Comparison between Thermophilic and Mesophilic Membrane-Aerated Biofilm Reactors—A Modeling Study
Abstract
:1. Introduction
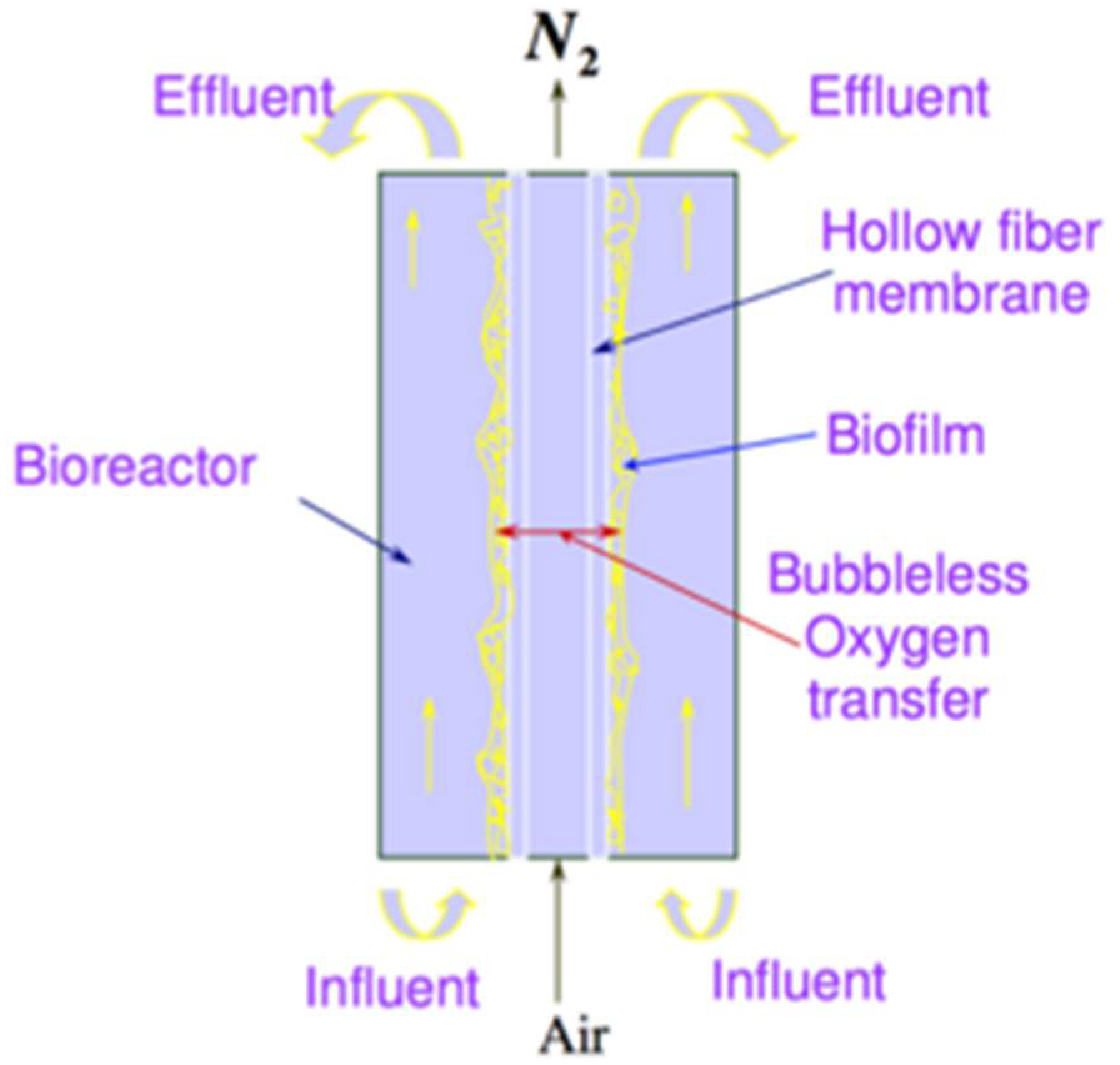
2. Materials and Methods
2.1. Theoretical Analysis of the Impact of Temperature on Biofilm, Water and Mass Transfer Characteristics
2.1.1. Impact of Temperature on Biofilm Properties
2.1.2. Impact of Temperature on Water and Gas Properties
2.1.3. Impact of Temperature on Membrane Properties
2.2. Mathematical Modelling of the Impact of Temperature on the Performance of MABR
2.3. Model Validation
3. Results and Discussion
3.1. Model Validation
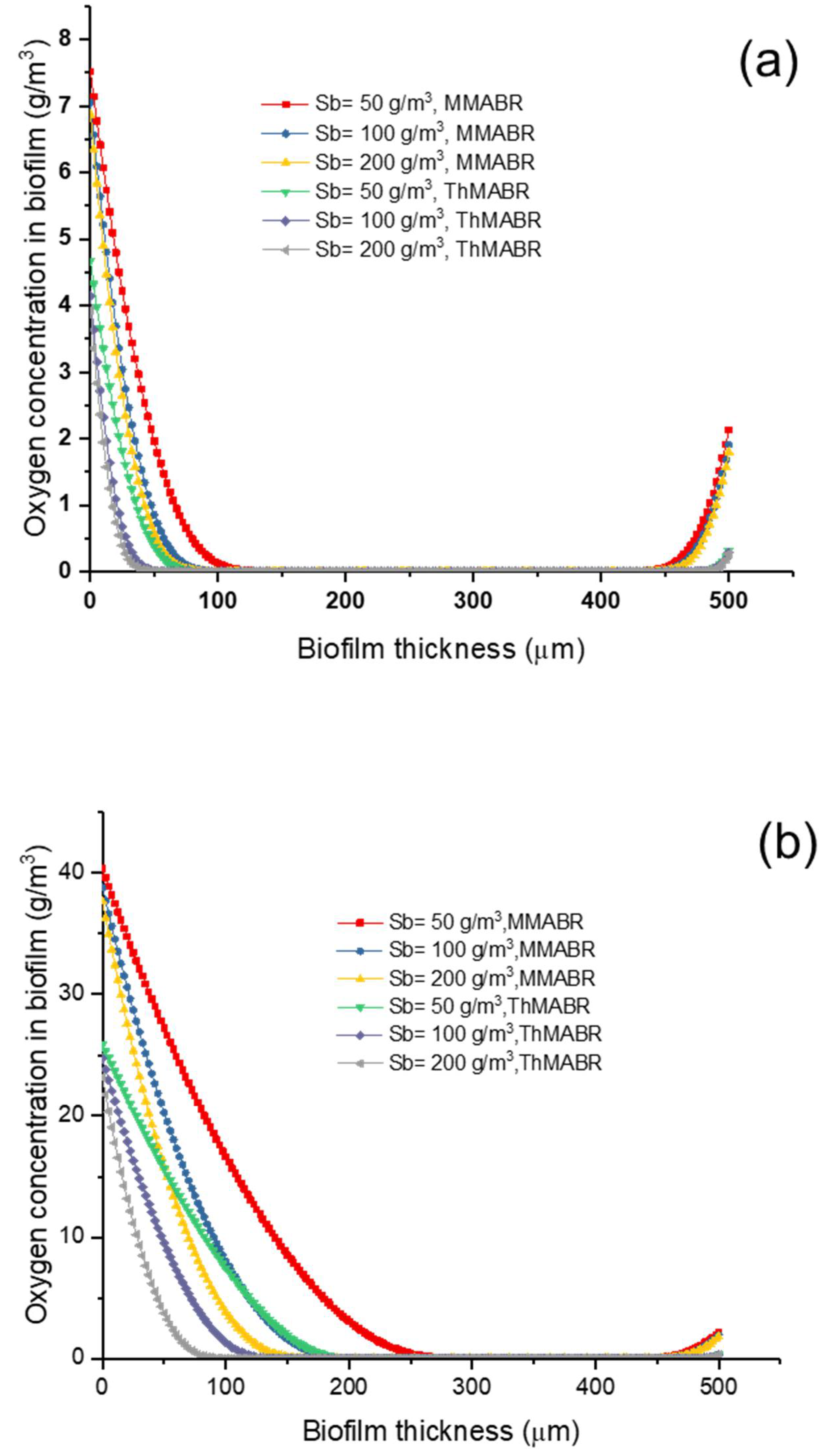
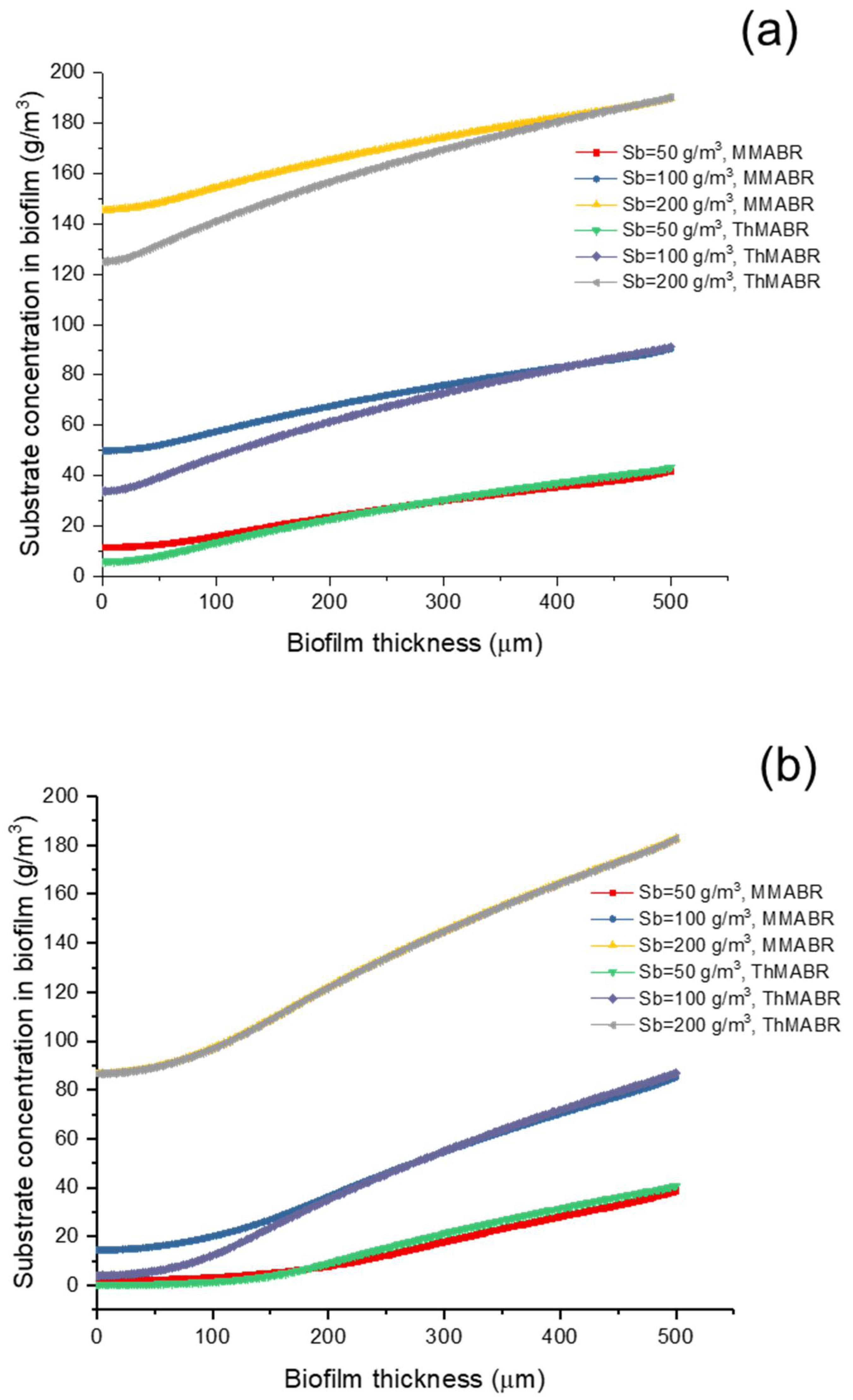
3.2. Impact of Temperature (Thermophilic vs. Mesophilic) on Oxygen and Substrate Concentration Profiles
3.3. Impact of Temperature on Oxygen Penetration Distance into Biofilms
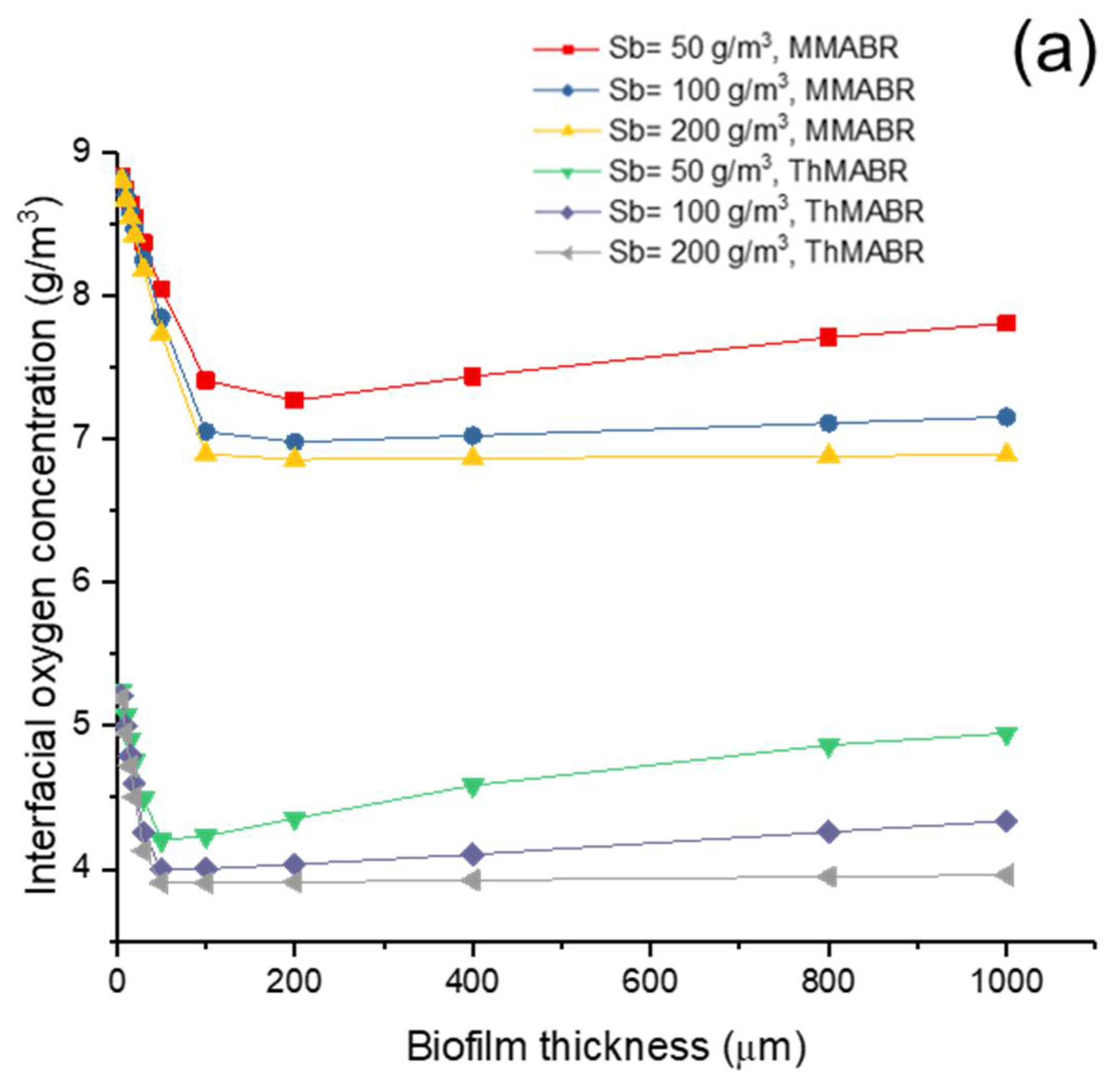
3.4. Impact of Temperature on Membrane–Biofilm Interfacial Oxygen Concentration
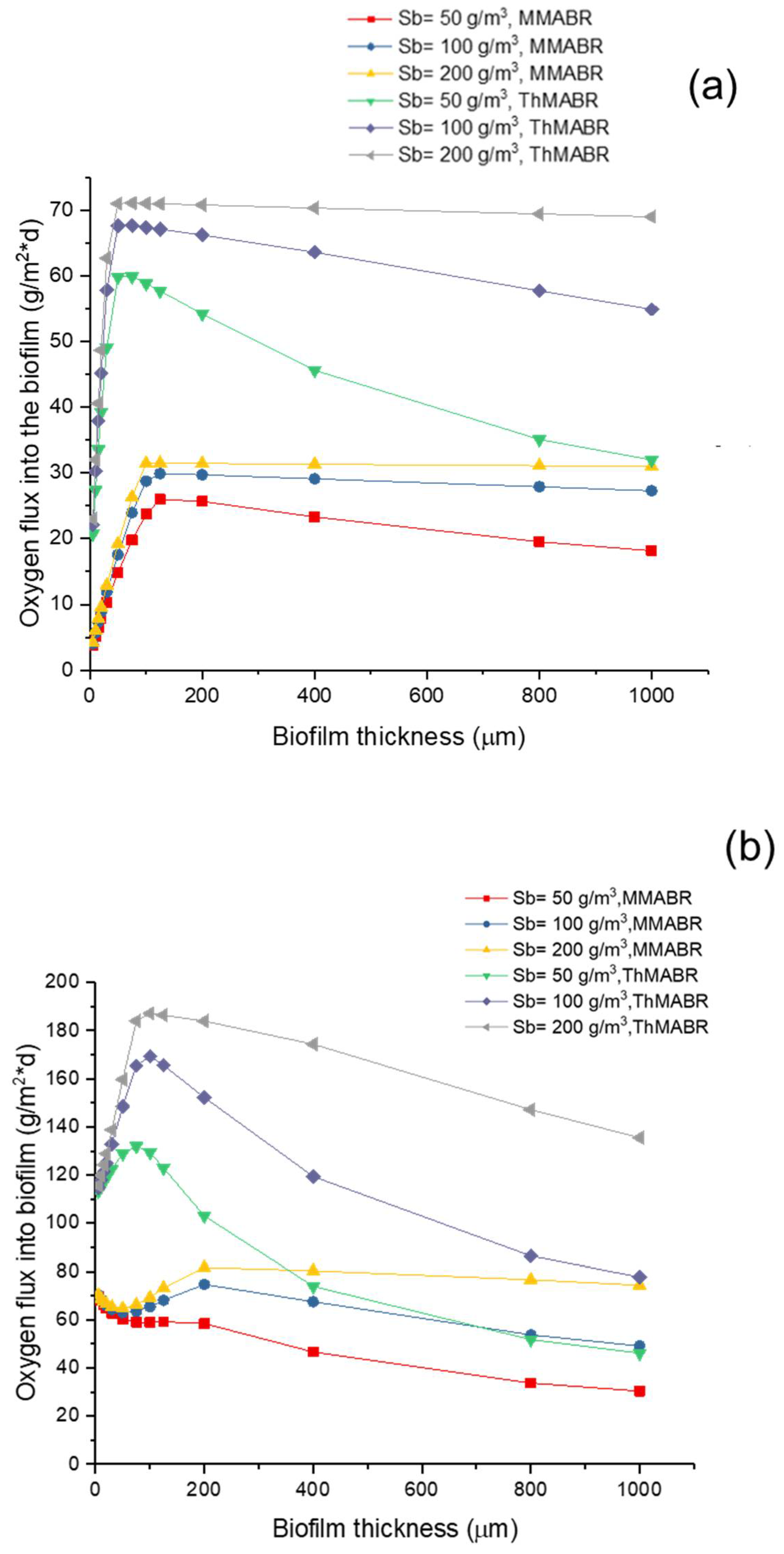
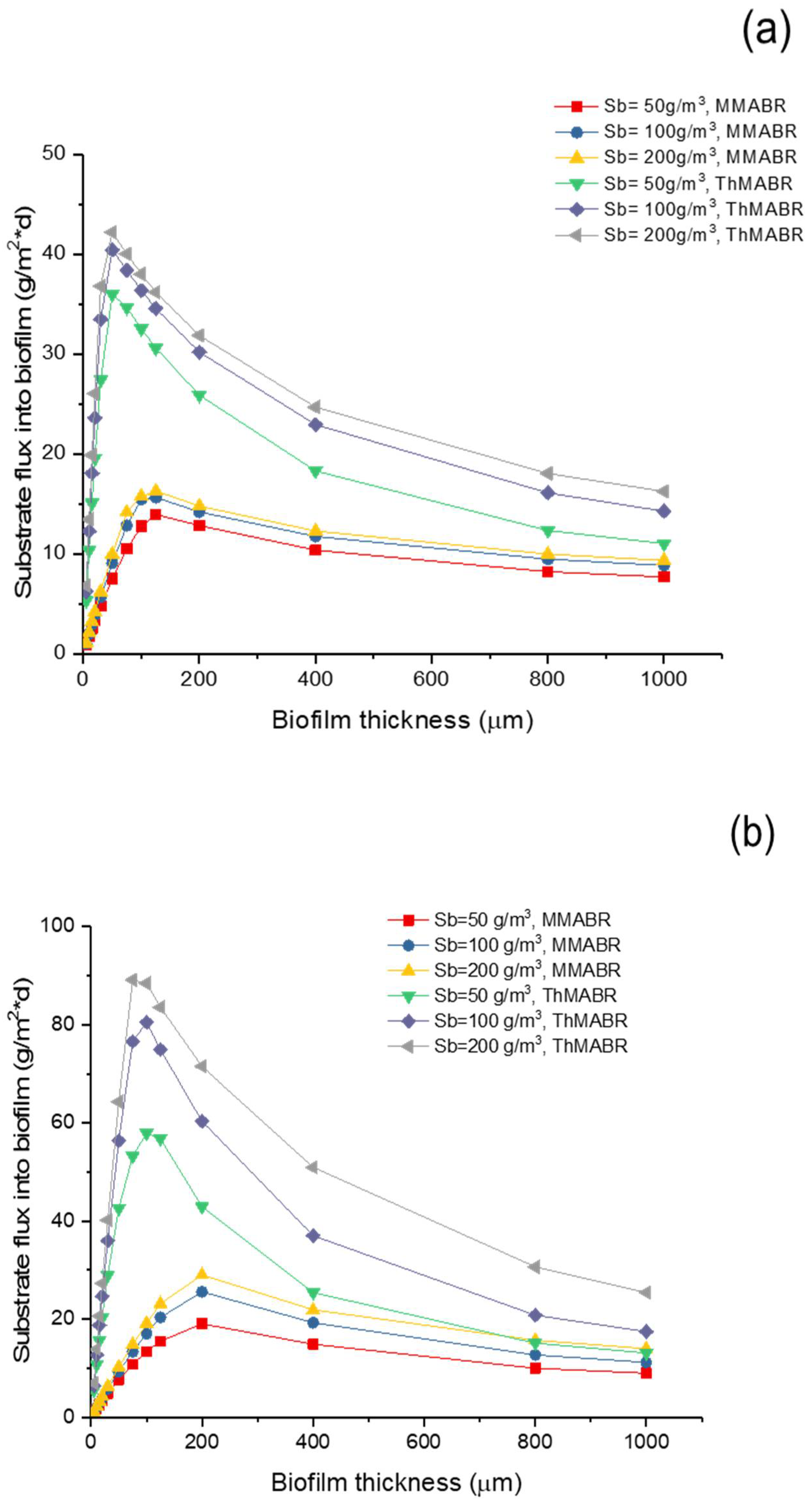
3.5. Impact of Temperature on Oxygen and Substrate Fluxes into Biofilms
3.6. Limitations of the Present Study
4. Conclusions
- (1)
- An increase in temperature from the mesophilic to the thermophilic range results in a significant increase in the oxygen and substrate fluxes into biofilms. The oxygen and substrate flux into biofilms at 60 °C is 2–3 times higher than that at 25 °C, respectively.
- (2)
- Under similar operating conditions, the oxygen penetration distance of ThMABRs is smaller than that of the MMABRs, implying that the control of biofilm thickness in ThMABRs is even more important than in MMABRs.
- (3)
- Under similar operating conditions, the membrane–biofilm interfacial oxygen concentration in ThMABR is lower than that in MMABRs.
- (4)
- An increase in oxygen partial pressure demonstrates that the advantages of the ThMABR are even superior to that of the MMABRs in treating high-strength wastewaters.
- (5)
- The general trend of the higher substrate removal rates observed in the modeling study of the ThMABR was partially verified by the literature experimental results, although they were not perfect. Well-controlled single-fiber MABR experiments should be designed together with biofilm microsensor techniques to verify the modeling results in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMABR | mesophilic membrane-aerated biofilm reactor |
