroenterol. 2016, 22, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Tóth, P.P.; Potter, D.; Ming, E.E. Prevalence of lipid abnormalities in the United States: The National Health and Nutrition Examination Survey 2003–2006. J. Clin. Lipidol. 2012, 6, 325–330. [Google Scholar] [CrossRef]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.-X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef]
- González-Amieva, A.; López-Miranda, J.; Marín, C.; Pérez-Martinez, P.; Gómez, P.; Paz-Rojas, E.; Arizón, J.M.; Jiménez-Perepérez, J.A.; Concha, M.; Pérez-Jiménez, F. The apo A-I gene promoter region polymorphism determines the severity of hyperlipidemia after heart transplantation. Clin. Transplant. 2003, 17, 56–62. [Google Scholar] [CrossRef]
- Taegtmeyer, A.B.; Breen, J.B.; Smith, J.; Rogers, P.; Kullak-Ublick, G.A.; Yacoub, M.H.; Banner, N.R.; Barton, P.J.R. Effect of ABCB1 Genotype on Pre- and Post-Cardiac Transplantation Plasma Lipid Concentrations. J. Cardiovasc. Transl. Res. 2011, 4, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Numakura, K.; Kagaya, H.; Yamamoto, R.; Komine, N.; Saito, M.; Hiroshi, T.; Akihama, S.; Inoue, T.; Narita, S.; Tsuchiya, N.; et al. Characterization of Clinical and Genetic Risk Factors Associated with Dyslipidemia after Kidney Transplantation. Dis. Markers 2015, 2015, 179434. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Balal, M.; Paydas, S.; Sertdemir, Y.; Erken, U. Dyslipidemia and Weight Gain Secondary to Lifestyle Changes in Living Renal Transplant Donors. Transplant. Proc. 2005, 37, 4176–4179. [Google Scholar] [CrossRef]
- Pinto, A.S.; Chedid, M.F.; Guerra, L.T.; Cabeleira, D.D.; Kruel, C.D.P. Dietary management for dyslipidemia in liver transplant recipients. Arq. Bras. Cir. Dig. 2016, 29, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Schlant, R.C.; Kobashigawa, J.; Kubo, S.; Renlund, D.G. Task force 5: Complications. J. Am. Coll. Cardiol. 1993, 22, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Ricoult, S.J.H.; Manning, B.D. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013, 14, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Riella, L.V.; Funakoshi, T. Risk of Metabolic Complications in Kidney Transplantation After Conversion to mTOR Inhibitor: A Systematic Review and Meta-Analysis. Am. J. Transplant. 2014, 14, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- Kurdi, A.; Martinet, W.; De Meyer, G. mTOR Inhibition and Cardiovascular Diseases. Transplantation 2018, 102, S44–S46. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Abagyan, R.; Tsunoda, S.M. Mtor inhibitors associated with higher cardiovascular adverse events—A large population database analysis. Clin. Transplant. 2021, 35, e14228. [Google Scholar] [CrossRef]
- Derfler, K.; Hayde, M.; Heinz, G.; Hirschl, M.M.; Steger, G.; Hauser, A.-C.; Balcke, P.; Widhalm, K. Decreased postheparin lipolytic activity in renal transplant recipients with cyclosporin A. Kidney Int. 1991, 40, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Princen, H.M.; Meijer, P.; Wolthers, B.G.; Vonk, R.J.; Kuipers, F. Cyclosporin A blocks bile acid synthesis in cultured hepatocytes by specific inhibition of chenodeoxycholic acid synthesis. Biochem. J. 1991, 275, 501–505. [Google Scholar] [CrossRef] [Green Version]
- De Groen, P.C. Cyclosporine, Low-Density Lipoprotein, and Cholesterol. Mayo Clin. Proc. 1988, 63, 1012–1021. [Google Scholar] [CrossRef]
