The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Solution
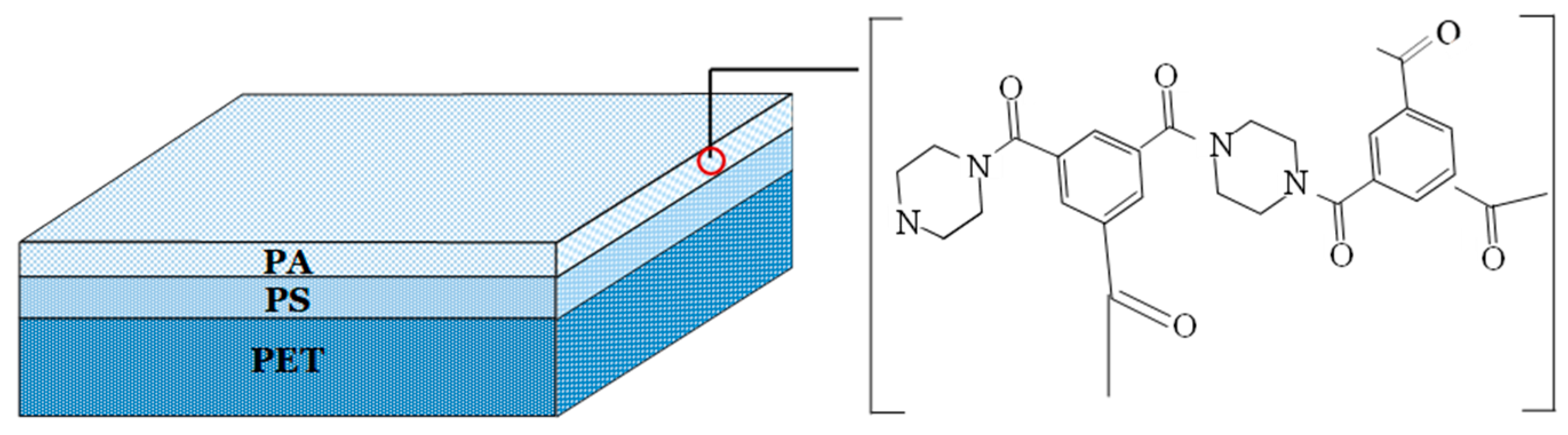
2.2. NF Set-Up
- (i)
- Sepa-CFII plate membrane module manufactured by GE Osmonics (Minnetonka, MN, USA) with the membrane active area equal to 0.015 m2; the channel in the module was filled with polypropylene net-spacer (50 mesh),
- (ii)
- 3CP Stainless Steel Plunger Pump model 3CP1221 (CAT PUMPS, Hampshire, England),