| ThMABR | thermophilic membrane-aerated biofilm reactor |
| TABT | thermophilic aerobic biological treatment |
| PDMS | Polydimethylsiloxane |
| J | flux (g/m2*d) |
| Kla | overall mass transfer coefficient (min−1) |
| T | absolute temperature of liquid under testing (K) |
| E | modulus of elasticity of water at temperature T, (kNm−2) |
| μ | dynamic viscosity of the solvent |
| ρ | density of water at temperature T, (kg m−3) |
| σ | interfacial surface tension of water at temperature T, (N m−1) |
| Po | saturation pressure at the equilibrium position (atm). |
| CO,g | dissolved oxygen concentrations in the membrane (g O2 m−3) |
| CO,0 | dissolved oxygen concentrations in the biofilm bottom (g O2 m−3) |
| Kd | the overall mass transfer coefficient of oxygen (m day−1) |
| Ko | oxygen half-saturation constant (mg/L) |
| Ks | substrate half-saturation constant (mg/L) |
| H | Henry’s constant (atm*m3/mole) |
| viscosity of water (Pa·s) | |
| viscosity of gas (Pa·s) | |
| oxygen solubility in gas phase (g/L) | |
| oxygen solubility in liquid phase (g/L) | |
| oxygen permeability in PDMS membrane (Barrer) | |
| Dw | diffusion coefficient in water (m2/s) |
| DAB | diffusion coefficient in air (m2/s) |
| porosity of biofilms | |
| tortuosity factor | |
| COD | chemical oxygen demand |
| m | maximum growth rate (1/s) |
| Yxo | biomass yield based on oxygen |
| Yxs | biomass yield based on substrate |
| Xbf | biofilm density (g/m3) |
| Pm | Permeability of oxygen gas (gmole*m/(m2*s*Pa) |
| Le | effective thickness of hollow fiber membrane (m) |
| Ls | stagnant layer of liquid (m) |
| Dsw | substrate diffusivity in water (m2/s) |
| Dow | oxygen diffusivity in water (m2/s) |
| rbf-in | outside radius of hollow fiber membrane (m) |
| rbf-out | outside radius of biofilm (m) |
References
- Kunetz, T.E.; Oskouie, A.; Poonsapaya, A.; Peeters, J.; Adams, N.; Long, Z.; Côté, P. Innovative membrane-aerated biofilm reactor pilot test to achieve low-energy nutrient removal at the Chicago MWRD, WEFTEC 2016—89th Water Environ. Proc. Water Environ. Fed. 2016, 2016, 2973–2987. [Google Scholar] [CrossRef]