- Montero, N.; Pascual, J. Immunosuppression and Post-transplant Hyperglycemia. Curr. Diabetes Rev. 2015, 11, 144–154. [Google Scholar] [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, G.A.; Goldberg, D.S.; Hwang, W.-T.; Judy, R.; Thomasson, A.; Kimmel, S.E.; Forde, K.A.; Lewis, J.D.; Yang, Y.-X. Sustained Posttransplantation Diabetes Is Associated with Long-Term Major Cardiovascular Events Following Liver Transplantation. Am. J. Transplant. 2017, 18, 207–215. [Google Scholar] [CrossRef]
- Cron, D.C.; Noon, K.A.; Cote, D.R.; Terjimanian, M.N.; Augustine, J.J.; Wang, S.C.; Englesbe, M.J.; Woodside, K.J. Using analytic morphomics to describe body composition associated with post-kidney transplantation diabetes mellitus. Clin. Transplant. 2017, 31, e13040. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Moore, R.; Baboolal, K. Influence of Lifestyle Modification in Renal Transplant Recipients With Postprandial Hyperglycemia. Transplantation 2008, 85, 353–358. [Google Scholar] [CrossRef]
- McCaughan, J.A.; McKnight, A.J.; Maxwell, A.P. Genetics of New-Onset Diabetes after Transplantation. J. Am. Soc. Nephrol. 2013, 25, 1037–1049. [Google Scholar] [CrossRef] [Green Version]
- Gervasini, G.; Luna, E.; García-Cerrada, M.; García-Pino, G.; Cubero, J.J. Risk factors for post-transplant diabetes mellitus in renal transplant: Role of genetic variability in the CYP450-mediated arachidonic acid metabolism. Mol. Cell. Endocrinol. 2016, 419, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, K. Insulin Resistance after Renal Transplantation: The Effect of Steroid Dose Reduction and Withdrawal. J. Am. Soc. Nephrol. 2004, 15, 3233–3239. [Google Scholar] [CrossRef] [Green Version]
- Schäcke, H.; Döcke, W.-D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Vincenti, F.; Friman, S.; Scheuermann, E.; Rostaing, L.; Jenssen, T.; Campistol, J.M.; Uchida, K.; Pescovitz, M.D.; Marchetti, P.; Tuncer, M.; et al. Results of an International, Randomized Trial Comparing Glucose Metabolism Disorders and Outcome with Cyclosporine Versus Tacrolimus. Am. J. Transplant. 2007, 7, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin Inhibition and New-Onset Diabetes Mellitus After Transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef]
- Fraenkel, M.; Ketzinel-Gilad, M.; Ariav, Y.; Pappo, O.; Karaca, M.; Castel, J.; Berthault, M.-F.; Magnan, C.; Cerasi, E.; Kaiser, N.; et al. mTOR Inhibition by Rapamycin Prevents β-Cell Adaptation to Hyperglycemia and Exacerbates the Metabolic State in Type 2 Diabetes. Diabetes 2008, 57, 945–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, O.; Rose, C.L.; Webster, A.C.; Gill, J.S. Sirolimus Is Associated with New-Onset Diabetes in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2008, 19, 1411–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, D.R.; Jenkins, G.H. Hypertension in transplant recipients. J. Hum. Hypertens. 1996, 10, 395–402. [Google Scholar]
- Sudhir, K.; MacGregor, J.S.; DeMarco, T.; De Groot, C.J.; Taylor, R.N.; Chou, T.M.; Yock, P.G.; Chatterjee, K. Cyclosporine impairs release of endothelium-derived relaxing factors in epicardial and resistance coronary arteries. Circulation 1994, 90, 3018–3023. [Google Scholar] [CrossRef] [Green Version]
- Ventura, H.O.; Mehra, M.; Stapleton, D.D.; Smart, F.W. Cyclosporine-induced hypertension in cardiac transplantation. Med. Clin. N. Am. 1997, 81, 1347–1357. [Google Scholar] [CrossRef]
