Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes?
Abstract
:1. General Features of Aquaporins

2. Aquaporins in Biomimetic Membranes
3. Functional Assays for Aquaporins
3.1. Stopped-Flow Water Permeability Assay
| Assay | System | Readout | Throughput | Characteristics | References |
|---|---|---|---|---|---|
| Stopped-flow water permeability assay | Suspended AQP-proteoliposomes, vesicles or cells (e.g., erythrocytes) | Light scattering or fluorescence changes | Low; about 10 samples per hour | Requires specialized instrumentation i.e., stopped-flow spectrometer | [ 52,53] |
| Transepithelial assay | Cell monolayers cultured on porous support | Dilution of indicator dye | Low; 12 wells per plate | Virtually free from artifacts Laborious to perform | [ 54] |
| Fluorescence-based assay | Cell monolayers cultured on solid support | Cytoplasmic fluorescence changes | Medium; 96-well plates | Potential artifacts related to interaction of compound with reporter fluorescence Easy to perform | [ 55–57] |
| Oocyte swelling assay | Oocytes from Xenopus laevis | Oocyte imaging | Low; about 2–5 samples per day | Prone to artifacts Technically challenging | [ 3] |
| Erythrocyte lysis assay | Erythrocytes | Cell lysis | High; 96-well plates | Only applicable for AQP1 | [ 58,59] |
| Yeast freeze-thaw assay | Yeast cells | Cell viability | High; 96-well plates | Generic Easy to perform | [ 60] |
3.2. Transepithelial Assay
3.3. Fluorescence-Based Assays
3.4. Oocyte Swelling Assay
3.5. Erythrocyte Lysis Assay
3.6. Yeast Freeze-Thaw Assay
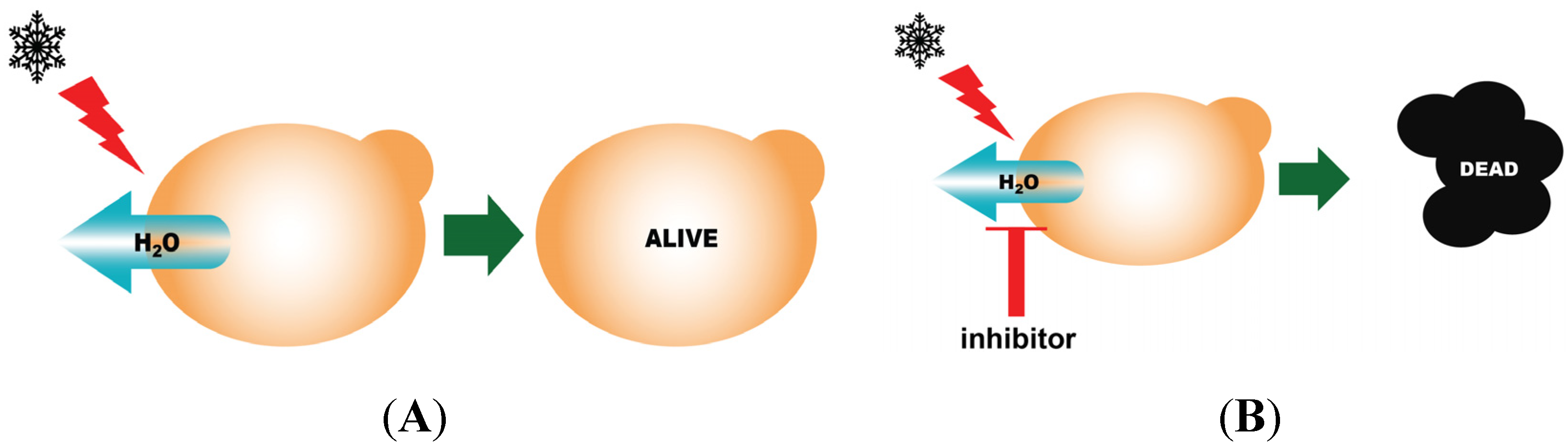
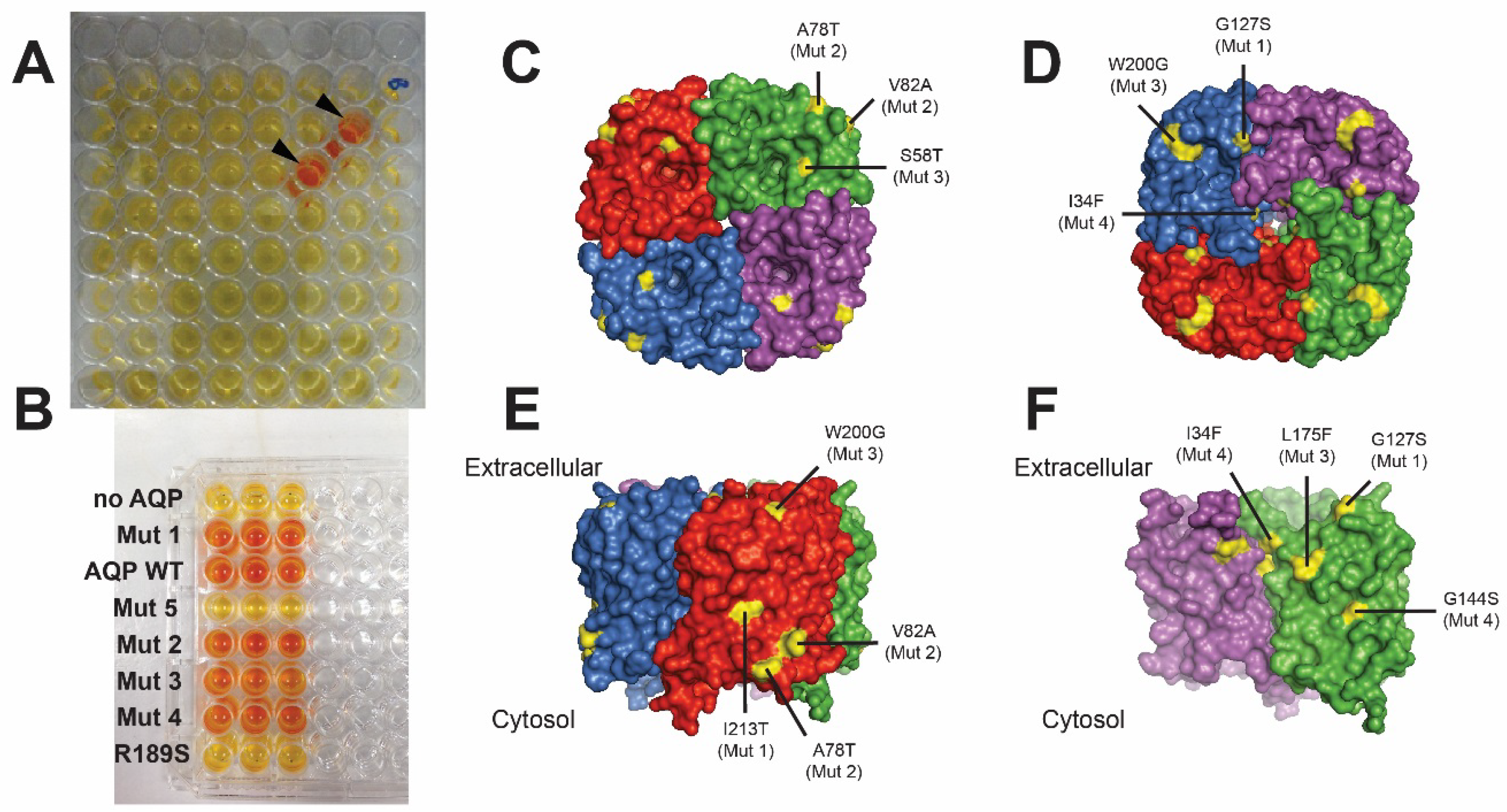
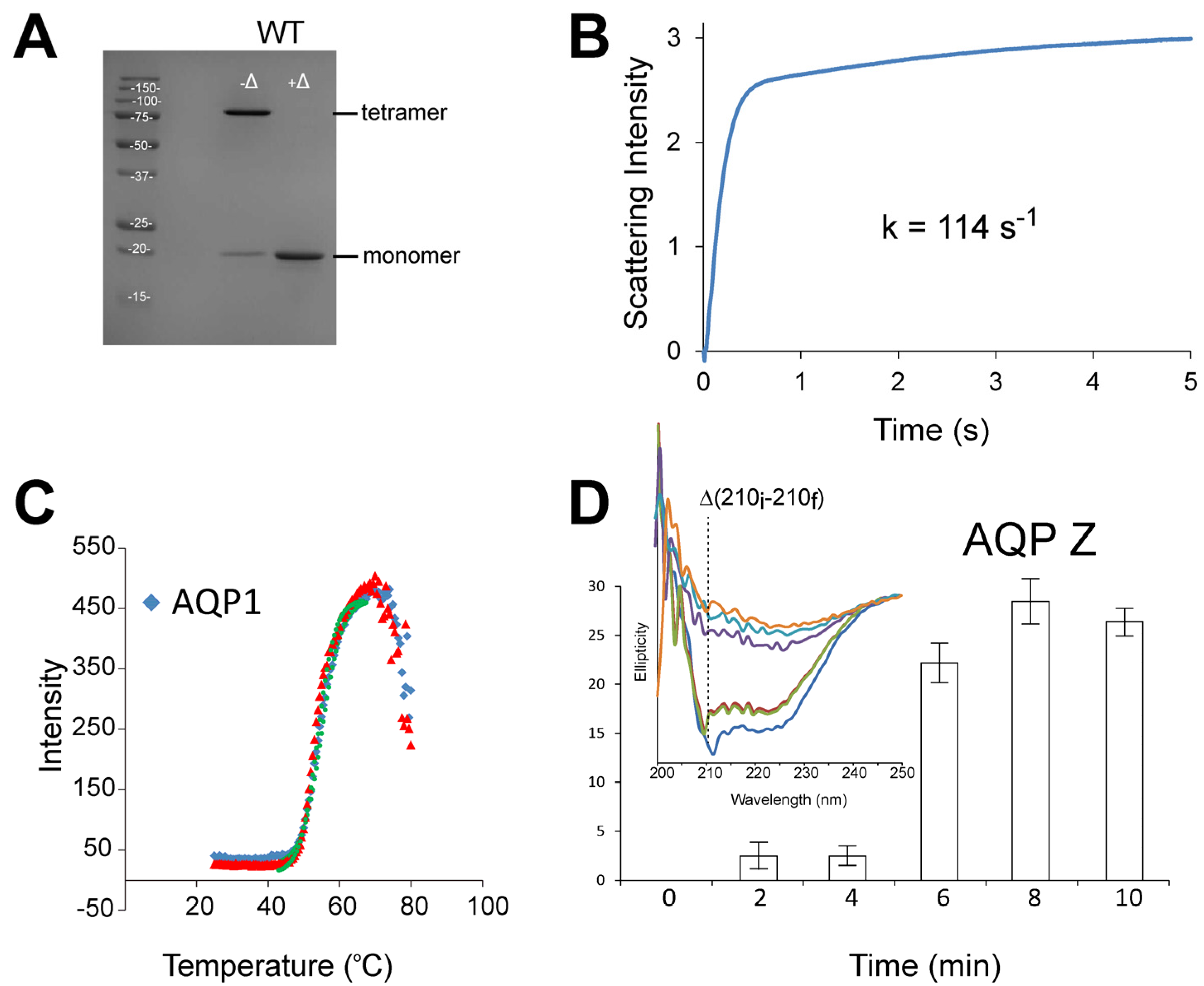
4. Stability of Aquaporins
5. Aquaporin Inhibitors
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benga, G.; Popescu, O.; Borza, V.; Pop, V.I.; Muresan, A.; Mocsy, I.; Brain, A.; Wrigglesworth, J.M. Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 1986, 41, 252–262. [Google Scholar] [PubMed]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel m(r) 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in xenopus oocytes expressing red cell chip28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Sliz, P.; Kistler, J.; Cheng, Y.; Walz, T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 2004, 429, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.-G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the aqp1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Hiroaki, Y.; Tani, K.; Kamegawa, A.; Gyobu, N.; Nishikawa, K.; Suzuki, H.; Walz, T.; Sasaki, S.; Mitsuoka, K.; Kimura, K.; et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006, 355, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Horsefield, R.; Norden, K.; Fellert, M.; Backmark, A.; Tornroth-Horsefield, S.; Terwisscha Van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed]
