Long-Term Safety in HBsAg-Negative, HBcAb-Positive Patients with Rheumatic Diseases Receiving Maintained Steroid Therapy after Pulse Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection
2.2.1. Baseline Characteristics
2.2.2. Serological and Virologic Tests for HBV
2.2.3. Glucocorticoid Therapy and Concurrent Medications
2.3. Outcome Definition
2.4. Statistical Analyses
3. Results
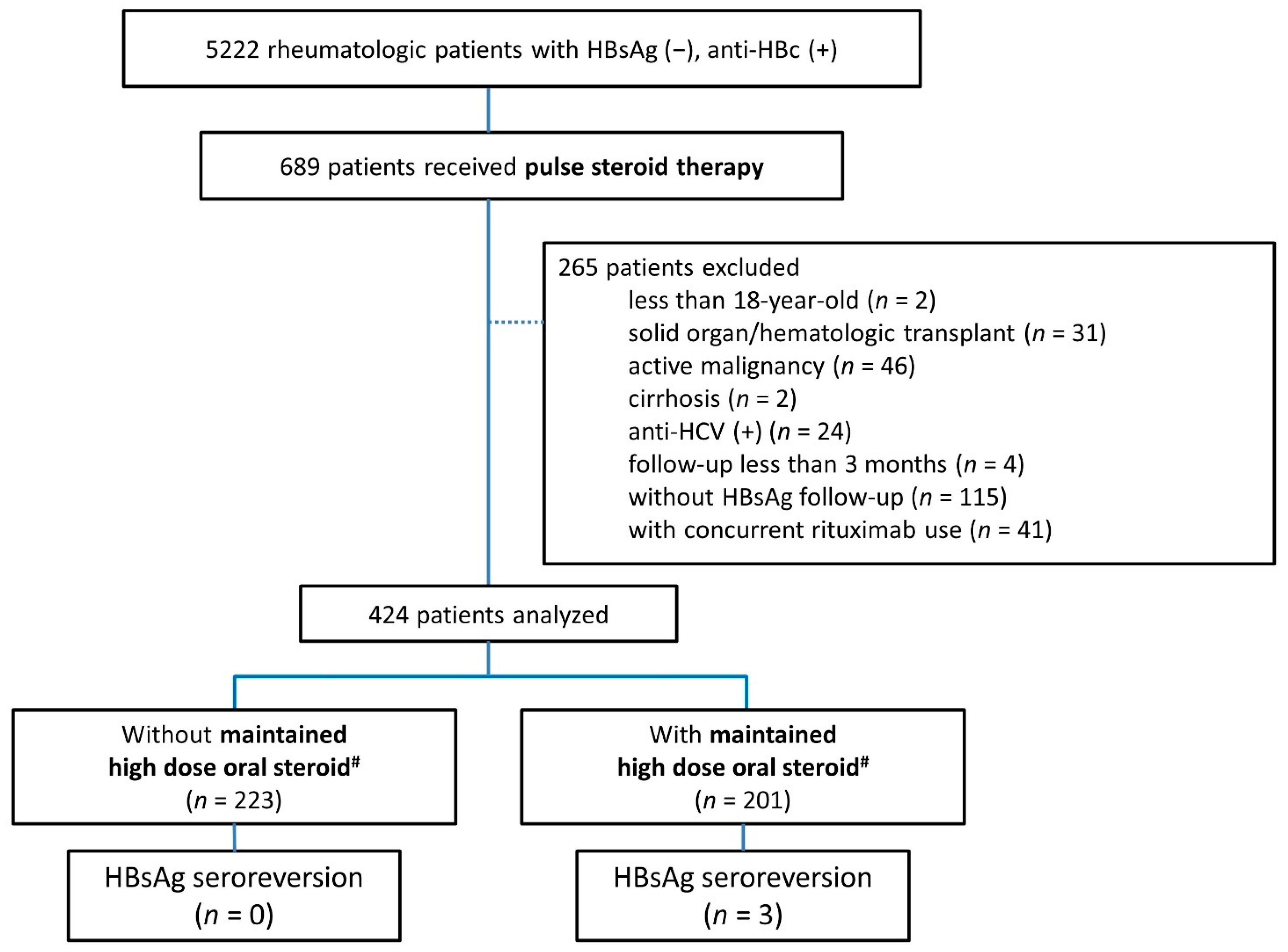
3.1. Enrolled Patients
3.2. The Occurrence of Hepatitis within the First Year after GC Pulse Therapy
3.3. The Ratio of HBsAg Seroreversion after GC Pulse Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepatitis, B. World Health Organization 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 23 January 2019).