- LaPara, T.M.; Alleman, J.E. Thermophilic aerobic biological wastewater treatment. Water Res. 1999, 33, 895–908. [Google Scholar] [CrossRef]
- Uri-Carreño, N.; Nielsen, P.H.; Gernaey, K.V.; Flores-Alsina, X. Long-term operation assessment of a full-scale membrane-aerated biofilm reactor under Nordic conditions. Sci. Total Environ. 2021, 779, 146366. [Google Scholar] [CrossRef]
- He, H.; Wagner, B.M.; Carlson, A.L.; Yang, C.; Daigger, G.T. Recent progress using membrane aerated biofilm reactors for wastewater treatment. Water Sci. Technol. 2021, 84, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- LaPara, T.M.; Alleman, J.E. Autothermal thermophilic aerobic waste treatment systems: A state-of-the-art review. In Proceedings of the 52nd Purdue Industrial Waste Conference, Purdue University, West Lafayette, Indiana, 5–7 May 1997; Ann Arbor Press: West Lafayette, IN, USA, 1998. [Google Scholar]
- Shaowei, H.; Fenglin, Y.; Cui, S.; Zhang, J.; Tonghua, W. Simultaneous removal of COD and nitrogen using a novel carbon-membrane aerated biofilm reactor. J. Environ. Sci. 2008, 20, 142–148. [Google Scholar]
- Subtil, E.; Mierzwa, J.; Hespanhol, I. Comparison between a conventional membrane bioreactor (C-MBR) and a biofilm membrane bioreactor (BF-MBR) for domestic wastewater treatment. Braz. J. Chem. Eng. 2014, 31, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Liao, B.; Zheng, M.; Ratana-Rueangsri, L.J.W.S. Treatment of synthetic kraft evaporator condensate using thermophilic and mesophilic membrane aerated biofilm reactors. Water Sci. Technol. 2010, 61, 1749–1756. [Google Scholar] [CrossRef]
- Das, J.; Ravishankar, H.; Lens, P.N. Biological biogas purification: Recent developments, challenges and future prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef]
- Syron, E. Innovative Energy Efficient Aerobic Bioreactors for Sewage Treatment. In Sewage Treatment Plants: Economic Evaluation of Innovative Technologies for Energy Efficiency; Stamatelatou, K., Tsagarakis, K.P., Eds.; IWA Publishing: London, UK, 2015. [Google Scholar]