- (iii)
- feed tank (volume 5 L),
- (iv)
- cooling bath (thermostatic Grundfos valve).
2.3. Experimental Protocol
2.4. Analytical Methods
3. Results and Discussion
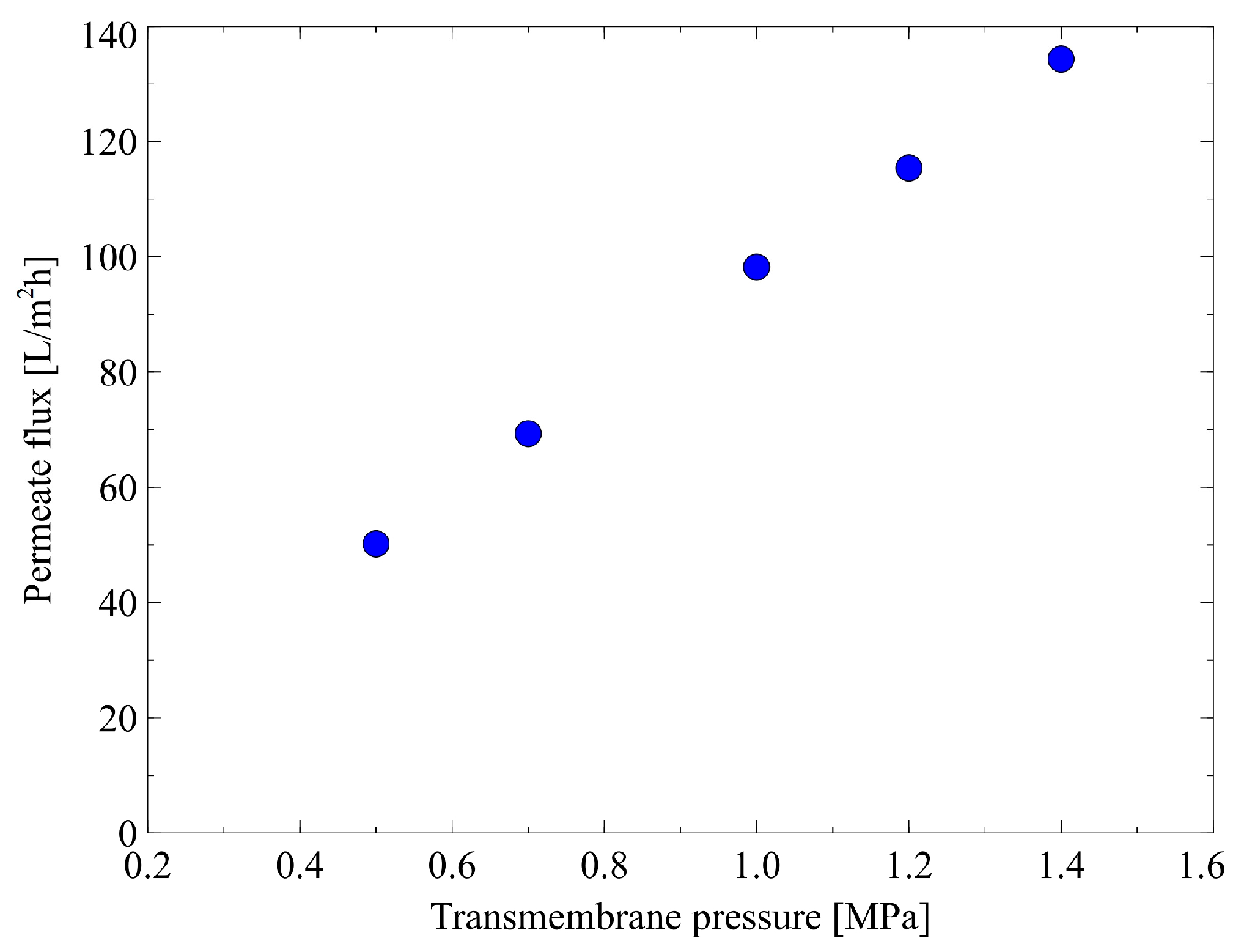
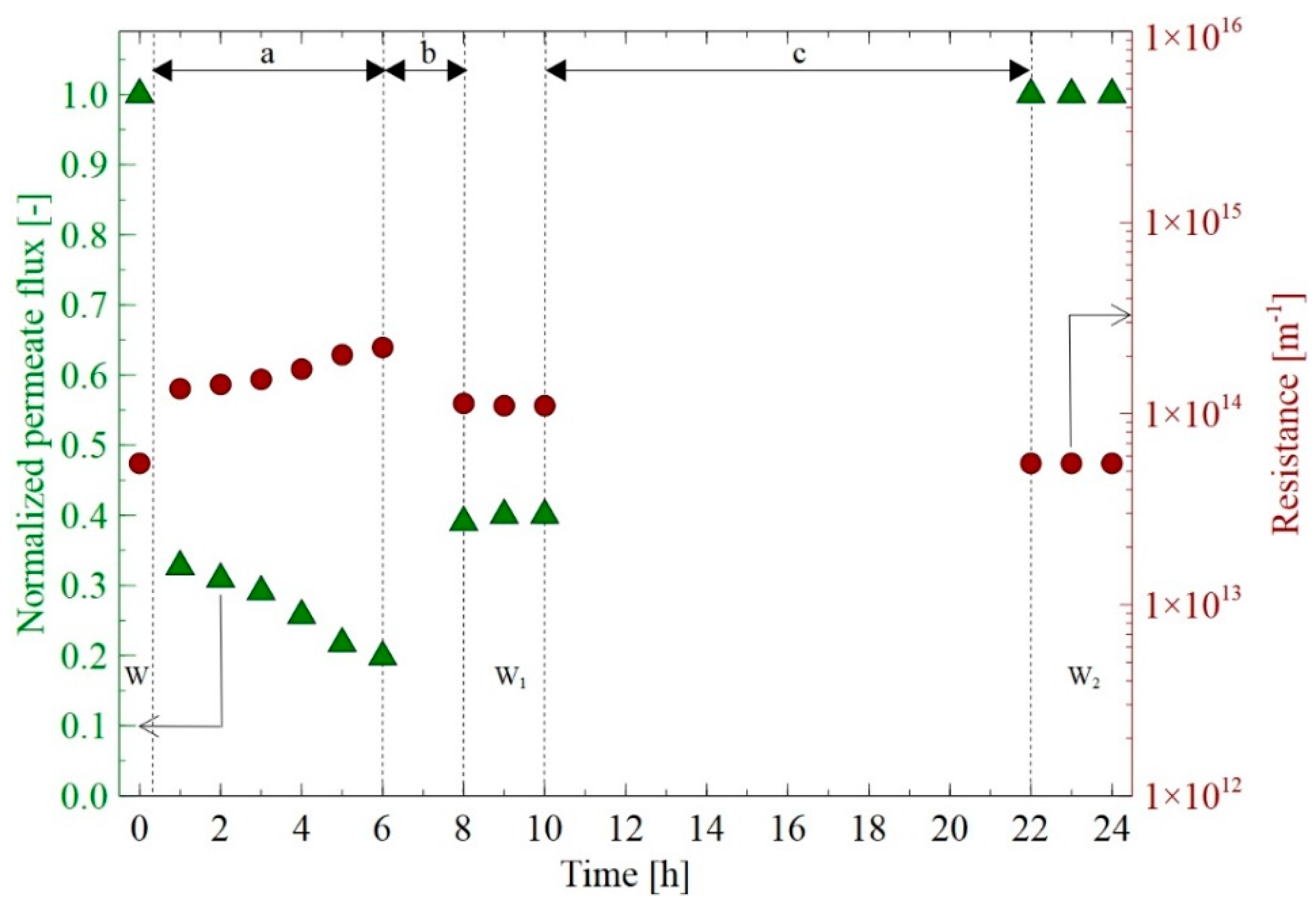
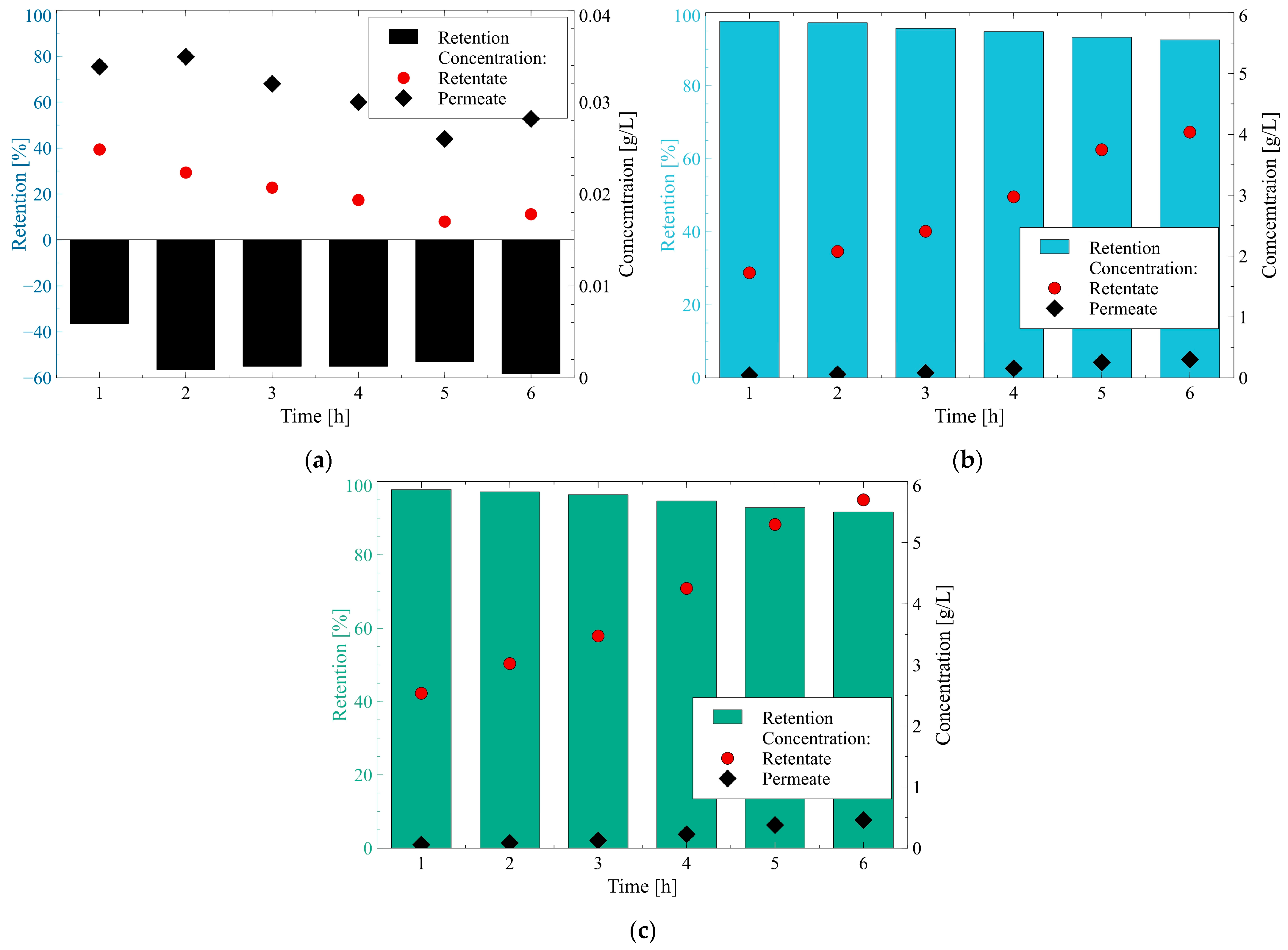
3.1. Membrane Performance
3.2. Separation of Organic Compounds
