Proton Conductivity of Nafion/Ex-Situ Sulfonic Acid-Modified Stöber Silica Nanocomposite Membranes As a Function of Temperature, Silica Particles Size and Surface Modification
Abstract
:1. Introduction
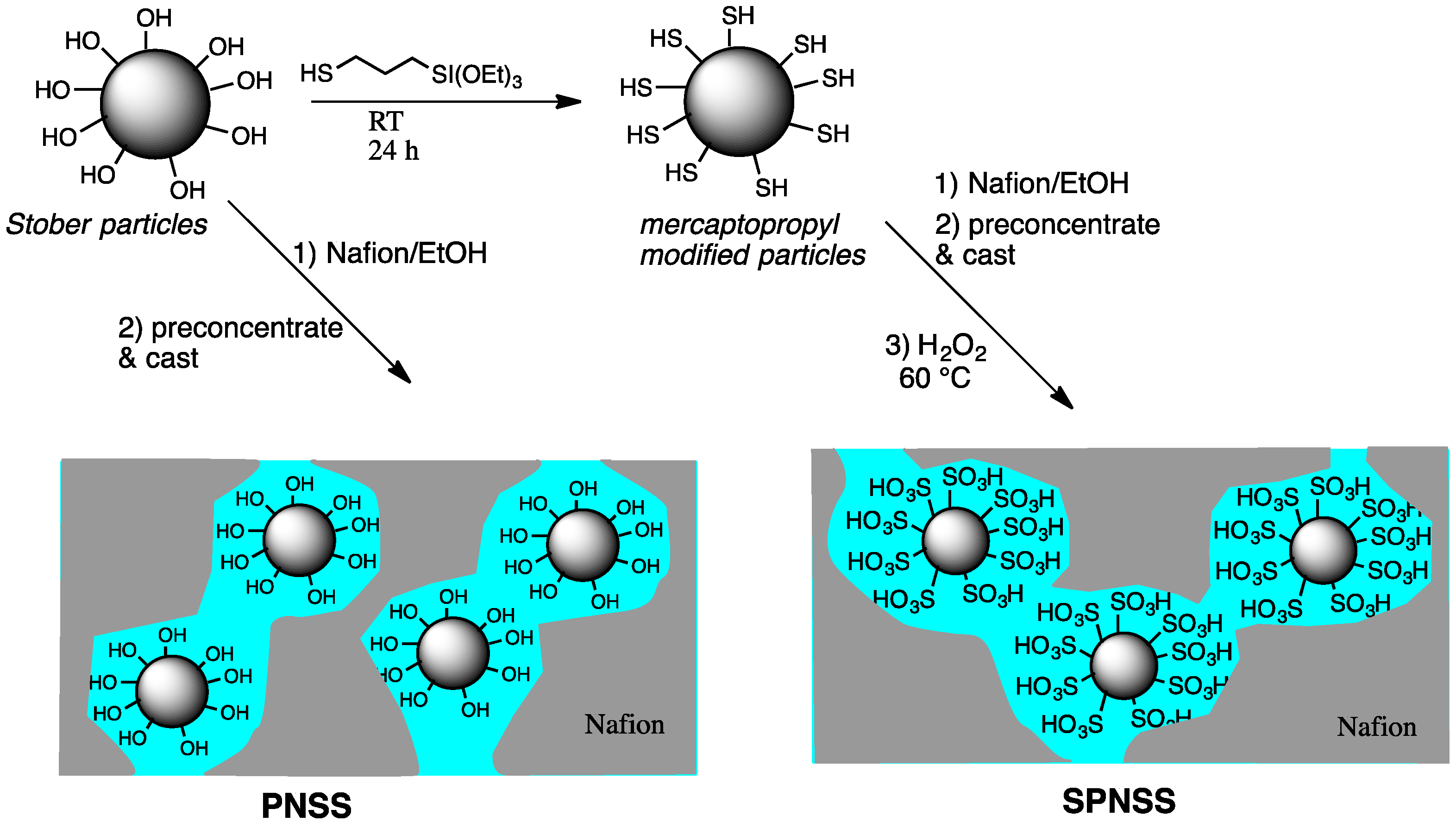
2. Results and Discussion
2.1. Nanoparticle Growth, Size, and Surface Modification
| Particles with Silanols (PNSS) | Sulfonic Acid Modified Particles (SPNSS) | ||||
|---|---|---|---|---|---|
| Particle Size (nm) | Titrated SiOH (mmol/gram) | Theoretical Surface * SiOH (mmol/gram) | Particle Size (nm) | Titrated Acidic Groups (mmol/gram) | Theoretical Surface SiOH (mmol/gram) |
| 50 ± 2 | 1.48 | 0.38 | 40 ± 5 | 3.86 | 0.47 |
| 120 ± 8 | 1.18 | 0.16 | 80 ± 8 | 2.24 | 0.24 |
| 232 ± 16 | 1.62 | 0.08 | 140 ± 10 | 2.05 | 0.13 |
| 388 ± 10 | 1.78 | 0.05 | 232 ± 16 | 1.8 | 0.08 |
2.2. Impact of Stöber Silica Particle Surface Modification on Nafion/ex-situ Sulfonated Silica Nanocomposite Membranes (SPNSS) Ionic Exchange Capacity
| Membranes | Experimental IECs | Theoretical IECs with Non Acidic Filler | Theoretical IECs with Acidic Filler from Table 1 |
|---|---|---|---|
| Nafion 117 | 0.93 | 0.91 (no filler) | 0.91(no filler) |