- Ruiz, M.; Medina, A.; Moreno, J.; Gómez, I.; Ruiz, N.; Bueno, P.; Asensio, C.; Osuna, A. Relationship Between Oxidative Stress Parameters and Atherosclerotic Signs in the Carotid Artery of Stable Renal Transplant Patients. Transplant. Proc. 2005, 37, 3796–3798. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Perek, B.; Rodzki, M.; Bociański, M.; Jemielity, M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021, 10, 3032. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Stehlik, J. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report—2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1155–1168. [Google Scholar] [CrossRef]
- Lu, W.-H.; Palatnik, K.; Fishbein, G.A.; Lai, C.; Levi, D.S.; Perens, G.; Alejos, J.; Kobashigawa, J.; Fishbein, M.C. Diverse morphologic manifestations of cardiac allograft vasculopathy: A pathologic study of 64 allograft hearts. J. Heart Lung Transplant. 2011, 30, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Holley, C. Cardiac Allograft Vasculopathy. J. Am. Coll. Cardiol. 2019, 74, 52–53. [Google Scholar] [CrossRef]
- Ghanem, H.; Dorpel, M.A.V.D.; Weimar, W.; Veld, A.J.M.I.; El-Kannishy, M.H.; Jansen, H. Increased low density lipoprotein oxidation in stable kidney transplant recipients. Kidney Int. 1996, 49, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Najafian, B.; Kasiske, B.L. Chronic allograft nephropathy. Curr. Opin. Nephrol. Hypertens. 2008, 17, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.-L.; Holvoet, P.; Dauwe, S.E.; Ysebaert, D.K.; Chapelle, T.; Jürgens, A.; Kovacic, V.; Van Marck, E.A.; De Broe, M.E.; Verpooten, G.A. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 11/2 years. Kidney Int. 2001, 59, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.; Teuteberg, J.; Shullo, M. Optimal low-density lipoprotein concentration for cardiac allograft vasculopathy prevention. Clin. Transplant. 2018, 32, e13248. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Pearce, L.S.; Wilkins, J.T.; Overington, J.; Hingorani, A.; Casas, J.P. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017, 4, CD011748. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Dhindsa, D.; Hirsh, B.; Jokhadar, M.; Cole, R.T.; Sperling, L.S. PCSK9 inhibition in patients with heart transplantation: A case series. J. Clin. Lipidol. 2019, 13, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, Y.; Kozuszko, S.; Knowles, J.W.; Chih, S.; Oro, G.; Lee, R.; Fearon, W.F.; Ross, H.J.; Teuteberg, J.J.; Khush, K.K. Safety and Efficacy of PCSK9 Inhibitors After Heart Transplantation. Can. J. Cardiol. 2018, 35, 104.e1–104.e3. [Google Scholar] [CrossRef] [PubMed]
- Sammour, Y.; Dezorzi, C.; Austin, B.A.; Borkon, A.M.; Everley, M.P.; Fendler, T.J.; Khumri, T.M.; Lawhorn, S.L.; Nassif, M.E.; Vodnala, D.; et al. PCSK9 Inhibitors in Heart Transplant Patients: Safety, Efficacy, and Angiographic Correlates. J. Card. Fail. 2021, 27, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Gude, E.; Karason, K.; Dellgren, G.; Rådegran, G.; Gjesdal, G.; Gustafsson, F.; Eiskjaer, H.; Lommi, J.; Pentikäinen, M.; et al. Cholesterol lowering with EVOLocumab to prevent cardiac allograft Vasculopathy in De-novo heart transplant recipients: Design of the randomized controlled EVOLVD trial. Clin. Transplant. 2020, 34, e13984. [Google Scholar] [CrossRef]
- Papasotiriou, M.; Ntrinias, T.; Savvidaki, E.; Papachristou, E.; Goumenos, D.S. Treatment of Mixed Dyslipidemia with Alirocumab in a Kidney Transplant Recipient: A Case Report. Transplant. Proc. 2021, 53, 2775–2778. [Google Scholar] [CrossRef]