- Lee, W.M. Hepatitis B Virus Infection. N. Engl. J. Med. 1997, 337, 1733–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.-M.; Hwang, J.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.R.; Beavers, K.L.; Hammond, S.; Lim, J.K.; Falck-Ytter, Y.T. American Gastroenterological Association Institute Guideline on the Prevention and Treatment of Hepatitis B Virus Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 2015, 148, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galbraith, R.; Williams, R.; Eddleston, A.; Zuckerman, A.; Bagshawe, K. Fulminant hepatic failure in leukaemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet 1975, 306, 528–530. [Google Scholar] [CrossRef]
- Perrillo, R.P.; Schiff, E.R.; Davis, G.L.; Bodenheimer, H.C.J.; Lindsay, K.; Payne, J.; Dienstag, J.; O’Brien, C.; Tamburro, C.; Jacobson, I.M.; et al. A Randomized, Controlled Trial of Interferon Alfa-2b Alone and after Prednisone Withdrawal for the Treatment of Chronic Hepatitis B. N. Engl. J. Med. 1990, 323, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.-F.; Tsai, S.-L.; Chien, R.-N.; Yeh, C.-T.; Chu, C.-M. Prednisolone priming enhances Th1 response and efficacy of sub-sequent lamivudine therapy in patients with chronic hepatitis B. Hepatology 2000, 32, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Lee, S.-W.; Yeh, H.-Z.; Chang, C.-S.; Yang, S.-S. The prevalence and risk factors of hepatitis B flares in chronic hepati-tis B patients receiving glucocorticoid pulse therapy. Int. J. Clin. Pharm. 2018, 40, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; da Silva, J.A.; Boers, M.; Burmester, G.R.; Cutolo, M.; Jacobs, J.; Kirwan, J.; Köhler, L.; Van Riel, P.; Vischer, T.; et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: Current ques-tions and tentative answers in rheumatology. Ann. Rheum. Dis. 2002, 61, 718–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aithal, G.P. Hepatotoxicity related to antirheumatic drugs. Nat. Rev. Rheumatol. 2011, 7, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Chan, T.C.; Leung, N.W.; Lam, W.Y.; Mo, F.K.; Chu, M.T.; Chan, H.L.; Hui, E.P.; Lei, K.I.; Mok, T.S.; et al. Hepa-titis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J. Clin. Oncol. 2009, 27, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.-K.; Cheung, W.-W.; Zhang, H.-Y.; Au, W.-Y.; Yueng, Y.-H.; Leung, A.-Y.; Leung, N.; Luk, J.-M.; Lie, A.-K.; Kwong, Y.-L.; et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006, 131, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.-K.; Chan, T.S.; Hwang, Y.-Y.; Wong, D.K.-H.; Fung, J.; Liu, K.S.-H.; Gill, H.; Lam, Y.-F.; Lie, A.K.; Lai, C.-L.; et al. Hepatitis B Reactivation in Patients With Previous Hepatitis B Virus Exposure Undergoing Rituximab-Containing Chemotherapy for Lymphoma: A Prospective Study. J. Clin. Oncol. 