| Recast Nafion | 0.90 ± 0.03 | 0.91 (no filler) | 0.91(no filler) |
| PNSS Membranes | 0.81 ± 0.06 | 0.874 | 0.94 |
| SPNSS-30nm Membranes | 0.88 ± 0.07 | 0.874 | 1.06 |
| SPNSS-150nm Membranes | 0.89 ± 0.2 | 0.874 | 0.97 |
| SPNSS-235nm Membranes | 0.80 ± 0.22 | 0.874 | 0.95 |
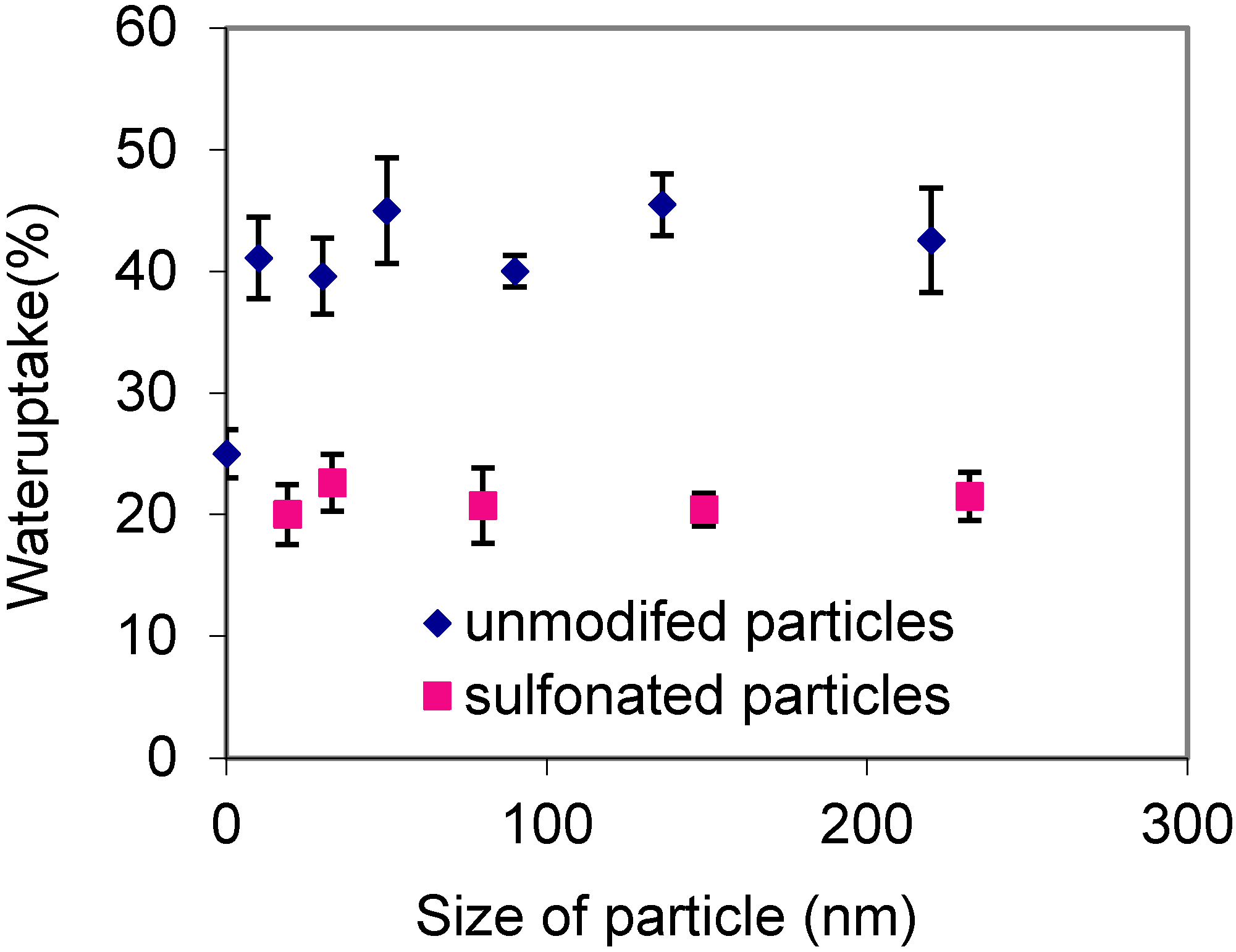
2.3. Water Uptake of SPNSS Nanocomposite Membranes at 60 °C
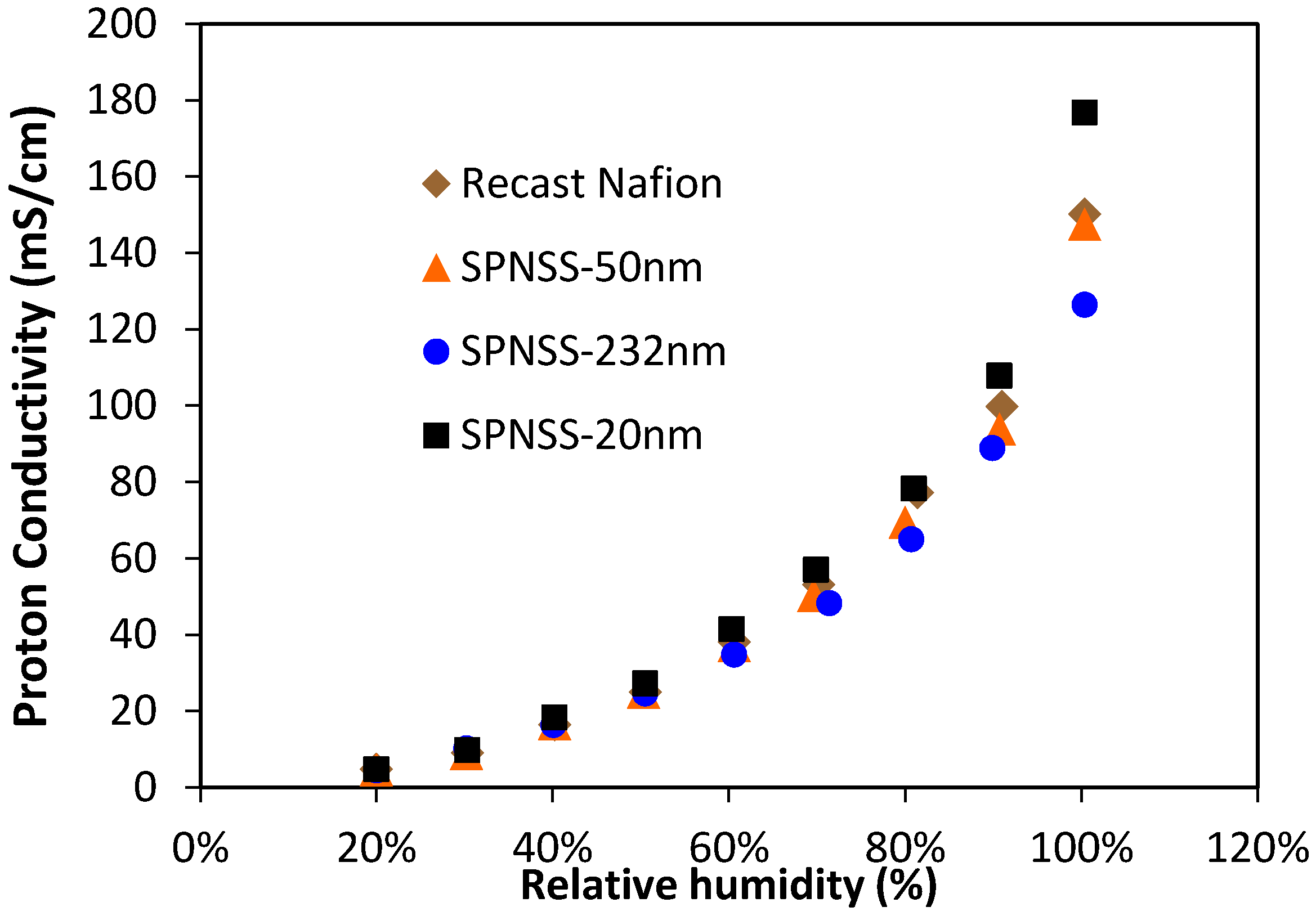
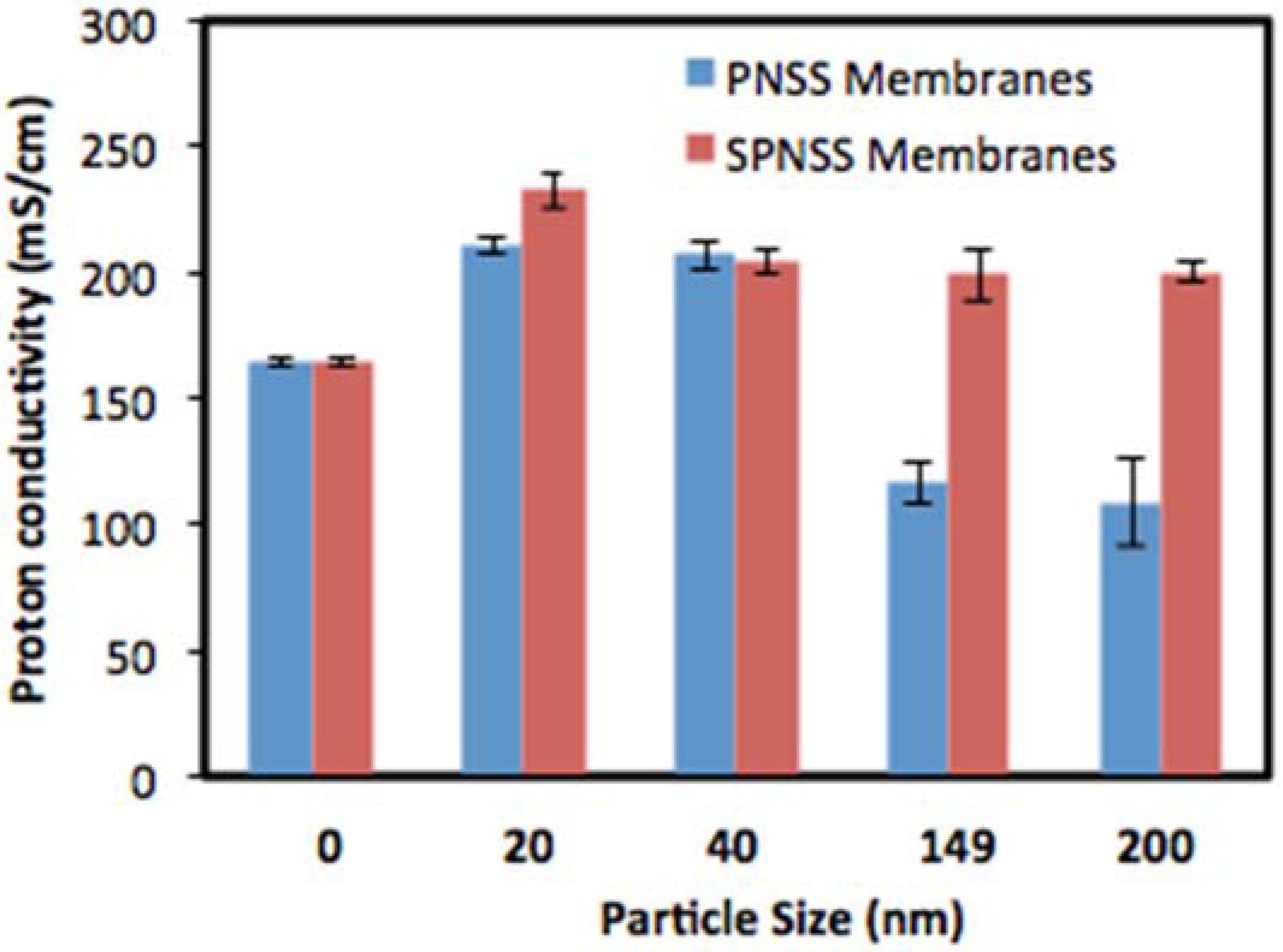
2.4. Proton Conductivity of SPNSS Nanocomposite Membranes at 80 °C
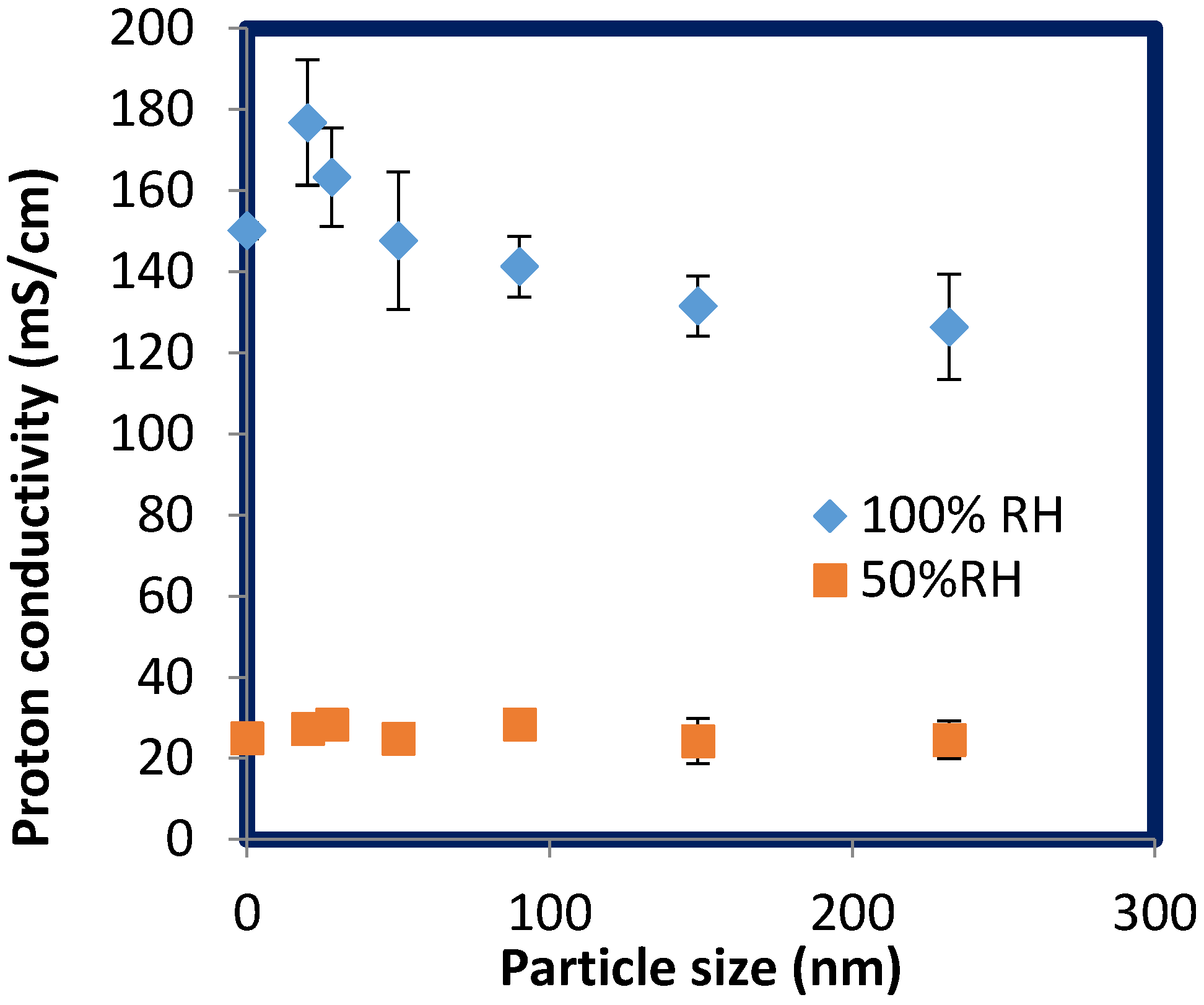
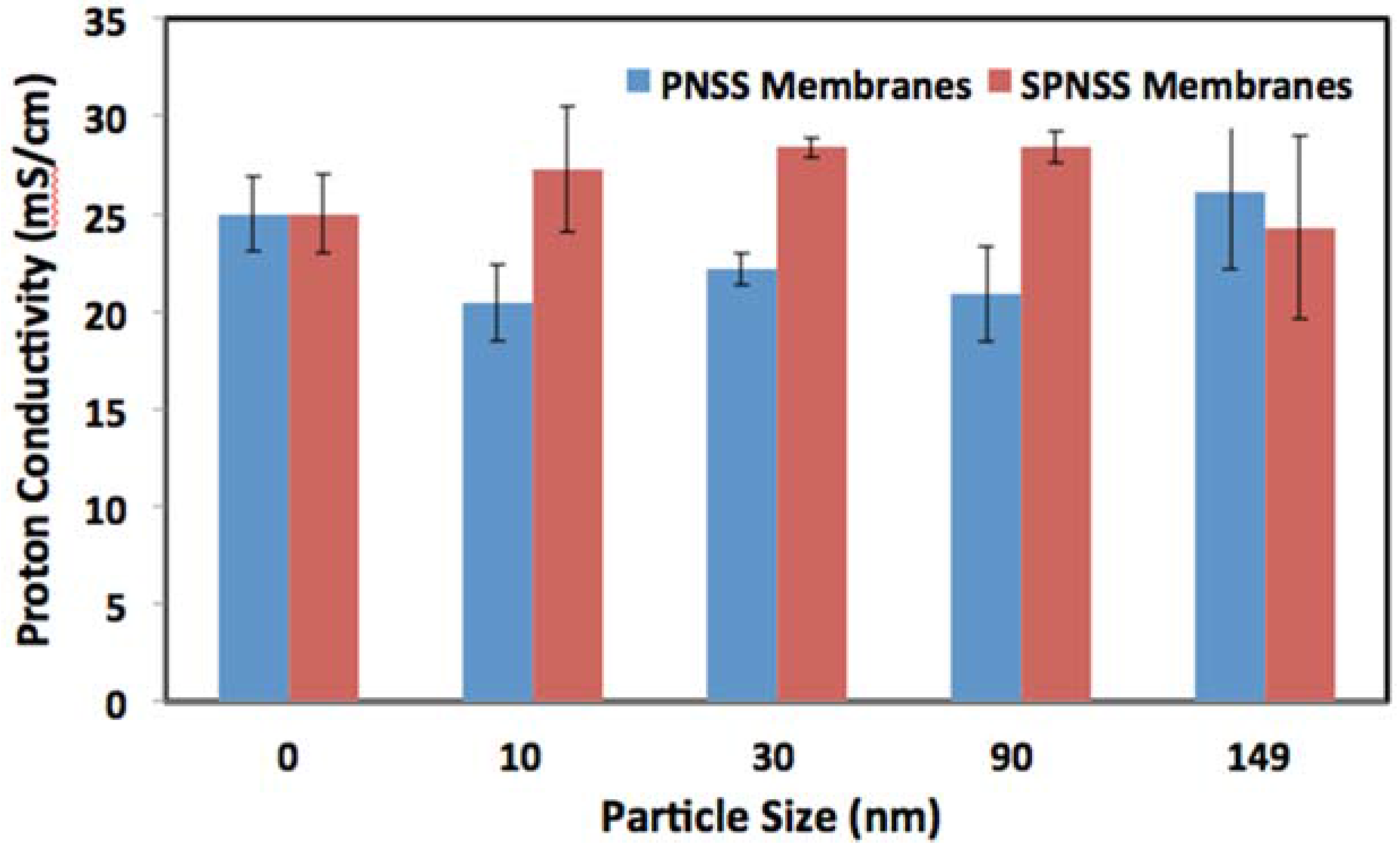
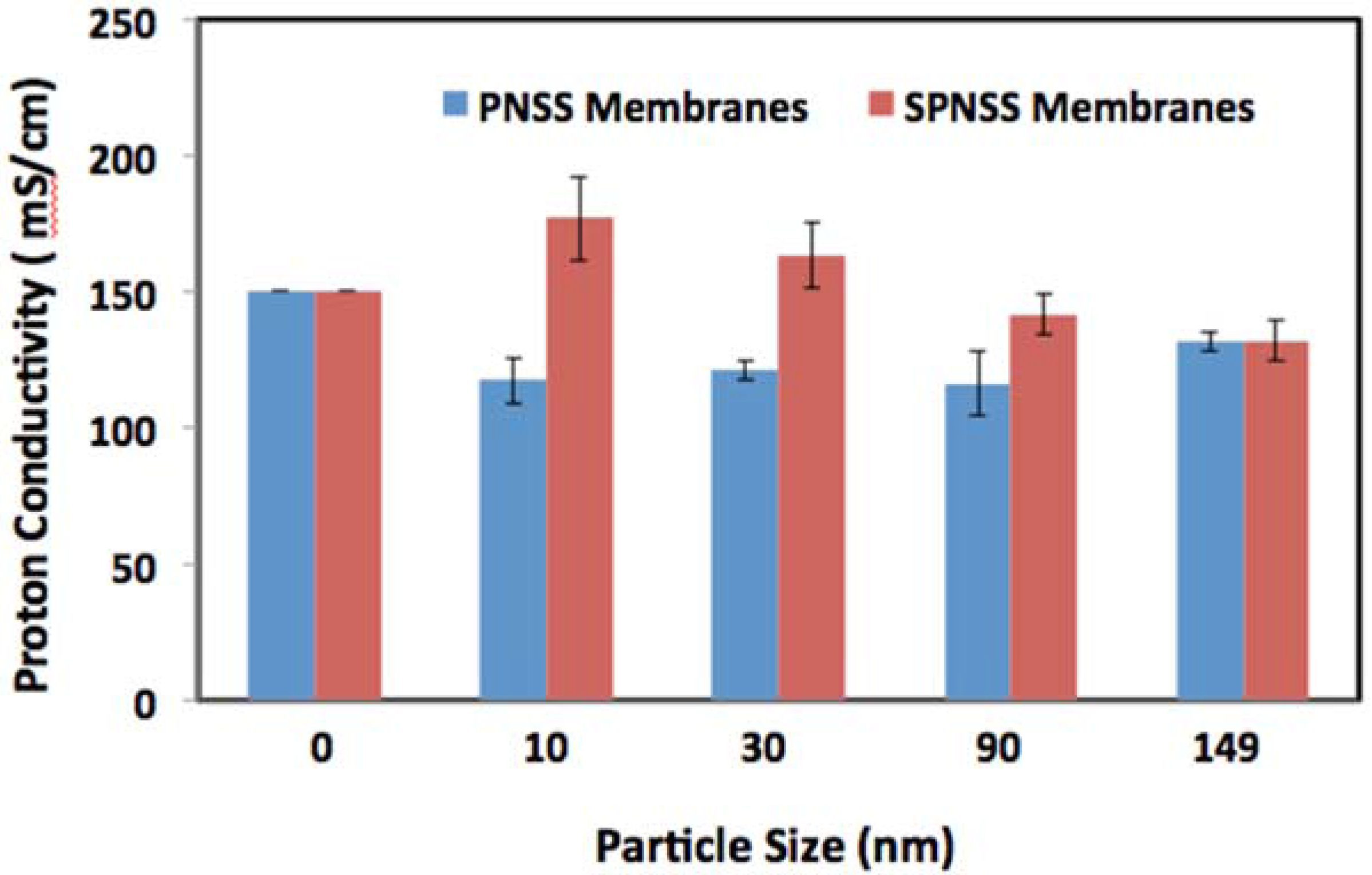
2.5. Proton Conductivity of SPNSS Nanocomposite Membranes at 120 °C
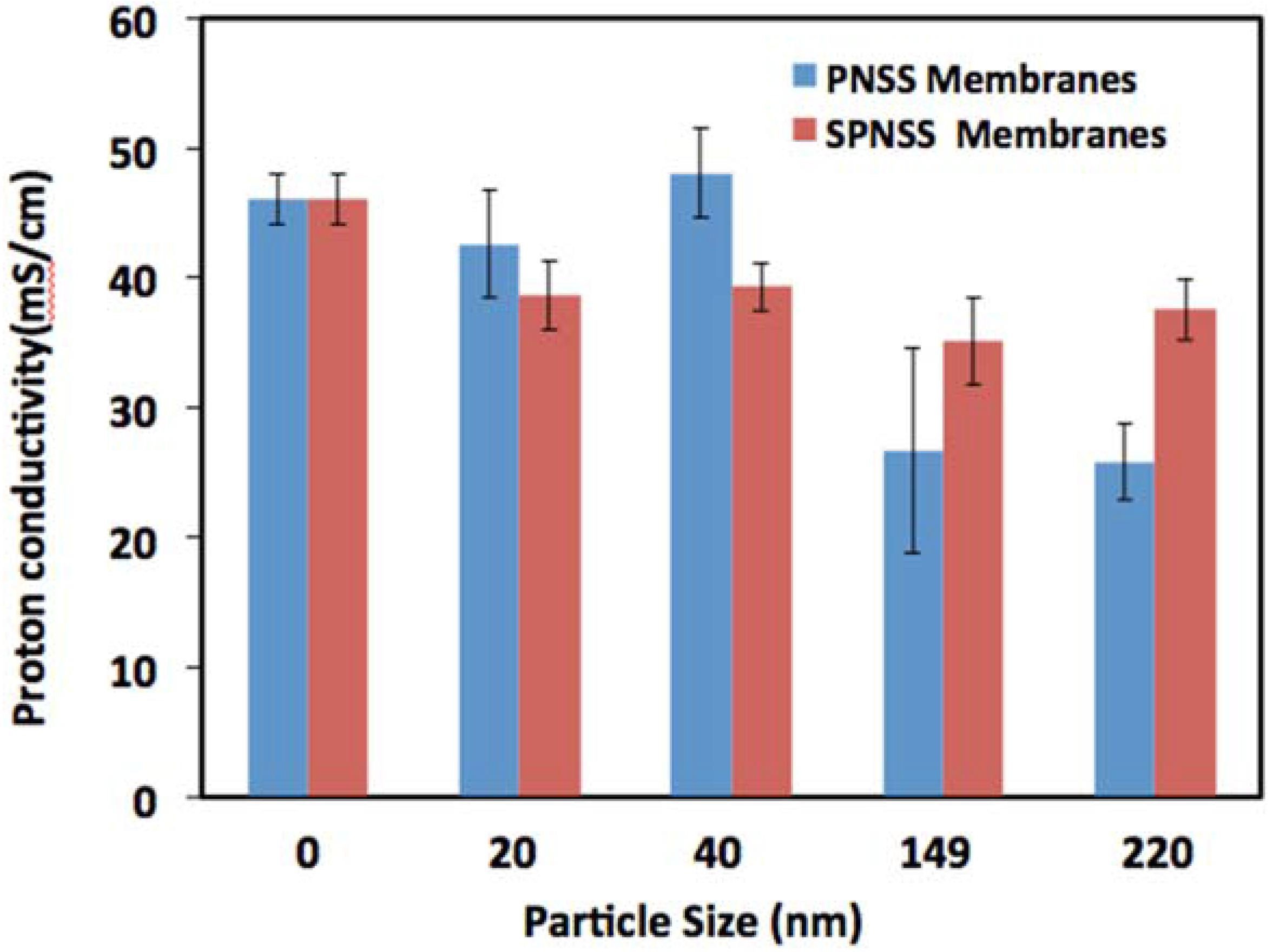
3. Experimental Section
3.1. General Methods
3.2. Stöber Synthesis
3.3. Nanocomposite Membranes
3.4. Water Up-Take Measurements
3.5. IEC Measurements
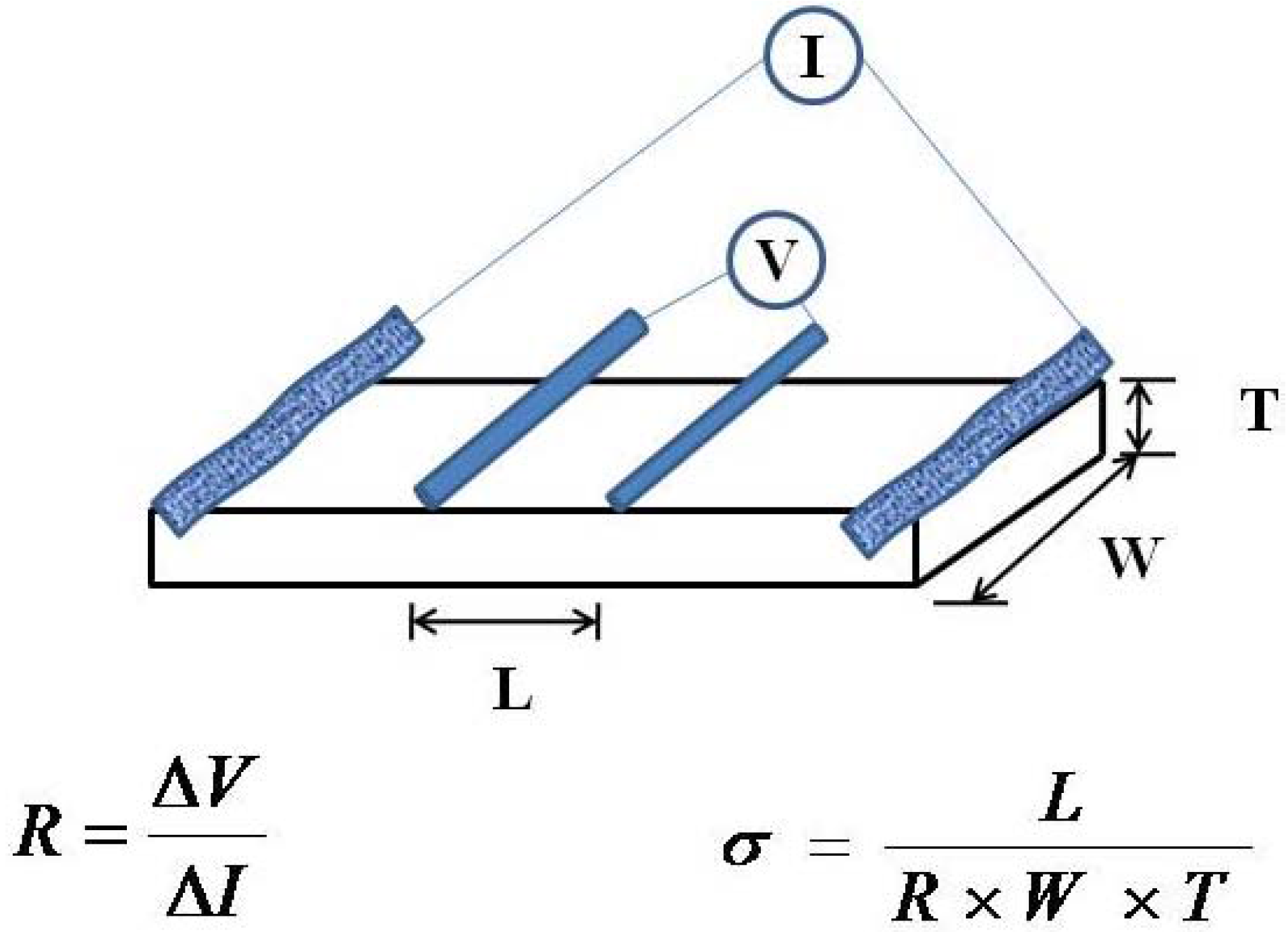
3.6. Proton Conductivity Measurements