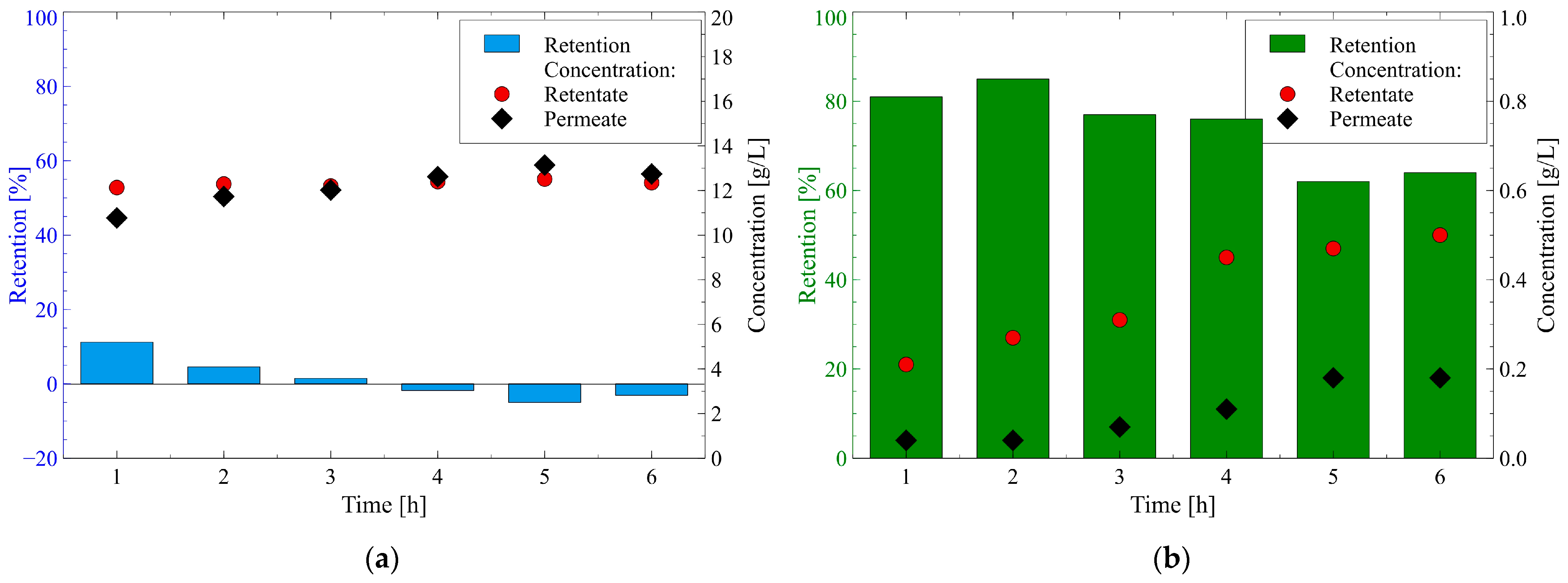
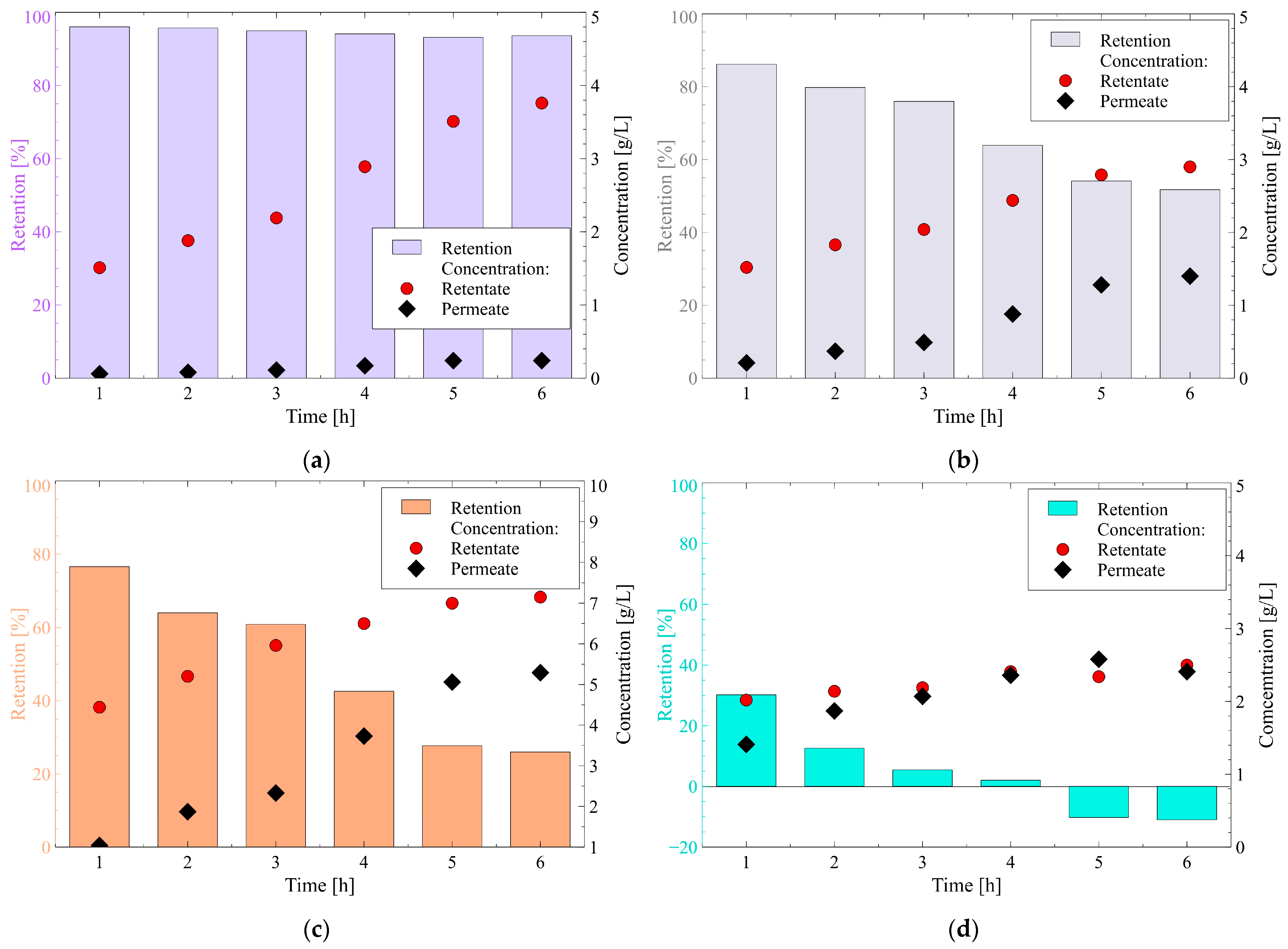
3.2.1. 1,3-Propanediol and Glycerol
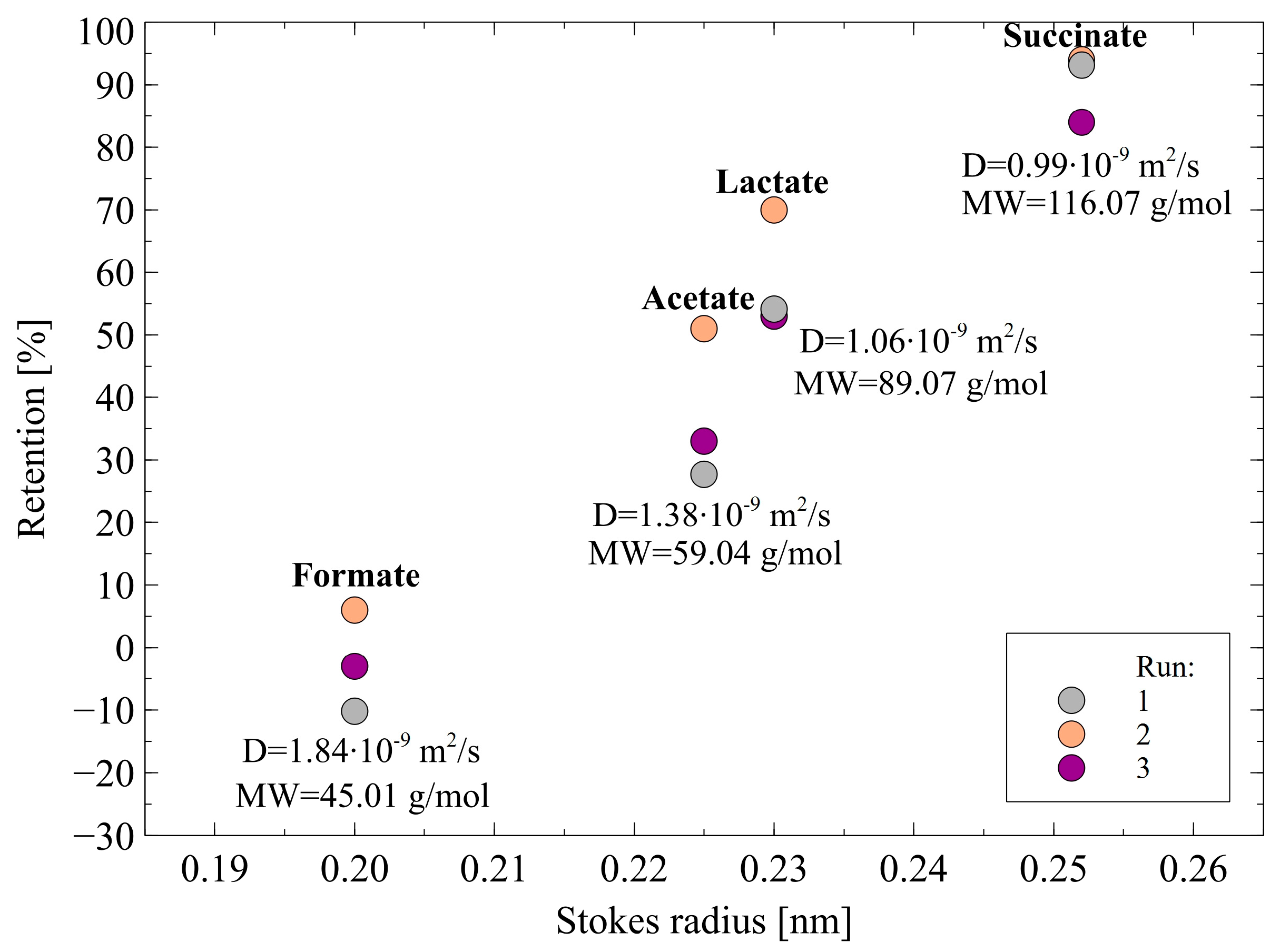
3.2.2. Carboxylic Acids and Ethanol
- (i)
- The highest rejection of succinic acid is related to the fact that it is a diprotic acid, hence, its separation was based on much stronger electrostatic interactions than in the cases of monoprotic acid (lactic, acetic, and formic acids).
- (ii)
- The Stokes radius and MW of charged acids ions: formate, acetate, lactate, and succinate are 0.200 nm and 45.01 g/mol, 0.225 nm and 59.04 g/mol, 0.230 nm and 89.07 g/mol, 0.255 nm and 116.07 g/mol, respectively. It indicates that the highest size ions were retained to a greater extent by the membrane, while the ions with the smallest size were characterized by the lowest retention. This finding indicated that the organic acids retention was based not only on the Donnan exclusion but also on the sieve mechanism.
- (iii)
- For anions with a larger radius, the charge center is farther from the surface and consequently, the electrostatic interactions between anions and negatively charged membrane surface are weaker [38].
- (iv)
- The diffusion coefficients of charged acids ions: formate, acetate, lactate, and succinate are 0.99·10−9, 1.06 10−9, 1.38·10−9, and 1.84 10−9 m/s2, respectively. Therefore, it can be indicated that the above-presented order of diffusion coefficients is inversely reflected in the rejection sequence. Indeed, ions characterized by the highest diffusion coefficient more easily flow through the membrane.