2014, 32, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Notarnicola, A.; Lopalco, G.; Viggiani, M.T.; Sebastiani, F.; Covelli, M.; Iannone, F.; Avolio, A.; Di Leo, A.; Cantarini, L.; et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015, 62, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Straub, R.H.; Wehling, M. Glucocorticoids in the treatment of rheumatic diseases: An update on the mechanisms of action. Arthritis Rheum. 2004, 50, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Cavalcanti, R.; Carnot, F.; Legendre, C.; Driss, F.; Chaix, M.L.; Thervet, E.; Chkoff, N.; Brechot, C.; Berthelot, P.; et al. Azathioprine hepatitis in kidney transplant recipients. Transplantation 1996, 61, 1774–1776. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Wong, V.W.-S.; Yuen, W.Y.; Tse, Y.-K.; Yip, T.C.-F.; Luk, H.W.-S.; Lui, C.Y.G.; Chan, H.L.-Y. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J. Hepatol. 2020, 72, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Chen, M.-H.; Chou, C.-T.; Hou, M.-C.; Tsai, C.-Y.; Huang, Y.-H. Low but Long-lasting Risk of Reversal of Sero-conversion in Patients with Rheumatoid Arthritis Receiving Immunosuppressive Therapy. Clin. Gastroenterol. Hepatol. 2020, 18, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Chen, H.-H.; Huang, W.-N.; Chen, Y.-H.; Hsieh, T.-Y.; Yang, S.-S.; Lan, J.-L.; Chen, D.-Y. Reactivation of hepatitis B virus infection following rituximab treatment in HBsAg-negative, HBcAb-positive rheumatoid arthritis patients: A long-term, real-world observation. Int. J. Rheum. Dis. 2019, 22, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chien, R.-N.; Huang, Y.-H.; Chen, D.-Y.; Lan, J.-L. Screening and management of hepatitis B infection in rheumat-ic patients scheduled for biologic therapy: Consensus recommen-dations from the Taiwan Rheumatology Association. J. Rheumatol. 2012, 26, 1–7. [Google Scholar]
| Characteristics | N = 424 | |
|---|---|---|
| Age (years) | 47.0 | (37.0–57.1) |
| Gender (M/F) | 126/298 | |
| Follow-up time (months) | 131.7 | (79.0–170.3) |
| Platelet (×103/L) | 266.5 | (207.3–342.3) |
| Albumin (mg/dL) | 3.45 | (3–3.9) |
| Total bilirubin (mg/dL) | 0.4 | (0.3–0.7) |
| ALT at baseline, U/L | 20 | (15–35) |
| Anti-HBs (+) (n = 371) | 311 | (83.8%) |
| Number of pulse steroid episodes | 2 | (1–3) |
| Underlying rheumatic disease | ||
| Rheumatoid arthritis | 140 | (33.0%) |
| Systemic lupus erythematosus | 133 | (31.4%) |
| Idiopathic inflammatory myopathy | 50 | (11.8%) |
| Ankylosing arthritis | 42 | (9.9%) |
| Psoriatic arthritis | 18 | (4.3%) |
| Adult onset Still’s disease | 15 | (3.5%) |
| Systemic sclerosis | 13 | (3.1%) |
| Vasculitis | 8 | (1.9%) |
| Sjogren’s syndrome | 5 | (1.2%) |
| Concurrent anti-rheumatic treatments | ||
| Anti-TNF-α | ||
| Etanercept | 11 | (2.6%) |
| Adalimumab | 23 | (5.4%) |
| Golimumab | 2 | (0.5%) |
| Abatacept | 3 | (0.7%) |
| Maintained oral glucocorticoid † | ||
| 10–19 mg/day | 140 | (33.0%) |
