The Role of the Clathrin Adaptor AP-1: Polarized Sorting and Beyond
Abstract
:1. Introduction
2. The AP Complex, an Evolutionarily-Conserved Clathrin Adaptor
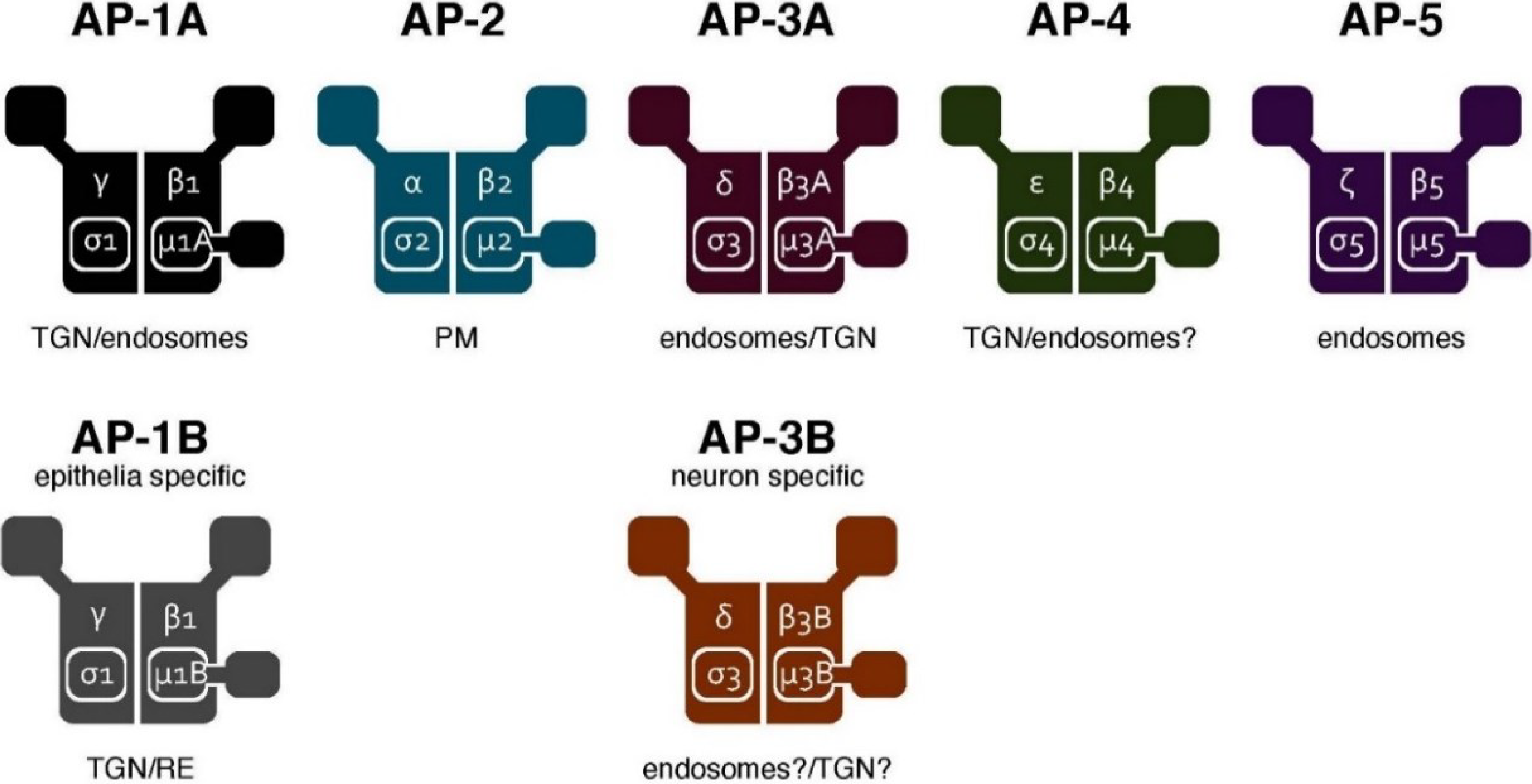
3. The AP Complex Regulates Cargo Sorting and Vesicle Formation

4. Discovery of the AP-1B Complex
5. AP-1B Regulates Polarized Sorting in Epithelial Cells
6. Possible Involvement of AP-1A in Polarized Sorting
7. The Role of AP-1B in Tissues and Organisms
7.1. Nematode (Caenorhabditis elegans)
7.1.1. Distinct Functions for the Two Different μ1 Subunits in C. elegans
7.1.2. The Role of AP-1 in Apical and Basolateral Polarized Sorting
7.2. Fruit Fly (Drosophila melanogaster)
7.2.1. AP-1 Controls Sensory Organ Development by Regulating the Basolateral Localization of Sanpodo
7.2.2. Numb Regulates the AP-1-Mediated Sorting
7.3. Zebrafish (Danio rerio)
7.3.1. The Role of D. rerio AP-1B in the Development of Kidney and Liver Tissues Containing Epithelial Cells
7.3.2. The Role of AP-1 in the Hair Sensory Epithelial Cells
7.4. Mouse
7.4.1. The Essential Role of AP-1A in the Mouse Embryonic Development
7.4.2. AP-1B Regulates Polarity and Integrity of the Intestinal Epithelial Cells in Mice
7.4.3. Ectopic Apical Formation in AP-1-Deficient Animals
7.5. Human
7.5.1. Crohn’s Disease
7.5.2. Colorectal Cancer
8. Concluding Remarks
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Palade, G. Intracellular aspects of the process of protein synthesis. Science 1975, 189, 347–358. [Google Scholar]
- Randy, W. Schekman—Nobel Lecture: Genetic and Biochemical Dissection of the Secretory Pathway. Available online: http://www.nobelprize.org/nobel_prizes/medicine/laureates/2013/schekman-lecture.html (accessed on 7 September 2014).
- Nelson, W.J. Adaptation of core mechanisms to generate cell polarity. Nature 2003, 422, 766–774. [Google Scholar]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar]
- Mellman, I.; Nelson, W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 833–845. [Google Scholar]
- Schuck, S.; Simons, K. Polarized sorting in epithelial cells: Raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 2004, 117, 5955–5964. [Google Scholar]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar]
- Bryant, D.M.; Mostov, K.E. From cells to organs: Building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008, 9, 887–901. [Google Scholar]
- Bonifacino, J.S. Adaptor proteins involved in polarized sorting. J. Cell Biol. 2014, 204, 7–17. [Google Scholar]
- Roth, T.F.; Porter, K.R. Yolk Protein Uptake in the Oocyte of the Mosquito Aedes Aegypti. L. J. Cell Biol. 1964, 20, 313–332. [Google Scholar]
- Friend, D.S.; Farquhar, M.G. Functions of coated vesicles during protein absorption in the rat vas deferens. J. Cell Biol. 1967, 35, 357–376. [Google Scholar]
- Keen, J.H.; Willingham, M.C.; Pastan, I.H. Clathrin-coated vesicles: Isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell 1979, 16, 303–312. [Google Scholar]
- Keen, J.H. Clathrin assembly proteins: Affinity purification and a model for coat assembly. J. Cell Biol. 1987, 105, 1989–1998. [Google Scholar]