- Ordóñez-Fernández, L.; Rodríguez-Ferreras, A.; Carriles, C.; Martínez-Torrón, A.; Lázaro-López, E.; Rosado-María, M.C. Pneumonia in a patient with kidney transplant treated with alirocumab and everolimus. Farm. Hosp. 2019, 43, 74–76. [Google Scholar] [CrossRef]
- Melexopoulou, C.; Marinaki, S.; Oikonomou, E.; Bonios, M.J.; Theofilis, P.; Miliou, A.; Siasos, G.; Tousoulis, D.; Boletis, J.N. PCSK9 and inflammatory biomarkers in the early post kidney transplantation period. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4762–4772. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-Q.; Shi, H.-W.; Li, J.-J. Proprotein Convertase Subtilisin/Kexin Type 9 and Inflammation: An Updated Review. Front. Cardiovasc. Med. 2022, 9, 763516. [Google Scholar] [CrossRef]
- Leander, K.; Mälarstig, A.; Hooft, F.M.V.; Hyde, C.; Hellénius, M.-L.; Troutt, J.S.; Konrad, R.J.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Zelle, D.M.; Sloan, J.H.; Gaillard, C.A.; Bakker, S.J.; Dullaart, R.P. High Serum PCSK9 Is Associated With Increased Risk of New-Onset Diabetes After Transplantation in Renal Transplant Recipients. Diabetes Care 2017, 40, 894–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, N.C. A comprehensive account of insulin and LDL receptor activity over the years: A highlight on their signaling and functional role. J. Biochem. Mol. Toxicol. 2021, 35, e22840. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Chen, C.; Han, S.; Ganda, A.; Murphy, A.J.; Haeusler, R.; Thorp, E.; Accili, D.; Horton, J.D.; Tall, A.R. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J. Clin. Investig. 2012, 122, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| BENEFITS SHOWED | Reduction in LDL levels in patients already at the highest possible dose of statins, without drug interferences with immunosuppressants. |
| Reduction in CVD incidence and atherosclerotic plaque progression. | |
| POSSIBLE BENEFITS | Reduction in cardiac allograft vasculopathy incidence. |
| Reduction in post-transplant diabetes mellitus incidence. | |
| Reduction in chronic allograft nephropathy incidence. | |
| Graft survival extension. | |
| Reduction in CVD through platelet aggregation inhibition. | |
| Reduction in CVD through FVIII clearance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuomo, G.; Cioffi, G.; Di Lorenzo, A.; Iannone, F.P.; Cudemo, G.; Iannicelli, A.M.; Pacileo, M.; D’Andrea, A.; Vigorito, C.; Iannuzzo, G.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients. J. Clin. Med. 2022, 11, 3247. https://doi.org/10.3390/jcm11113247
Cuomo G, Cioffi G, Di Lorenzo A, Iannone FP, Cudemo G, Iannicelli AM, Pacileo M, D’Andrea A, Vigorito C, Iannuzzo G, et al. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients. Journal of Clinical Medicine. 2022; 11(11):3247. https://doi.org/10.3390/jcm11113247
Chicago/Turabian StyleCuomo, Gianluigi, Giuseppe Cioffi, Anna Di Lorenzo, Francesca Paola Iannone, Giuseppe Cudemo, Anna Maria Iannicelli, Mario Pacileo, Antonello D’Andrea, Carlo Vigorito, Gabriella Iannuzzo, and et al. 2022. "Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients" Journal of Clinical Medicine 11, no. 11: 3247. https://doi.org/10.3390/jcm11113247
APA StyleCuomo, G., Cioffi, G., Di Lorenzo, A., Iannone, F. P., Cudemo, G., Iannicelli, A. M., Pacileo, M., D’Andrea, A., Vigorito, C., Iannuzzo, G., & Giallauria, F. (2022). Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Use for Atherogenic Dyslipidemia in Solid Organ Transplant Patients. Journal of Clinical Medicine, 11(11), 3247. https://doi.org/10.3390/jcm11113247