| ≥20 mg/day | 201 | (47.4%) |
| DMARDs | ||
| Methotrexate | 232 | (54.7%) |
| Sulfasalazine | 165 | (38.9%) |
| Azathioprine | 152 | (35.9%) |
| Cyclosporine | 142 | (33.5%) |
| Leflunomide | 49 | (11.6%) |
| Mycophenolate | 34 | (8.0%) |
| Cyclophosphamide | 98 | (23.11%) |
| Hepatitis (+) (n = 28) | Hepatitis (−) (n = 396) | p Value | |||
|---|---|---|---|---|---|
| Age (years) | 55.22 | (43.59–61.7) | 46.41 | (36.98–56.55) | 0.047 * |
| Gender (M/F) | 12/16 | 114/282 | 0.174 | ||
| Platelet (×103/L) | 246 | (202–330) | 267 | (208–353) | 0.351 |
| Albumin (mg/dL) | 3.3 | (2.6–3.7) | 3.5 | (3.05–3.9) | 0.222 |
| Total bilirubin (mg/dL) | 0.7 | (0.4–1.2) | 0.4 | (0.3–0.6) | 0.006 ** |
| ALT at baseline, U/L | 28.5 | (16–42.25) | 20 | (15–33.5) | 0.189 |
| Anti-HBs (+) (n = 411) | 20 | (76.92%) | 291 | (84.35%) | 0.404 |
| Anti-HBs titer (n = 89) | 70.1 | (7.3–635.9) | 51.9 | (13.0–659.3) | 0.819 |
| Time to hepatitis (days) | 84.8 | (17.2–247.3) | |||
| Peak ALT when hepatitis, U/L | 157.0 | (124.5–206.3) | |||
| Peak ALP when hepatitis, U/L | 86 | (119–140) | |||
| Seroreversion when hepatitis | 0 | (0%) | |||
| HBV DNA detected when hepatitis (n = 27) | 0 | (0%) | |||
| Number of pulse steroid episodes | 2.5 | (1–4) | 2 | (1–3) | 0.490 |
| Underlying rheumatic disease | 0.027 * | ||||
| Rheumatoid arthritis | 4 | (14.29%) | 136 | (34.34%) | |
| Systemic lupus erythematosus | 9 | (32.14%) | 124 | (31.31%) | |
| Idiopathic inflammatory myopathy | 8 | (28.57%) | 42 | (10.61%) | |
| Ankylosing arthritis | 1 | (3.57%) | 41 | (10.35%) | |
| Psoriatic arthritis | 3 | (10.71%) | 15 | (3.79%) | |
| Adult onset Still’s disease | 2 | (7.14%) | 13 | (3.28%) | |
| Systemic sclerosis | 0 | (0%) | 13 | (3.28%) | |
| Vasculitis | 1 | (3.57%) | 7 | (1.77%) | |
| Sjogren’s syndrome | 0 | (0%) | 5 | (1.26%) | |
| Concurrent anti-rheumatic treatments | |||||
| Anti-TNF-α | |||||
| Adalimumab f | 1 | (3.57%) | 10 | (2.53%) | 0.533 |
| Etanercept f | 1 | (3.57%) | 22 | (5.56%) | 1.000 |
| Golimumab f | 0 | (0%) | 2 | (0.51%) | 1.000 |
| Abatacept f | 0 | (0%) | 3 | (0.76%) | 1.000 |
| Maintained oral glucocorticoid doses † | 1.000 | ||||
| 10–19 mg/day | 10 | (41.67%) | 130 | (41.01%) | |
| ≥20 mg/day | 14 | (58.33%) | 187 | (58.99%) | |
| DMARDs | |||||
| Methotrexate | 8 | (28.57%) | 224 | (56.57%) | 0.007 ** |
| Azathioprine | 16 | (57.14%) | 136 | (34.34%) | 0.026 * |
| Leflunomide f | 4 | (14.29%) | 45 | (11.36%) | 0.550 |
| Cyclosporine | 12 | (42.86%) | 130 | (32.83%) | 0.379 |
| Sulfasalazine | 4 | (14.29%) | 161 | (40.66%) | 0.010 * |
| Mycophenolate f | 6 | (21.43%) | 28 | (7.07%) | 0.018 * |
| Cyclophasphamide | 11 | (39.29%) | 87 | (21.97%) | 0.062 |
| Cyclophosphamide | 11 | (34.38%) | 96 | (22.17%) | 0.172 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p Value | OR | (95% CI) | p Value | |
| Age | 1.03 | (1.00–1.05) | 0.069 | 1.04 | (1.01–1.07) | 0.021 * |