- Kanaseki, T.; Kadota, K. The “vesicle in a basket”. A morphological study of the coated vesicle isolated from the nerve endings of the guinea pig brain, with special reference to the mechanism of membrane movements. J. Cell Biol. 1969, 42, 202–220. [Google Scholar]
- Keen, J.H. Clathrin and associated assembly and disassembly proteins. Annu. Rev. Biochem. 1990, 59, 415–438. [Google Scholar]
- Pearse, B.M.; Robinson, M.S. Clathrin, adaptors, and sorting. Annu. Rev. Cell Biol. 1990, 6, 151–171. [Google Scholar]
- Pearse, B.M.; Bretscher, M.S. Membrane recycling by coated vesicles. Annu. Rev. Biochem. 1981, 50, 85–101. [Google Scholar]
- Boehm, M.; Bonifacino, J.S. Genetic analyses of adaptin function from yeast to mammals. Gene 2002, 286, 175–186. [Google Scholar]
- Nakatsu, F.; Ohno, H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct. Funct. 2003, 28, 419–429. [Google Scholar]
- Hirst, J.; Irving, C.; Borner, G.H.H. Adaptor protein complexes AP-4 and AP-5: New players in endosomal trafficking and progressive spastic paraplegia. Traffic 2013, 14, 153–164. [Google Scholar]
- Robinson, M.S.; Bonifacino, J.S. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001, 13, 444–453. [Google Scholar]
- Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004, 14, 167–174. [Google Scholar]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar]
- Rothman, J.E. Mechanisms of intracellular protein transport. Nature 1994, 372, 55–63. [Google Scholar]
- Robinson, M.S. Coats and vesicle budding. Trends Cell Biol. 1997, 7, 99–102. [Google Scholar]
- Bonifacino, J.S.; Dell’Angelica, E.C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 1999, 145, 923–926. [Google Scholar]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar]
- Matter, K.; Mellman, I. Mechanisms of cell polarity: Sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 2004, 6, 545–554. [Google Scholar]
- Ohno, H.; Tomemori, T.; Nakatsu, F.; Okazaki, Y.; Aguilar, R.C.; Foelsch, H.; Mellman, I.; Saito, T.; Shirasawa, T.; Bonifacino, J.S. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999, 449, 215–220. [Google Scholar]
- Schreiner, R.; Frindt, G.; Diaz, F.; Carvajal-Gonzalez, J.M.; Perez Bay, A.E.; Palmer, L.G.; Marshansky, V.; Brown, D.; Philp, N.J.; Rodriguez-Boulan, E. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 2010, 78, 382–388. [Google Scholar]
- Diaz, F.; Gravotta, D.; Deora, A.; Schreiner, R.; Schoggins, J.; Falck-Pedersen, E.; Rodriguez-Boulan, E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 11143–11148. [Google Scholar]
- Fölsch, H.; Ohno, H.; Bonifacino, J.S.; Mellman, I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 1999, 99, 189–198. [Google Scholar]
- Gravotta, D.; Deora, A.; Perret, E.; Oyanadel, C.; Soza, A.; Schreiner, R.; Gonzalez, A.; Rodriguez-Boulan, E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc. Natl. Acad. Sci. USA 2007, 104, 1564–1569. [Google Scholar]
- Gan, Y.; McGraw, T.E.; Rodriguez-Boulan, E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 2002, 4, 605–609. [Google Scholar]
- Fölsch, H.; Pypaert, M.; Maday, S.; Pelletier, L.; Mellman, I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003, 163, 351–362. [Google Scholar]
- Ang, A.L.; Taguchi, T.; Francis, S.; Fölsch, H.; Murrells, L.J.; Pypaert, M.; Warren, G.; Mellman, I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004, 167, 531–543. [Google Scholar]