3.7. Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Hamrock, S.J.; Yandrasits, M.A. Proton exchange membranes for fuel cell applications. Polym. Rev. 2006, 46, 219–244. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Lee, S.J.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Silicon oxide Nafion composite membranes for proton-exchange membrane fuel cell operation at 80–140 degrees C. J. Electrochem. Soc. 2002, 149, A256–A261. [Google Scholar] [CrossRef]
- Muriithi, B.; Loy, D.A. Processing, Morphology, and Water Uptake of Nafion/Ex situ Stober Silica Nanocomposite Membranes As a Function of Particle Size. ACS Appl. Mater. Interfaces 2012, 4, 6765–6772. [Google Scholar] [CrossRef] [PubMed]
- Muriithi, B.; Loy, D.A. Proton conductivity of Nafion/ex situ Stober silica nanocomposite membranes as a function of silica particle size and temperature. J. Mater. Sci. 2014, 49, 1566–1573. [Google Scholar] [CrossRef]
- Miyake, N.; Wainright, J.S.; Savinell, R.F. Evaluation of a sol-gel derived Nafion/silica hybrid membrane for proton electrolyte membrane fuel cell applications-I. Proton conductivity and water content. J. Electrochem. Soc. 2001, 148, A898–A904. [Google Scholar] [CrossRef]
