Aspects of Mathematical Modelling of Pressure Retarded Osmosis
Abstract
:1. Introduction
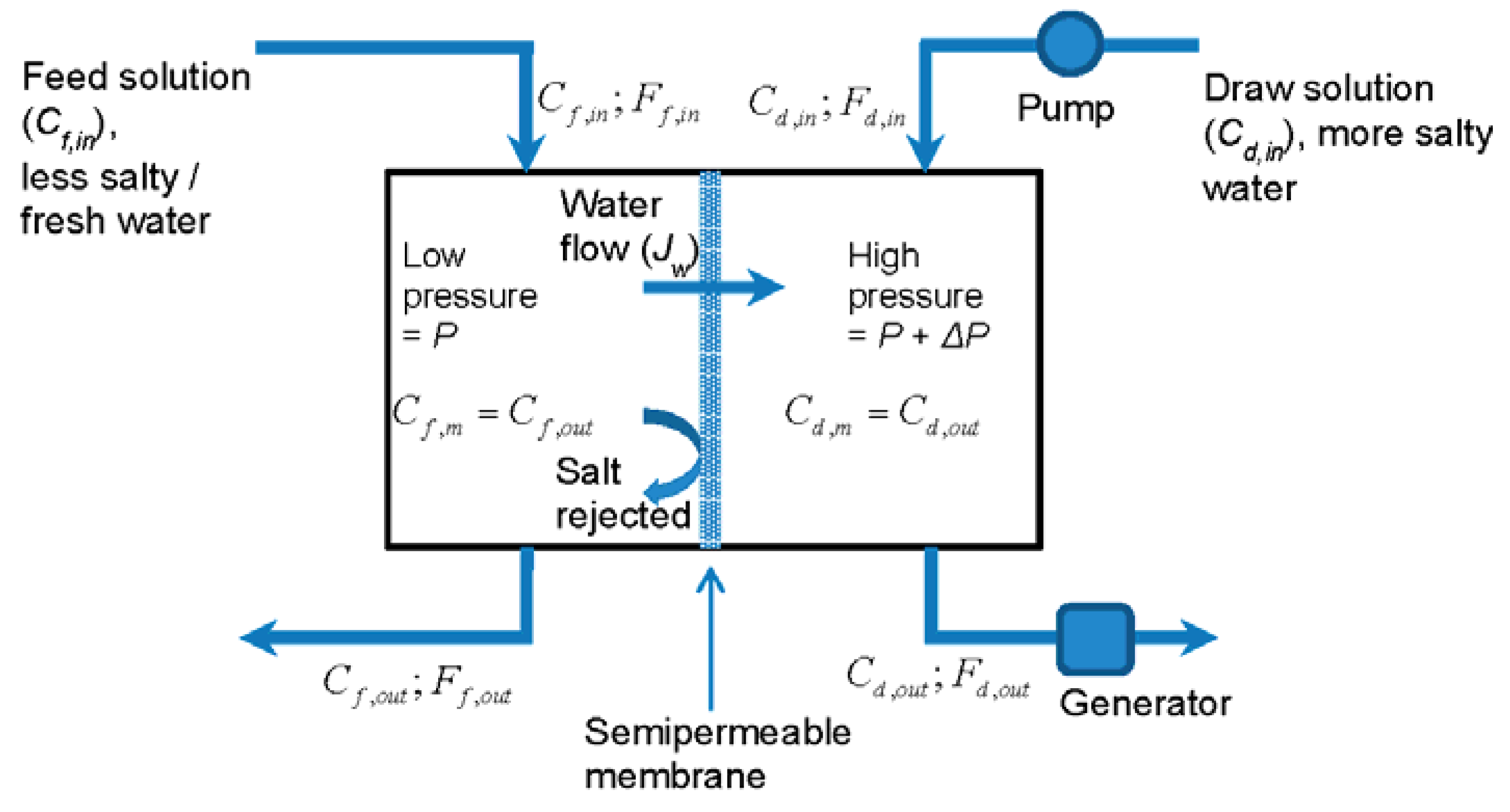
2. Ideal Membrane
2.1. Compartmental Configuration
2.2. Counterflow Configuration

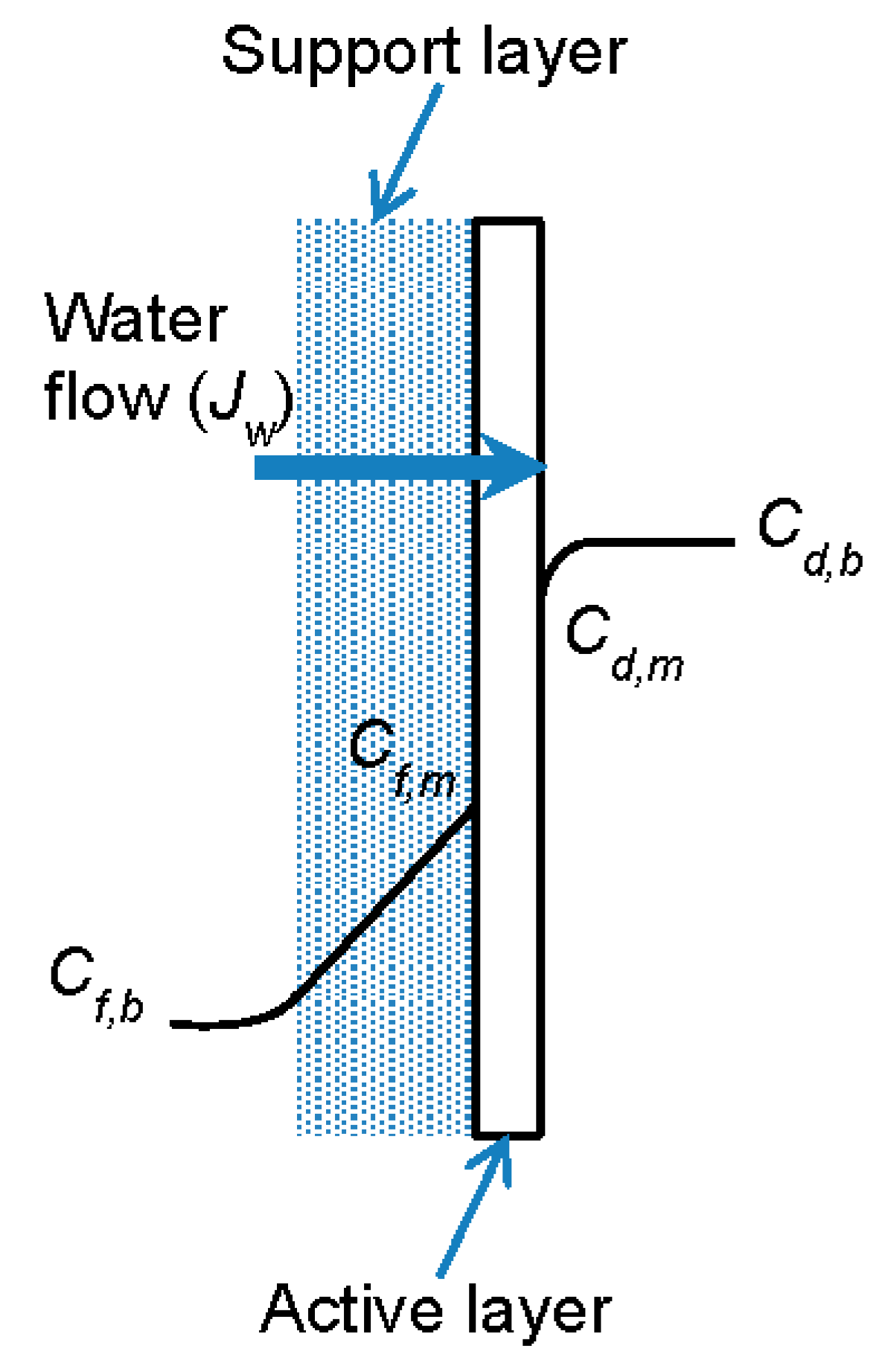
3. Concentration Polarization
4. Module-Scale Analysis of PRO
5. Other Aspects of Mathematical Modelling of PRO
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Pattle, R.E. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Loeb, S. Osmotic power-plants. Science 1975, 189, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Baker, R.W.; Lonsdale, H.K. Membranes for power-generation by pressure-retarded osmosis. J. Membr. Sci. 1981, 8, 141–171. [Google Scholar] [CrossRef]
- McGinnis, R.L.; McCutcheon, J.R.; Elimelech, M. A novel ammonia-carbon dioxide osmotic heat engine for power generation. J. Membr. Sci. 2007, 305, 13–19. [Google Scholar] [CrossRef]
- Hon, K.C.; Zhao, C.L.; Yang, C.; Low, S.C. A method of producing electrokinetic power through forward osmosis. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Lin, S.H.; Yip, N.Y.; Cath, T.Y.; Osuji, C.O.; Elimelech, M. Hybrid pressure retarded osmosis-membrane distillation system for power generation from low-grade heat: Thermodynamic analysis and energy efficiency. Environ. Sci. Technol. 2014, 48, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Helfer, F.; Lemckert, C.; Anissimov, Y.G. Osmotic power with pressure retarded osmosis: Theory, performance and trends—A review. J. Membr. Sci. 2014, 453, 337–358. [Google Scholar] [CrossRef]
- Zhao, S.F.; Zou, L.; Tang, C.Y.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Altaee, A.; Zaragoza, G.; Sharif, A. Pressure retarded osmosis for power generation and seawater desalination: Performance analysis. Desalination 2014, 344, 108–115. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A. Pressure retarded osmosis: Advancement in the process applications for power generation and desalination. Desalination 2015, 356, 31–46. [Google Scholar] [CrossRef]
- Straub, A.P.; Deshmukh, A.; Elimelech, M. Pressure-retarded osmosis for power generation from salinity gradients: Is it viable? Energy Environ. Sci. 2016, 9, 31–48. [Google Scholar] [CrossRef]