- Cancino, J.; Torrealba, C.; Soza, A.; Yuseff, M.I.; Gravotta, D.; Henklein, P.; Rodriguez-Boulan, E.; Gonzalez, A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol. Biol. Cell 2007, 18, 4872–4884. [Google Scholar]
- Gravotta, D.; Carvajal-Gonzalez, J.M.; Mattera, R.; Deborde, S.; Banfelder, J.R.; Bonifacino, J.S.; Rodriguez-Boulan, E. The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 2012, 22, 811–823. [Google Scholar]
- Rodriguez-Boulan, E.; Perez-Bay, A.; Schreiner, R.; Gravotta, D. Response: The “tail” of the twin adaptors. Dev. Cell 2013, 27, 247–248. [Google Scholar]
- Guo, X.; Mattera, R.; Ren, X.; Chen, Y.; Retamal, C.; Gonzalez, A.; Bonifacino, J.S. The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev. Cell 2013, 27, 353–366. [Google Scholar]
- Gonzalez, A.; Rodriguez-Boulan, E. Clathrin and AP1B: Key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009, 583, 3784–3795. [Google Scholar]
- Fölsch, H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005, 15, 222–228. [Google Scholar]
- Zizioli, D.; Meyer, C.; Guhde, G.; Saftig, P.; von Figura, K.; Schu, P. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 1999, 274, 5385–5390. [Google Scholar]
- Meyer, C.; Zizioli, D.; Lausmann, S.; Eskelinen, E.L.; Hamann, J.; Saftig, P.; von Figura, K.; Schu, P. mu1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000, 19, 2193–2203. [Google Scholar]
- Nakatsu, F.; Okada, M.; Mori, F.; Kumazawa, N.; Iwasa, H.; Zhu, G.; Kasagi, Y.; Kamiya, H.; Harada, A.; Nishimura, K.; et al. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J. Cell Biol. 2004, 167, 293–302. [Google Scholar] [Green Version]
- Dell’Angelica, E.C.; Shotelersuk, V.; Aguilar, R.C.; Gahl, W.A.; Bonifacino, J.S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell 1999, 3, 11–21. [Google Scholar]
- Kantheti, P.; Qiao, X.; Diaz, M.E.; Peden, A.A.; Meyer, G.E.; Carskadon, S.L.; Kapfhamer, D.; Sufalko, D.; Robinson, M.S.; Noebels, J.L.; et al. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 1998, 21, 111–122. [Google Scholar]
- Matsuda, S.; Miura, E.; Matsuda, K.; Kakegawa, W.; Kohda, K.; Watanabe, M.; Yuzaki, M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 2008, 57, 730–745. [Google Scholar]
- Shim, J.; Sternberg, P.W.; Lee, J. Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol. Biol. Cell 2000, 11, 2743–2756. [Google Scholar]
- Zhang, H.; Kim, A.; Abraham, N.; Khan, L.A.; Hall, D.H.; Fleming, J.T.; Gobel, V. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 2012, 139, 2071–2083. [Google Scholar]
- Shafaq-Zadah, M.; Brocard, L.; Solari, F.; Michaux, G. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 2012, 139, 2061–2070. [Google Scholar]
- Sternberg, P.W.; LESA, G.; LEE, J.H.; Katz, W.S.; Yoon, C.; Clandinin, T.R.; Huang, L.S.; Chamberlin, H.M.; Jongeward, G. Let-23-Mediated Signal-Transduction During Caenorhabditis-Elegans Development. Mol. Reprod. Dev. 1995, 42, 523–528. [Google Scholar]
- Lee, J.; Jongeward, G.D.; Sternberg, P.W. unc-101, a gene required for many aspects of Caenorhabditiselegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994, 8, 60–73. [Google Scholar]