- Jiang, R.C.; Kunz, H.R.; Fenton, J.M. Composite silica/Nafion (R) membranes prepared by tetraethylorthosilicate sol-gel reaction and solution casting for direct methanol fuel cells. J. Membr. Sci. 2006, 272, 116–124. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Shi, Z.Q.; Holdcroft, S. Transport properties of composite membranes containing silicon dioxide and Nafion (R). J. Membr. Sci. 2008, 325, 346–356. [Google Scholar] [CrossRef]
- Pereira, F.; Valle, K.; Belleville, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced mesostructured hybrid silica-nafion membranes for high-performance PEM fuel cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Domingues, E.M.; Sousa, N.; Ferreira, P.; Figueiredo, F.M.L. Meso-structured organosilicas as fillers for Nafion membranes. Solid State Ionics 2014, 262, 324–327. [Google Scholar] [CrossRef]
- Lin, Y.F.; Yen, C.Y.; Ma, C.C.M.; Liao, S.H.; Lee, C.H.; Hsiao, Y.H.; Lin, H.P. High proton-conducting Nafion (R)/-SO3H functionalized mesoporous silica composite membranes. J. Power Sour. 2007, 171, 388–395. [Google Scholar] [CrossRef]
- Ren, S.Z.; Sun, G.Q.; Li, C.N.; Liang, Z.X.; Wu, Z.M.; Jin, W.; Qin, X.; Yang, X.F. Organic silica/Nafion (R) composite membrane for direct methanol fuel cells. J. Membr. Sci. 2006, 282, 450–455. [Google Scholar] [CrossRef]