- Sen, D.; Randall, C.W.; Brink, W.; Farren, G.; Pehrson, D.; Flournoy, W.; Copithorn, R.R. Understanding the importance of aerobic mixing, biofilm thickness control and modeling on the success or failure of IFAS systems for biological nutrient removal. Proc. Water Environ. Fed. 2007, 2, 1098–1126. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Du, C.; Sun, L.; Li, B. Pilot-scale study of an integrated membrane-aerated biofilm reactor system on urban river remediation. Ind. Eng. Chem. Res. 2016, 55, 8373–8382. [Google Scholar] [CrossRef]
- Hou, F.; Li, B.; Xing, M.; Wang, Q.; Hu, L.; Wang, S. Surface modification of PVDF hollow fiber membrane and its application in membrane aerated biofilm reactor (MABR). Bioresour. Technol. 2013, 140, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, D.; Zhang, Y.; Sun, L.; Zhang, H.; Lian, M.; Li, B.J.C.E.J. Oil-field wastewater treatment by hybrid membrane-aerated biofilm reactor (MABR) system. Chem. Eng. J. 2015, 264, 595–602. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, H.; Li, P.; Sun, L.; Hou, F.; Li, B. Treatment of pharmaceutical wastewater for reuse by coupled membrane-aerated biofilm reactor (MABR) system. RSC Adv. 2015, 5, 69829–69838. [Google Scholar] [CrossRef]
- Osborne, M.; Aryasomayajula, A.; Shakeri, A.; Selvaganapathy, P.R.; Didar, T.F. Suppression of biofouling on a permeable membrane for dissolved oxygen sensing using a lubricant-infused coating. ACS Sens. 2019, 4, 687–693. [Google Scholar] [CrossRef]
- Desmond, P.; Huisman, K.T.; Sanawar, H.; Farhat, N.M.; Traber, J.; Fridjonsson, E.O.; Johns, M.L.; Flemming, H.-C.; Picioreanu, C.; Vrouwenvelder, J.S. Controlling the hydraulic resistance of membrane biofilms by engineering biofilm physical structure. Water Res. 2022, 210, 118031. [Google Scholar] [CrossRef]
- Casey, E.; Glennon, B.; Hamer, G. Review of membrane aerated biofilm reactors. Resour. Conserv. Recycl. 1999, 27, 203–215. [Google Scholar] [CrossRef]
- Zheng, M.; Liao, B. Membrane aerated biofilm reactors for thermomechanical pulping pressate treatment. Int. J. Chem. React. Eng. 2016, 14, 1017–1024. [Google Scholar] [CrossRef]
- Liao, B.; Liss, S.N. A comparative study between thermophilic and mesophilic membrane aerated biofilm reactors. J. Environ. Eng. Sci. 2007, 6, 247–252. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, Z.; Guo, Z.; Li, Z.; Hou, X.; Jin, C. The performance and microbial community identification in mesophilic and atmospheric anaerobic membrane bioreactor for municipal wastewater treatment associated with different hydraulic retention times. Water 2019, 11, 160. [Google Scholar] [CrossRef] [Green Version]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Reij, M.W.; Keurentjes, J.T.; Hartmans, S. Membrane bioreactors for waste gas treatment. J. Biotechnol. 1998, 59, 155–167. [Google Scholar] [CrossRef]
- Lu, D.; Bai, H.; Kong, F.; Liss, S.N.; Liao, B. Recent advances in membrane aerated biofilm reactors. Rev. Environ. Sci. Biotechnol. 2021, 51, 649–703. [Google Scholar] [CrossRef]
- Zhang, T.C.; Bishop, P.L. Density, porosity, and pore structure of biofilms. Water Res. 1994, 28, 2267–2277. [Google Scholar] [CrossRef]
- Bonilla, M.R.; Bhatia, S.K. Diffusion in pore networks: Effective self-diffusivity and the concept of tortuosity. J. Phys. Chem. C 2013, 117, 3343–3357. [Google Scholar] [CrossRef]
- López, L.G.; Veiga, M.; Nogueira, R.; Aparicio, A.; Melo, L. A technique using a membrane flow cell to determine average mass transfer coefficients and tortuosity factors in biofilms. Water Sci. Technol. 2003, 47, 61–67. [Google Scholar] [CrossRef]
- Al-Shemmeri, T. Engineering Fluid Mechanics; Ventus Publishing ApS: Copenhagen, Denmark, 2012; pp. 17–18. ISBN 978-87-403-0114-4. [Google Scholar]
- Smits, A.J.; Dussauge, J.-P. Turbulent Shear Layers in Supersonic Flow; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- LaPara, T.M.; Nakatsu, C.H.; Pantea, L.; Alleman, J.E. Phylogenetic analysis of bacterial communities in mesophilic and thermophilic bioreactors treating pharmaceutical wastewater. Appl. Environ. Microbiol. 2000, 66, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Essila, N.J. Contrasting Behavior of Biofilms Grown on Gas-Permeable Membranes with Those Grown on Solid Surfaces: A Model Study; University of Minnesota: Minneapolis, MN, USA; St. Paul, MI, USA, 1997. [Google Scholar]
- Baker, R.; Wilkinson, D.P.; Zhang, J. Electrocatalytic activity and stability of substituted iron phthalocyanines towards oxygen reduction evaluated at different temperatures. Electrochim. Acta 2008, 53, 6906–6919. [Google Scholar] [CrossRef]