- Weisz, O.A.; Rodriguez-Boulan, E. Apical trafficking in epithelial cells: Signals, clusters and motors. J. Cell. Sci. 2009, 122, 4253–4266. [Google Scholar]
- Bellaïche, Y.; Schweisguth, F. Lineage diversity in the Drosophila nervous system. Curr. Opin. Genet. Dev. 2001, 11, 418–423. [Google Scholar]
- Jan, Y.N.; Jan, L.Y. Asymmetric cell division in the Drosophila nervous system. Nat. Rev. Neurosci. 2001, 2, 772–779. [Google Scholar]
- Gönczy, P. Mechanisms of asymmetric cell division: Flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 2008, 9, 355–366. [Google Scholar]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar]
- O’Connor-Giles, K.M.; Skeath, J.B. Numb inhibits membrane localization of Sanpodo, a four-passtransmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 2003, 5, 231–243. [Google Scholar]
- Benhra, N.; Lallet, S.; Cotton, M.; le Bras, S.; Dussert, A.; le Borgne, R. AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 2011, 21, 87–95. [Google Scholar]
- Uemura, T.; Shepherd, S.; Ackerman, L.; Jan, L.Y.; Jan, Y.N. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 1989, 58, 349–360. [Google Scholar]
- Guo, M.; Jan, L.Y.; Jan, Y.N. Control of daughter cell fates during asymmetric division: Interaction of Numb and Notch. Neuron 1996, 17, 27–41. [Google Scholar]
- Spana, E.P.; Doe, C.Q. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 1996, 17, 21–26. [Google Scholar]
- Frise, E.; Knoblich, J.A.; Younger-Shepherd, S.; Jan, L.Y.; Jan, Y.N. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc. Natl. Acad. Sci. USA 1996, 93, 11925–11932. [Google Scholar]
- Cotton, M.; Benhra, N.; le Borgne, R. Numb inhibits the recycling of Sanpodo in Drosophila sensory organ precursor. Curr. Biol. 2013, 23, 581–587. [Google Scholar]
- Cayouette, M.; Raff, M. Asymmetric segregation of Numb: A mechanism for neural specification from Drosophila to mammals. Nat. Neurosci. 2002, 5, 1265–1269. [Google Scholar]
- Gariano, G.; Guarienti, M.; Bresciani, R.; Borsani, G.; Carola, G.; Monti, E.; Giuliani, R.; Rezzani, R.; Bonomini, F.; Preti, A.; et al. Analysis of three μ1-AP1 subunits during zebrafish development. Dev. Dyn. 2014, 243, 299–314. [Google Scholar]
- Zizioli, D.; Forlanelli, E.; Guarienti, M.; Nicoli, S.; Fanzani, A.; Bresciani, R.; Borsani, G.; Preti, A.; Cotelli, F.; Schu, P. Characterization of the AP-1 μ1A and μ1B adaptins in zebrafish (Danio rerio). Dev. Dyn. 2010, 239, 2404–2412. [Google Scholar]
- Hase, K.; Nakatsu, F.; Ohmae, M.; Sugihara, K.; Shioda, N.; Takahashi, D.; Obata, Y.; Furusawa, Y.; Fujimura, Y.; Yamashita, T.; et al. AP-1BLMediated Protein Sorting Regulates Polarity and Proliferation of Intestinal Epithelial Cells in Mice. Gastroenterology 2013, 145, 625–635. [Google Scholar]
- Granato, M.; van Eeden, F.J.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.P.; Jiang, Y.J.; et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 1996, 123, 399–413. [Google Scholar]
- Clemens Grisham, R.; Kindt, K.; Finger-Baier, K.; Schmid, B.; Nicolson, T. Mutations in ap1b1 cause mistargeting of the Na(+)/K(+)-ATPase pump in sensory hair cells. PLoS One 2013, 8, e60866. [Google Scholar]