- Lavorgna, M.; Mascia, L.; Mensitieri, G.; Gilbert, M.; Scherillo, G.; Palomba, B. Hybridization of Nafion membranes by the infusion of functionalized siloxane precursors. J. Membr. Sci. 2007, 294, 159–168. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Lee, C.-H.; Lin, Y.-F.; Lin, H.-L.; Hsiao, Y.-H.; Liao, S.-H.; Chuang, C.-Y.; Ma, C.-C.M. Sol-gel derived sulfonated-silica/Nafion composite membrane for direct methanol fuel cell. J. Power Sour. 2007, 173, 36–44. [Google Scholar] [CrossRef]
- Alekseev, S.A.; Zaitsev, V.N.; Alekseev, A.N.; Kochkin, Y.N.; Evans, J. Effect of silanol groups on the acidic and catalytic properties of alkylsulphoacidic silicas and SiO2/Nafion nanocomposites. Adsorpt. Sci. Technol. 2004, 22, 615–625. [Google Scholar] [CrossRef]
- Yuan, J.; Guangbin, Z.; Hongting, P. Preparation and properties of Nafion®/hollow silica spheres composite membranes. J. Membr. Sci. 2008, 325, 742–748. [Google Scholar] [CrossRef]
- Yuan, J.J.; Pu, H.T.; Yang, Z.L. Studies on Sulfonic Acid Functionalized Hollow Silica Spheres/Nafion (R) Composite Proton Exchange Membranes. J. Polym. Sci. Part a-Polym. Chem. 2009, 47, 2647–2655. [Google Scholar] [CrossRef]