- Tajkhorshid, E.; Nollert, P.; Jensen, M.Ã.; Miercke, L.J.W.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kozono, D.; Remis, J.; Kitagawa, Y.; Agre, P.; Stroud, R.M. Structural basis for conductance by the archaeal aquaporin aqpm at 1.68 ã. Proc. Natl. Acad. Sci. USA 2005, 102, 18932–18937. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Stroud, R.M. Structural basis of aquaporin inhibition by mercury. J. Mol. Biol. 2007, 368, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.R.; Ying, J.T.Y.; Darwis, D.; Soon, C.H.; Cornvik, T.; Torres, J.; Lescar, J. Crystallization and preliminary crystallographic analysis of human aquaporin 1 at a resolution of 3.28 angstrom. Acta Crystallogr. Sec. F Struct. Biol. Commun. 2014, 70, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom resolution x-ray structure details aquaporin-water interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Egea, P.F.; Robles-Colmenares, Y.; O’Connell Iii, J.D.; Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 å x-ray structure of aquaporin z. PLoS Biol. 2003, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Libson, A.; Miercke, L.J.W.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Harries, W.E.C.; Akhavan, D.; Miercke, L.J.W.; Khademi, S.; Stroud, R.M. The channel architecture of aquaporin 0 at a 2.2-å resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 14045–14050. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Eriksson, U.K.; De Mattia, F.; Öberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.T.; Törnroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.C.; Robbins, R.A.; Miercke, L.J.W.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 a and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Fan, Y.; Munro, R.; Ladizhansky, V.; Brown, L.S. Yeast-expressed human membrane protein aquaporin-1 yields excellent resolution of solid-state mas nmr spectra. J. Biomol. NMR 2013, 55, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of aqpz, the e. Coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Werten, P.J.L.; Hasler, L.; Koenderink, J.B.; Klaassen, C.H.W.; de Grip, W.J.; Engel, A.; Deen, P.M.T. Large-scale purification of functional recombinant human aquaporin-2. FEBS Lett. 2001, 504, 200–205. [Google Scholar] [CrossRef]
- Nyblom, M.; Oberg, F.; Lindkvist-Petersson, K.; Hallgren, K.; Findlay, H.; Wikstrom, J.; Karlsson, A.; Hansson, O.; Booth, P.J.; Bill, R.M.; et al. Exceptional overproduction of a functional human membrane protein. Prot. Exp. Purif. 2007, 56, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bomholt, J.; Hélix-Nielsen, C.; Scharff-Poulsen, P.; Pedersen, P.A. Recombinant production of human aquaporin-1 to an exceptional high membrane density in saccharomyces cerevisiae. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Felgner, J.H.; Kumar, R.; Sridhar, C.N.; Wheeler, C.J.; Tsai, Y.J.; Border, R.; Ramsey, P.; Martin, M.; Felgner, P.L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994, 269, 2550–2561. [Google Scholar] [PubMed]