- Sivertsen, E.; Holt, T.; Thelin, W.R.; Brekke, G. Iso-watt diagrams for evaluation of membrane performance in pressure retarded osmosis. J. Membr. Sci. 2015, 489, 299–307. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Banchik, L.D.; Lienhard, J.H. Effectiveness-mass transfer units (epsilon-mtu) model of an ideal pressure retarded osmosis membrane mass exchanger. J. Membr. Sci. 2013, 445, 211–219. [Google Scholar] [CrossRef]
- Banchik, L.D.; Sharqawy, M.H.; Lienhard, J.H. Limits of power production due to finite membrane area in pressure retarded osmosis. J. Membr. Sci. 2014, 468, 81–89. [Google Scholar] [CrossRef]
- Mehta, G.D.; Loeb, S. Internal polarization in the porous substructure of a semipermeable membrane under pressure-retarded osmosis. J. Membr. Sci. 1978, 4, 261–265. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a loeb-sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffrnan, J.D.; Hoover, L.A.; Kim, Y.C.; Elimelech, M. Thin-film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients. Environ. Sci. Technol. 2011, 45, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Chou, S.R.; Wang, R.; Shi, L.; She, Q.H.; Tang, C.Y.; Fane, A.G. Thin-film composite hollow fiber membranes for pressure retarded osmosis (pro) process with high power density. J. Membr. Sci. 2012, 389, 25–33. [Google Scholar] [CrossRef]
- Straub, A.P.; Lin, S.H.; Elimelech, M. Module-scale analysis of pressure retarded osmosis: Performance limitations and implications for full-scale operation. Environ. Sci. Technol. 2014, 48, 12435–12444. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by ammonia-carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Reimund, K.K.; McCutcheon, J.R.; Wilson, A.D. Thermodynamic analysis of energy density in pressure retarded osmosis: The impact of solution volumes and costs. J. Membr. Sci. 2015, 487, 240–248. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Optimal hydrostatic counter-pressure in pressure-retarded osmosis with composite/asymmetric membranes. J. Membr. Sci. 2015, 477, 157–160. [Google Scholar] [CrossRef]