- Simon, A.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Effects of caustic cleaning on pore size of nanofiltration membranes and their rejection of trace organic chemicals. J. Membr. Sci. 2013, 447, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Robb, W. Thin silicone membranes-their permeation properties and some applications. Ann. N. Y. Acad. Sci. 1968, 146, 119–137. [Google Scholar] [CrossRef]
- Merkel, T.; Bondar, V.; Nagai, K.; Freeman, B.; Pinnau, I. Gas sorption, diffusion, and permeation in poly (dimethylsiloxane). J. Polym. Sci. B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes; Springer: Dordrecht, The Netherlands, 2015; Volume 10, pp. 973–978. ISBN 3319010956. [Google Scholar]
- Casey, E.; Glennon, B.; Hamer, G. Oxygen mass transfer characteristics in a membrane-aerated biofilm reactor. Biotechnol. Bioeng. 1999, 62, 183–192. [Google Scholar] [CrossRef]
- Essila, N.J.; Semmens, M.J.; Voller, V.R. Modeling Biofilms on Gas-Permeable Supports: Concentration and Activity Profiles. J. Environ. Eng. 2000, 126, 250–257. [Google Scholar] [CrossRef]
- Syron; Kelly, H.; Casey, E. Studies on the effect of concentration of a self-inhibitory substrate on biofilm reaction rate under co-diffusion and counter-diffusion configurations. J. Membr. Sci. 2009, 335, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.P.; Zhang, J.J. Oxygen solubility, diffusion coefficients, and solution viscosty. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G.P., Zhang, J.J., Eds.; Elsiver Publisher: New York, NY, USA, 2014; Chapter 1. [Google Scholar]
- Rittmann, B. Comparative performance of biofilm reactor types. Biotechnol. Bioeng. 1982, 24, 1341–1370. [Google Scholar] [CrossRef] [PubMed]
- Wanner, O.; Debus, O.; Reichert, P. Modelling the spatial distribution and dynamics of a xylene-degrading microbial population in a membrane-bound biofilm. Water Sci. Technol. 1994, 29, 243. [Google Scholar] [CrossRef]
- Chen, G.; Ozaki, H.; Terashima, Y. Modelling of the simultaneous removal of organic substances and nitrogen in a biofilm. Water Sci. Technol. 1989, 21, 791–804. [Google Scholar] [CrossRef]
- Tanase, C.; Chirvase, A.A.; Ungureanu, C.; Caramihai, M.; Muntean, O. Study of double-substrate limited growth of Pseudomonas aeruginosa in aerobic bioprocess. Rev. Roum. Chem. 2011, 56, 1147–1153. [Google Scholar]