- Blitzer, B.L.; Boyer, J.L. Cytochemical localization of Na+, K+-ATPase in the rat hepatocyte. J. Clin. Investig. 1978, 62, 1104–1108. [Google Scholar]
- Rostgaard, J.; Møller, O. Localization of Na+, K+ -ATPase to the inside of the basolateral cell membranes of epithelial cells of proximal and distal tubules in rabbit kidney. Cell Tissue Res. 1980, 212, 17–28. [Google Scholar]
- Gundersen, D.; Orlowski, J.; Rodriguez-Boulan, E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J. Cell Biol. 1991, 112, 863–872. [Google Scholar]
- Farr, G.A.; Hull, M.; Mellman, I.; Caplan, M.J. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J. Cell Biol. 2009, 186, 269–282. [Google Scholar]
- Glyvuk, N.; Tsytsyura, Y.; Geumann, C.; D’Hooge, R.; Hüve, J.; Kratzke, M.; Baltes, J.; Boening, D.; Böning, D.; Klingauf, J.; et al. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010, 29, 1318–1330. [Google Scholar]
- Baltes, J.; Larsen, J.V.; Radhakrishnan, K.; Geumann, C.; Kratzke, M.; Petersen, C.M.; Schu, P. σ1B adaptin regulates adipogenesis by mediating the sorting of sortilin in adipose tissue. J. Cell. Sci. 2014, 127, 3477–3487. [Google Scholar]
- Eskelinen, E.-L.; Meyer, C.; Ohno, H.; von Figura, K.; Schu, P. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep. 2002, 3, 471–477. [Google Scholar]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar]
- Simmen, T.; Höning, S.; Icking, A.; Tikkanen, R.; Hunziker, W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 2002, 4, 154–159. [Google Scholar]
- Sato, T.; Mushiake, S.; Kato, Y.; Sato, K.; Sato, M.; Takeda, N.; Ozono, K.; Miki, K.; Kubo, Y.; Tsuji, A.; et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 2007, 448, 366–369. [Google Scholar]
- Totong, R.; Achilleos, A.; Nance, J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 2007, 134, 1259–1268. [Google Scholar]
- Zhang, H.; Abraham, N.; Khan, L.A.; Hall, D.H.; Fleming, J.T.; Gobel, V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 2011, 13, 1189–1201. [Google Scholar]
- Takahashi, D.; Hase, K.; Kimura, S.; Nakatsu, F.; Ohmae, M.; Mandai, Y.; Sato, T.; Date, Y.; Ebisawa, M.; Kato, T.; et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 2011, 141, 621–632. [Google Scholar]
- McCaffrey, L.M.; Macara, I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011, 21, 727–735. [Google Scholar]
- Mimura, M.; Masuda, A.; Nishiumi, S.; Kawakami, K.; Fujishima, Y.; Yoshie, T.; Mizuno, S.; Miki, I.; Ohno, H.; Hase, K.; et al. AP1B plays an important role in intestinal tumorigenesis with the truncating mutation of an APC gene. Int. J. Cancer 2012, 130, 1011–1020. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatsu, F.; Hase, K.; Ohno, H. The Role of the Clathrin Adaptor AP-1: Polarized Sorting and Beyond. Membranes 2014, 4, 747-763. https://doi.org/10.3390/membranes4040747
Nakatsu F, Hase K, Ohno H. The Role of the Clathrin Adaptor AP-1: Polarized Sorting and Beyond. Membranes. 2014; 4(4):747-763. https://doi.org/10.3390/membranes4040747
Chicago/Turabian StyleNakatsu, Fubito, Koji Hase, and Hiroshi Ohno. 2014. "The Role of the Clathrin Adaptor AP-1: Polarized Sorting and Beyond" Membranes 4, no. 4: 747-763. https://doi.org/10.3390/membranes4040747