- Thorsen, T.; Holt, T. The potential for power production from salinity gradients by pressure retarded osmosis. J. Membr. Sci. 2009, 335, 103–110. [Google Scholar] [CrossRef]
- Trettin, D.R.; Doshi, M.R. Limiting flux in ultrafiltration of macromolecular solutions. Chem. Eng. Commun. 1980, 4, 507–522. [Google Scholar] [CrossRef]
- Skilhagen, S.E. Osmotic power—A new, renewable energy source. Desalination Water Treat. 2010, 15, 271–278. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, Y.; Oh, D.; Lee, K.H. Experimental investigation of a spiral-wound pressure-retarded osmosis membrane module for osmotic power generation. Environ. Sci. Technol. 2013, 47, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Peng, X.Y.; Tang, C.Y.Y.; Fu, Q.S.A.; Nie, S.Z. Effect of draw solution concentration and operating conditions on forward osmosis and pressure retarded osmosis performance in a spiral wound module. J. Membr. Sci. 2010, 348, 298–309. [Google Scholar] [CrossRef]
- Lin, S.H.; Straub, A.P.; Elimelech, M. Thermodynamic limits of extractable energy by pressure retarded osmosis. Energy Environ. Sci. 2014, 7, 2706–2714. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 2012, 46, 5230–5239. [Google Scholar] [CrossRef] [PubMed]
- Seppala, A.; Lampinen, M.J. Thermodynamic optimizing of pressure-retarded osmosis power generation systems. J. Membr. Sci. 1999, 161, 115–138. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: An experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Bruening, M.L.; Bernal, E.E.L. Solution-diffusion-electro-migration model and its uses for analysis of nanofiltration, pressure-retarded osmosis and forward osmosis in multi-ionic solutions. J. Membr. Sci. 2013, 447, 463–476. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anissimov, Y.G. Aspects of Mathematical Modelling of Pressure Retarded Osmosis. Membranes 2016, 6, 13. https://doi.org/10.3390/membranes6010013
Anissimov YG. Aspects of Mathematical Modelling of Pressure Retarded Osmosis. Membranes. 2016; 6(1):13. https://doi.org/10.3390/membranes6010013
Chicago/Turabian StyleAnissimov, Yuri G. 2016. "Aspects of Mathematical Modelling of Pressure Retarded Osmosis" Membranes 6, no. 1: 13. https://doi.org/10.3390/membranes6010013