- Nicolella, C.; Pavasant, P.; Livingston, A.G. Substrate counterdiffusion and reaction in membrane-attached biofilms: Mathematical analysis of rate limiting mechanisms. Chem. Eng. Sci. 2000, 55, 1385–1398. [Google Scholar] [CrossRef]
- Metcalf, L.; Eddy, H.P.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal, and Reuse, 3rd ed.; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Stewart, P.S.; Zhang, T.; Xu, R.; Pitts, B.; Walters, M.C.; Roe, F.; Kikhney, J.; Moter, A. Reaction–diffusion theory explains hypoxia and heterogeneous growth within microbial biofilms associated with chronic infections. NPJ Biofilms Microbiomes 2016, 2, 16012. [Google Scholar] [CrossRef]
- Li, S.; Chen, G.H. Modeling the organic removal and oxygen consumption by biofilms in an open-channel flow. Water Sci. Technol. 1994, 30, 53. [Google Scholar] [CrossRef]
- Ntwampe, S.K.O.; Sheldon, M.S.; Volschenk, H. Oxygen mass transfer for an immobilised biofilm of Phanerochaete chrysosporium in a membrane gradostat reactor. Braz. J. Chem. Eng. 2008, 25, 649–664. [Google Scholar] [CrossRef]
- Matsumoto, S.; Terada, A.; Tsuneda, S. Modeling of membrane-aerated biofilm: Effects of C/N ratio, biofilm thickness and surface loading of oxygen on feasibility of simultaneous nitrification and denitrification. Biochem. Eng. J. 2007, 37, 98–107. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, F.; Wang, Y.; Lewandowski, Z. Determining the optimal transmembrane gas pressure for nitrification in membrane-aerated biofilm reactors based on oxygen profile analysis. Appl. Microbiol. Biotechnol. 2016, 100, 7699–7711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syron, E.; Casey, E.J.B. Bioengineering, Model-based comparative performance analysis of membrane aerated biofilm reactor configurations. Biotechnol. Bioeng. 2008, 99, 1361–1373. [Google Scholar] [CrossRef] [Green Version]
- Motlagh, A.R.A.; Voller, V.R.; Semmens, M.J. Advective flow through membrane-aerated biofilms: Modeling results. J. Membr. Sci. 2006, 273, 143–151. [Google Scholar]
- Duncan, J.; Bokhary, A.; Fatehi, P.; Kong, F.; Lin, H.; Liao, B. Thermophilic membrane bioreactors: A review. Bioresour. Technol. 2017, 243, 1180–1193. [Google Scholar] [CrossRef]

| Parameters | Symbol | Unit | Typical Value | Typical Value | Typical Value |
|---|---|---|---|---|---|
| Oxygen diffusivity in biofilm | Doeff | m2/s | [41] | (Equation (5)) | (Equation (5)) |