- Yen, C.Y.; Lee, C.H.; Lin, Y.F.; Lin, H.L.; Hsiao, Y.H.; Liao, S.H.; Chuang, C.Y.; Ma, C.C.M. Sol-gel derived sulfonated-silica/Nafion (R) composite membrane for direct methanol fuel cell. J. Power Sour. 2007, 173, 36–44. [Google Scholar] [CrossRef]
- Mosa, J.; Duran, A.; Aparicio, M. Sulfonic acid-functionalized hybrid organic-inorganic proton exchange membranes synthesized by sol-gel using 3-mercaptopropyl trimethoxysilane (MPTMS). J. Power Sour. 2015, 297, 208–216. [Google Scholar] [CrossRef]
- Khiterer, M.; Loy, D.A.; Cornelius, C.J.; Fujimoto, C.H.; Small, J.H.; McIntire, T.M.; Shea, K.J. Hybrid Polyelectrolyte Materials for Fuel Cell Applications: Design, Synthesis, and Evaluation of Proton-Conducting Bridged Polysilsesquioxanes. Chem. Mater. 2006, 18, 3665–3673. [Google Scholar] [CrossRef]
- Ke, C.-C.; Li, X.-J.; Qu, S.-G.; Shao, Z.-G.; Yi, B.-L. Preparation and properties of Nafion/SiO2 composite membrane derived via in situ sol-gel reaction: size controlling and size effects of SiO2 nano-particles. Polym. Adv. Technol. 2012, 23, 92–98. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Tominaga, Y.; Hong, I.C.; Asai, S.; Sumita, M. Proton conduction in Nafion composite membranes filled with mesoporous silica. J. Power Sour. 2007, 171, 530–534. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Knott, R.B.; Martin, D.J.; da Costa, J.C.D.; Lu, G.Q. Nafion-MPMDMS nanocomposite membranes with low methanol permeability. Electrochem. Commun. 2007, 9, 781–786. [Google Scholar] [CrossRef]
- Li, C.N.; Sun, G.Q.; Ren, S.Z.; Liu, J.; Wang, Q.; Wu, Z.M.; Sun, H.; Jin, W. Casting Nafion-sulfonated organosilica nano-composite membranes used in direct methanol fuel cells. J. Membr. Sci. 2006, 272, 50–57. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss Mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Marx, D. Proton transfer 200 years after von Grotthuss: Insights from ab initio simulations (vol 7, pg 1848, 2006). ChemPhysChem 2007, 8, 209–210. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Rabenau, A.; Weppner, W. Vehicle Mechanism, a New Model for the Interpretation of the Conductivity of Fast Proton Conductors. Angew. Chem. Int. Ed. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Rahman, I.A.; Vejayakumaran, P.; Sipaut, C.S.; Ismail, J.; Abu Bakar, M.; Adnan, R.; Chee, C.K. An optimized sol-gel synthesis of stable primary equivalent silica particles. Colloids Surfaces Physicochem. Eng. Asp. 2007, 294, 102–110. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriithi, B.; Loy, D.A. Proton Conductivity of Nafion/Ex-Situ Sulfonic Acid-Modified Stöber Silica Nanocomposite Membranes As a Function of Temperature, Silica Particles Size and Surface Modification. Membranes 2016, 6, 12. https://doi.org/10.3390/membranes6010012
Muriithi B, Loy DA. Proton Conductivity of Nafion/Ex-Situ Sulfonic Acid-Modified Stöber Silica Nanocomposite Membranes As a Function of Temperature, Silica Particles Size and Surface Modification. Membranes. 2016; 6(1):12. https://doi.org/10.3390/membranes6010012
Chicago/Turabian StyleMuriithi, Beatrice, and Douglas A. Loy. 2016. "Proton Conductivity of Nafion/Ex-Situ Sulfonic Acid-Modified Stöber Silica Nanocomposite Membranes As a Function of Temperature, Silica Particles Size and Surface Modification" Membranes 6, no. 1: 12. https://doi.org/10.3390/membranes6010012