- Öberg, F.; Sjöhamn, J.; Conner, M.T.; Bill, R.M.; Hedfalk, K. Improving recombinant eukaryotic membrane protein yields in pichia pastoris: The importance of codon optimization and clone selection. Mol. Membr. Biol. 2011, 28, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Junge, F.; Shirokov, V.A.; Durst, F.; Schwarz, D.; Dötsch, V.; Bernhard, F. Membrane protein expression in cell-free systems. Methods Mol. Biol. 2010, 601, 165–186. [Google Scholar] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-free protein expression based on extracts from cho cells. Biotechnol. Bioeng. 2014, 111, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Kaldenhoff, R.; Lian, J.; Zhu, X.; Dötsch, V.; Bernhard, F.; Cen, P.; Xu, Z. Preparative scale production of functional mouse aquaporin 4 using different cell-free expression modes. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Hovijitra, N.T.; Wu, J.J.; Peaker, B.; Swartz, J.R. Cell-free synthesis of functional aquaporin z in synthetic liposomes. Biotechnol. Bioeng. 2009, 104, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein aquaporin z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhou, H.; Li, Y.; Jeyaseelan, K.; Armugam, A.; Chung, T.S. A novel method of aquaporinz incorporation via binary-lipid langmuir monolayers. Colloids Surf. B. Biointerfaces 2012, 89, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin z incorporation. Colloids Surf. B. Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Wang, R.; Wicaksana, F.; Tang, C.Y.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (nf) membranes incorporated with aquaporin z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, F.; Wang, B.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.Q.; Li, X.S.; Vararattanavech, A.; Shen, W.M.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and ro performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Kaufman, Y.; Freger, V. Supported biomimetic membranes for pressure-driven water purification. In On Biomimetics; Pramatarova, L.D., Ed.; In Tech Europe: Rijeka, Croatia, 2011. [Google Scholar]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Helix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.Z.; Ding, S.H.; Cai, J.; Zhang, D.P.; Xu, Z.N.; Wang, X.N. Improving aquaporin z expression in escherichia coli by fusion partners and subsequent condition optimization. Appl. Microbiol. Biotechnol. 2009, 82, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Habel, J.E.O.; Shen, Y.X.; Meier, W.P.; Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 2012, 134, 18631–18637. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. Stabilization and immobilization of aquaporin reconstituted lipid vesicles for water purification. Colloid Surface B 2013, 102, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bowie, J.U. Building a thermostable membrane protein. J. Biol. Chem. 2000, 275, 6975–6979. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; Kummer, L.; Tremmel, D.; Pluckthun, A. Stabilizing membrane proteins through protein engineering. Curr. Opin. Chem. Biol. 2013, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U. Stabilizing membrane proteins. Curr. Opin. Struct. Biol. 2001, 11, 397–402. [Google Scholar] [CrossRef]
- Asial, I.; Cheng, Y.X.; Engman, H.; Dollhopf, M.; Wu, B.; Nordlund, P.; Cornvik, T. Engineering protein thermostability using a generic activity-independent biophysical screen inside the cell. Nat. Commun. 2013, 4, 2901. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Cobo, A.G.; Echevarria, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zeidel, M.L.; Albalak, A.; Grossman, E.; Carruthers, A. Role of glucose carrier in human erythrocyte water permeability. Biochemistry 1992, 31, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Wilson, M.T. Stopped flow spectroscopy. In Spectrophotometry and Spectrofluorimetry; Gore, M., Ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Van Hoek, A.N.; Verkman, A.S. Functional reconstitution of the isolated erythrocyte water channel chip28. J. Biol. Chem. 1992, 267, 18267–18269. [Google Scholar] [PubMed]
- Levin, M.H.; Sullivan, S.; Nielson, D.; Yang, B.; Finkbeiner, W.E.; Verkman, A.S. Hypertonic saline therapy in cystic fibrosis: Evidence against the proposed mechanism involving aquaporins. J. Biol. Chem. 2006, 281, 25803–25812. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.; Kiilgaard, J.F.; Litman, T.; Alvarez-Leefmans, F.J.; Winther, B.R.; Zeuthen, T. Measurement of cell volume changes by fluorescence self-quenching. J. Fluoresc. 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Galietta, L.J.V.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef]
- Mola, M.G.; Nicchia, G.P.; Svelto, M.; Spray, D.C.; Frigeri, A. Automated cell-based assay for screening of aquaporin inhibitors. Anal. Chem. 2009, 81, 8219–8229. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.H.; de La Fuente, R.; Verkman, A.S. Urearetics: A small molecule screen yields nanomolar potency inhibitors of urea transporter ut-b. FASEB J. 2007, 21, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- To, J.; Yeo, C.Y.; Soon, C.H.; Torres, J. A generic high-throughput assay to detect aquaporin functional mutants: Potential application to discovery of aquaporin inhibitors. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, A.; van Dijck, P.; Dumortier, F.; Teunissen, A.; Hohmann, S.; Thevelein, J.M. Aquaporin expression correlates with freeze tolerance in baker's yeast, and overexpression improves freeze tolerance in industrial strains. Appl. Environ. Microbiol. 