| Substrate diffusivity in biofilm | Dseff | m2/s | [42] | (Equation (5)) | (Equation (5)) |
| Oxygen half-saturation constant | KO | g/m3 | 0.2 [44] | 0.2 [44] | 0.2 [44] |
| Substrate half-saturation constant | KS | g/m3 | 20 [44] | 20 [44] | 20 [44] |
| Maximum growth rate | 1/s | [2] | [2] | [2] | |
| Biomass yield based on oxygen | Yxo | / | 0.2 [45] | 0.2 [45] | 0.2 [45] |
| Biomass yield based on substrate | Yxs | mg/mg substrate | 0.45 [2] | 0.35 [2] | 0.35 [2] |
| Biofilm density | Xbf | g/m3 | 55,000 [31] | 55,000 [31] | 55,000 [31] |
| Permeability | Pm | gmole*m/(m2*s*pa) | [45] | [Equation (9)] | [Equation (9)] |
| Effective thickness of hollow fiber membrane | Le | m | [37] | [37] | [37] |
| Substrate diffusivity in water | Dsw | m2/s | [43] | (Equation (5)) | [Equation (5)] |
| oxygen diffusivity in water | Dow | m2/s | [40] | [40] | [40] |
| Outside radius of hollow fiber membrane | r0 | m | [37] | [37] | [37] |
| Outside radius of biofilm | rb | m | (This study) | (This study) | (This study) |
| Henry’s constant | H | atm*m3/mole | 0.769 [46] | 1.15761 [46] | 1.09767 [46] |
| Biofilm Reactor | Outside Radius of Hollow Fiber (μm) | Inner Radius of Hollow Fiber (μm) | Biofilm Thickness (μm) | Simulate COD Removal Rate (g/d) | Experiment COD Removal Rate (g/d) | Relative Error |
|---|---|---|---|---|---|---|
| 320 | 200 | 1080 [20] | 2.5780 | 1.1625 [20] | 121.7% | |
| 320 | 200 | 1080 [20] | 2.6466 | 1.2375 [20] | 113.8% | |
| 320 | 200 | 280 [20] | 8.5929 | 1.6532 [20] | 419.8% | |
| 320 | 200 | 280 [20] | 9.0763 | 1.6826 [20] | 439.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Bai, H.; Liao, B. Comparison between Thermophilic and Mesophilic Membrane-Aerated Biofilm Reactors—A Modeling Study. Membranes 2022, 12, 418. https://doi.org/10.3390/membranes12040418
Lu D, Bai H, Liao B. Comparison between Thermophilic and Mesophilic Membrane-Aerated Biofilm Reactors—A Modeling Study. Membranes. 2022; 12(4):418. https://doi.org/10.3390/membranes12040418
Chicago/Turabian StyleLu, Duowei, Hao Bai, and Baoqiang Liao. 2022. "Comparison between Thermophilic and Mesophilic Membrane-Aerated Biofilm Reactors—A Modeling Study" Membranes 12, no. 4: 418. https://doi.org/10.3390/membranes12040418