| Concurrent anti-rheumatic treatments | ||||||
| Maintained oral glucocorticoid † | ||||||
| 10–19 mg/day | Reference | |||||
| ≥20 mg/day | 0.97 | (0.42–2.26) | 0.950 | |||
| DMARDs | ||||||
| Methotrexate | 0.31 | (0.13–0.71) | 0.006 ** | 0.54 | (0.21–1.42) | 0.211 |
| Azathioprine | 2.55 | (1.17–5.54) | 0.018 * | 2.50 | (1.03–6.06) | 0.043 * |
| Leflunomide | 1.30 | (0.43–3.92) | 0.641 | |||
| Cyclosporine | 1.53 | (0.71–3.34) | 0.280 | |||
| Sulfasalazine | 0.24 | (0.08–0.71) | 0.010 * | 0.42 | (0.12–1.43) | 0.165 |
| Mycophenolate | 3.58 | (1.34–9.56) | 0.011 * | 2.83 | (0.94–8.52) | 0.064 |
| Cyclophosphamide | 2.30 | (1.04–5.09) | 0.040 * | 1.02 | (0.41–2.54) | 0.963 |
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age/Gender | 66/F | 25/F | 59/F |
| Diagnosis | Idiopathic Inflammatory myopathy | SLE | RA |
| Baseline anti-HBs | Positive | Negative | Positive |
| anti-HBs loss at seroreversion | yes | N/A | yes |
| Baseline ALT (U/L) | 17 | 36 | 30 |
| Peak ALT at seroreversion (U/L) | 26 | 694 | 71 |
| Baseline total Bilirubin (mg/dL) | 0.3 | 0.3 | 0.4 |
| Peak Bilirubin at seroreversion (mg/dL) | 0.4 | 0.1 | 0.4 |
| Viral loads at baseline (IU/mL) | N/A | 3.48 × 102 | Undetectable |
| Viral loads at reactivation (IU/mL) | 1.03 × 108 | >1.7 × 108 | 8.79 × 107 |
| Concomitant drugs | |||
| Anti-TNF-α biologics | |||
| Maintained oral glucocorticoid ≥20 mg/day † | + | + | + |
| Time to seroreversion (months) | 97 | 66 | 168 |
| NUCs usage at seroreversion | ETV | ETV | ETV |
| Outcomes | Stable | Stable | Stable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Chen, Y.-J.; Lee, S.-W.; Lee, T.-Y.; Chen, Y.-H.; Huang, W.-N.; Yang, S.-S.; Chen, Y.-M. Long-Term Safety in HBsAg-Negative, HBcAb-Positive Patients with Rheumatic Diseases Receiving Maintained Steroid Therapy after Pulse Therapy. J. Clin. Med. 2021, 10, 3296. https://doi.org/10.3390/jcm10153296
Lin Y-C, Chen Y-J, Lee S-W, Lee T-Y, Chen Y-H, Huang W-N, Yang S-S, Chen Y-M. Long-Term Safety in HBsAg-Negative, HBcAb-Positive Patients with Rheumatic Diseases Receiving Maintained Steroid Therapy after Pulse Therapy. Journal of Clinical Medicine. 2021; 10(15):3296. https://doi.org/10.3390/jcm10153296
Chicago/Turabian StyleLin, Ying-Cheng, Yen-Ju Chen, Shou-Wu Lee, Teng-Yu Lee, Yi-Hsing Chen, Wen-Nan Huang, Sheng-Shun Yang, and Yi-Ming Chen. 2021. "Long-Term Safety in HBsAg-Negative, HBcAb-Positive Patients with Rheumatic Diseases Receiving Maintained Steroid Therapy after Pulse Therapy" Journal of Clinical Medicine 10, no. 15: 3296. https://doi.org/10.3390/jcm10153296
APA StyleLin, Y.-C., Chen, Y.-J., Lee, S.-W., Lee, T.-Y., Chen, Y.-H., Huang, W.-N., Yang, S.-S., & Chen, Y.-M. (2021). Long-Term Safety in HBsAg-Negative, HBcAb-Positive Patients with Rheumatic Diseases Receiving Maintained Steroid Therapy after Pulse Therapy. Journal of Clinical Medicine, 10(15), 3296. https://doi.org/10.3390/jcm10153296