2002, 68, 5981–5989. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, A.; Van Dijck, P.; Colavizza, D.; Thevelein, J.M. Aquaporin-mediated improvement of freeze tolerance of saccharomyces cerevisiae is restricted to rapid freezing conditions. Appl. Environ. Microbiol. 2004, 70, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Vedadi, M.; Niesen, F.H.; Allali-Hassani, A.; Fedorov, O.Y.; Finerty, P.J.; Wasney, G.A.; Yeung, R.; Arrowsmith, C.; Ball, L.J.; Berglund, H.; et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc. Natl. Acad. Sci. USA 2006, 103, 15835–15840. [Google Scholar] [CrossRef] [PubMed]
- Senisterra, G.A.; Ghanei, H.; Khutoreskaya, G.; Dobrovetsky, E.; Edwards, A.M.; Prive, G.G.; Vedadi, M. Assessing the stability of membrane proteins to detect ligand binding using differential static light scattering. J. Biomol. Screen. 2010, 15, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, I.; Survery, S.; Ibragimova, S.; Hansen, J.S.; Kjellbom, P.; Helix-Nielsen, C.; Johanson, U.; Mouritsen, O.G. Structure and stability of the spinach aquaporin sopip2;1 in detergent micelles and lipid membranes. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Stigliano, C.; Sparaneo, A.; Rossi, A.; Frigeri, A.; Svelto, M. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 2013, 91, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of hgcl2 with candidate aquaporin-1 inhibitors dmso, gold, tea+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; van Hoek, A.N.; Biwersi, J.; Verkman, A.S. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel chip28k. Biochemistry 1993, 32, 2938–2941. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Jin Sup, J.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the chip28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [PubMed]
- Huebert, R.C.; Vasdev, M.M.; Shergill, U.; Das, A.; Huang, B.Q.; Charlton, M.R.; LaRusso, N.F.; Shah, V.H. Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis. Hepatology 2010, 52, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Yukutake, Y.; Tsuji, S.; Hirano, Y.; Adachi, T.; Takahashi, T.; Fujihara, K.; Agre, P.; Yasui, M.; Suematsu, M. Mercury chloride decreases the water permeability of aquaporin-4-reconstituted proteoliposomes. Biol. Cell 2008, 100, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tetraethylammonium: Involvement of the loop e pore region. Mol. Pharmacol. 2000, 57, 1021–1026. [Google Scholar] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tea. FASEB J. 1999, 13, A394. [Google Scholar]
- Detmers, F.J.M.; de Groot, B.L.; Müller, E.M.; Hinton, A.; Konings, I.B.M.; Sze, M.; Flitsch, S.L.; Grubmüller, H.; Deen, P.M.T. Quaternary ammonium compounds as water channel blockers: Specificity, potency, and site of action. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef] [PubMed]
- Yukutake, Y.; Hirano, Y.; Suematsu, M.; Yasui, M. Rapid and reversible inhibition of aquaporin-4 by zinc. Biochemistry 2009, 48, 12059–12061. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ma, B.; Li, T.; Gao, J.W.; Yu, H.M.; Li, X.J. Acetazolamide inhibits aquaporin-1 protein expression and angiogenesis. Acta Pharmacol. Sin. 2004, 25, 812–816. [Google Scholar] [PubMed]
- Ma, B.; Xiang, Y.; Mu, S.M.; Li, T.; Yu, H.M.; Li, X.J. Effects of acetazolamide and anordiol on osmotic water permeability in aqp1-crna injected xenopus oocyte. Acta Pharmacol. Sin. 2004, 25, 90–97. [Google Scholar] [PubMed]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Kwee, I.L.; Nakada, T. Inhibition of aquaporin 4 by antiepileptic drugs. Biorg. Med. Chem. 2009, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Ciancetta, A.; deAlmeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin inhibition by gold(iii) compounds: New insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, tgn-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Hayashi, M.K.; Aizu, S.; Yukutake, Y.; Takeda, J.; Yasui, M. A general anaesthetic propofol inhibits aquaporin-4 in the presence of Zn2+. Biochem. J. 2013, 454, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.G.; Ritchie, A.K. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2+-activated K+ channels in a pituitary cell line. J. Physiol. 1990, 425, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, X.; Chang, Y.; Zhang, J.; Song, Q.; Yu, H.; Li, X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006, 350, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, R.; Zeuthen, T. Test of blockers of aqp1 water permeability by a high-resolution method: No effects of tetraethylammonium ions or acetazolamide. Pflugers Archiv. Eur. J. Physiol. 2008, 456, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; Zapater, C.; Krenc, D.; Haddoub, R.; Flitsch, S.; Beitz, E.; Cerdà, J.; de Groot, B.L. Discovery of novel human aquaporin-1 blockers. ACS Chem. Biol. 2013, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, J.; Torres, J. Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes? Membranes 2015, 5, 352-368. https://doi.org/10.3390/membranes5030352
To J, Torres J. Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes? Membranes. 2015; 5(3):352-368. https://doi.org/10.3390/membranes5030352
Chicago/Turabian StyleTo, Janet, and Jaume Torres. 2015. "Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes?" Membranes 5, no. 3: 352-368. https://doi.org/10.3390/membranes5030352
APA StyleTo, J., & Torres, J. (2015). Can Stabilization and Inhibition of Aquaporins Contribute to Future Development of Biomimetic Membranes? Membranes, 5(3), 352-368. https://doi.org/10.3390/membranes5030352