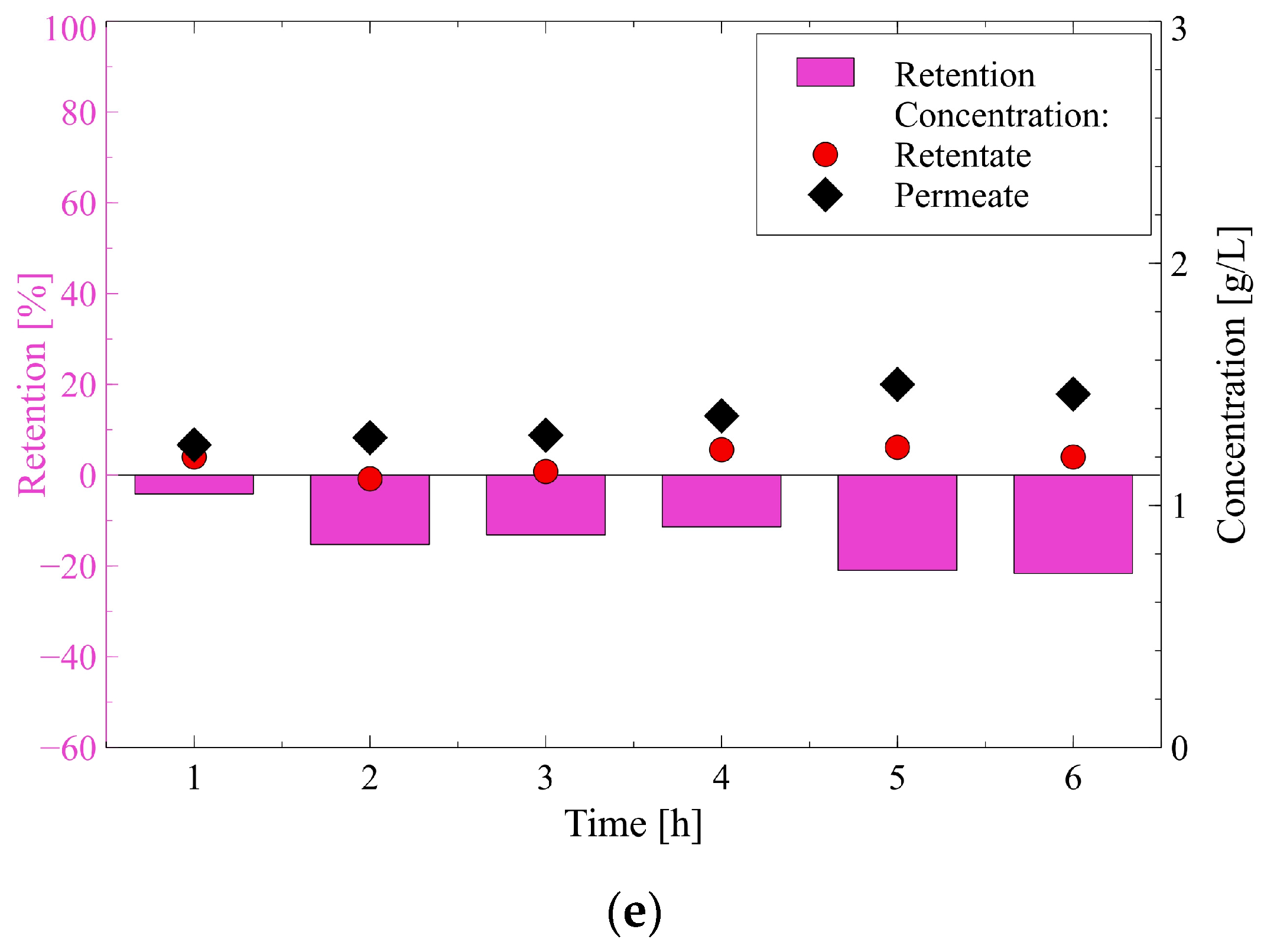
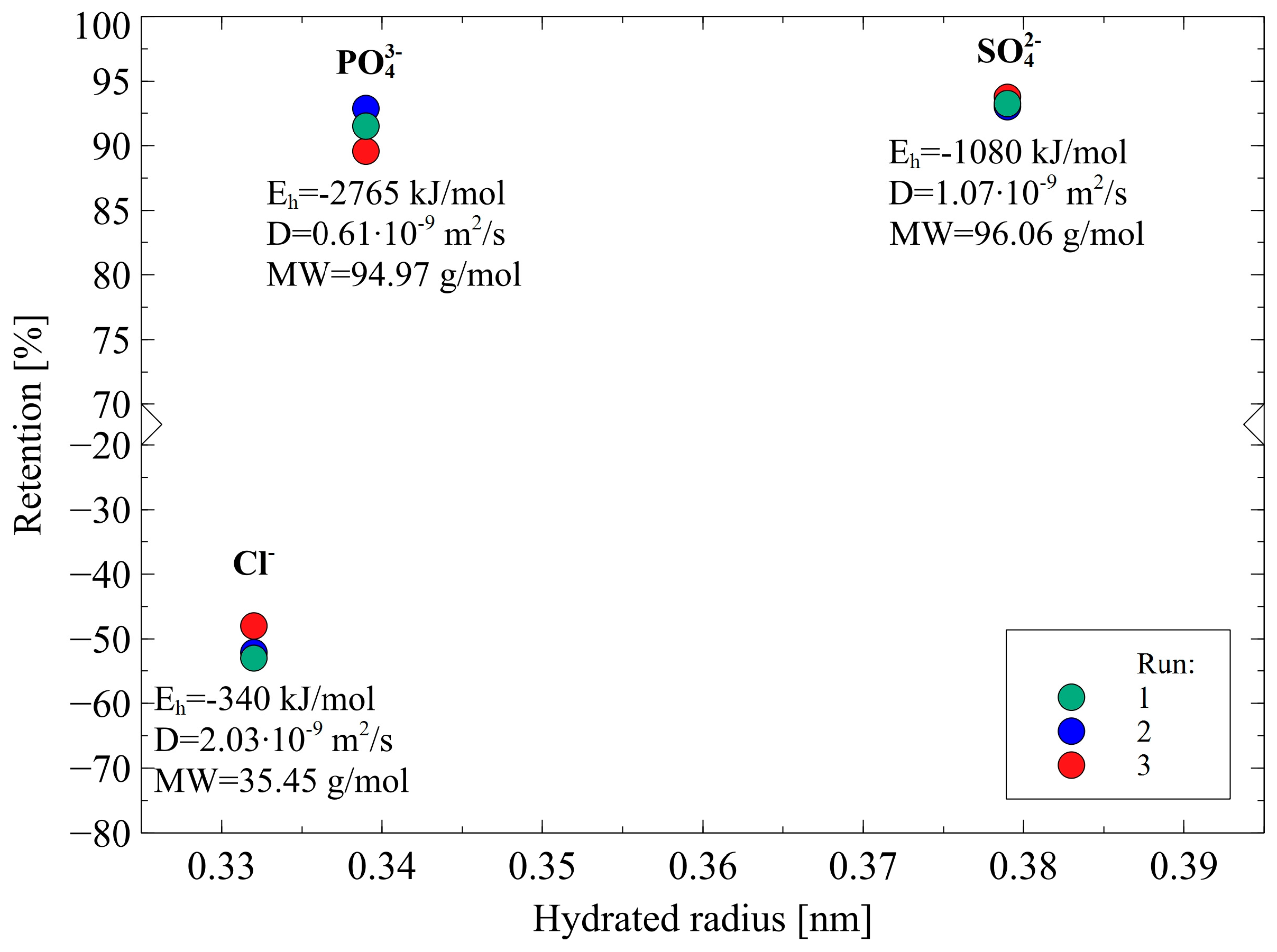
3.3. Separation of Ions
3.3.1. Anions
- (i)
- The rejection of divalent SO42− and trivalent PO43− anions was higher than that of monovalent ions Cl− due to the fact that they are characterized by higher charge density. This observation indicates that the Donnan effect was the key mechanism of the separation of anions present in 1,3-PD fermentation broths.
- (ii)
- The Stokes radius and MW of anions: Cl-, NO3−, PO43− and SO42− are 0.332 nm and 35.45 g/mol, 0.335 nm and 62 g/mol, 0.339 nm and 94.97 g/mol, 0.379 nm and 96.06 g/mol, respectively. It indicates that the highest retention was obtained for anions that present the highest both radius and MW. Therefore, it can be concluded that the steric hindrance effects also played a significant role in the anion’s separation. It is confirmed by the fact that for anions Cl−, negative retention has been reported.
- (iii)
- In the case of ions with a larger radius, the charge center is at a greater distance from the surface and consequently, the electrostatic interactions between ions and the negative membrane surface are weaker [38].
- (iv)
- In light of dielectric exclusion theory, ions Cl− have significantly lower hydration-free energy than PO43− and SO42−, hence, they can easily reduce the number of water molecules in the hydration shell:
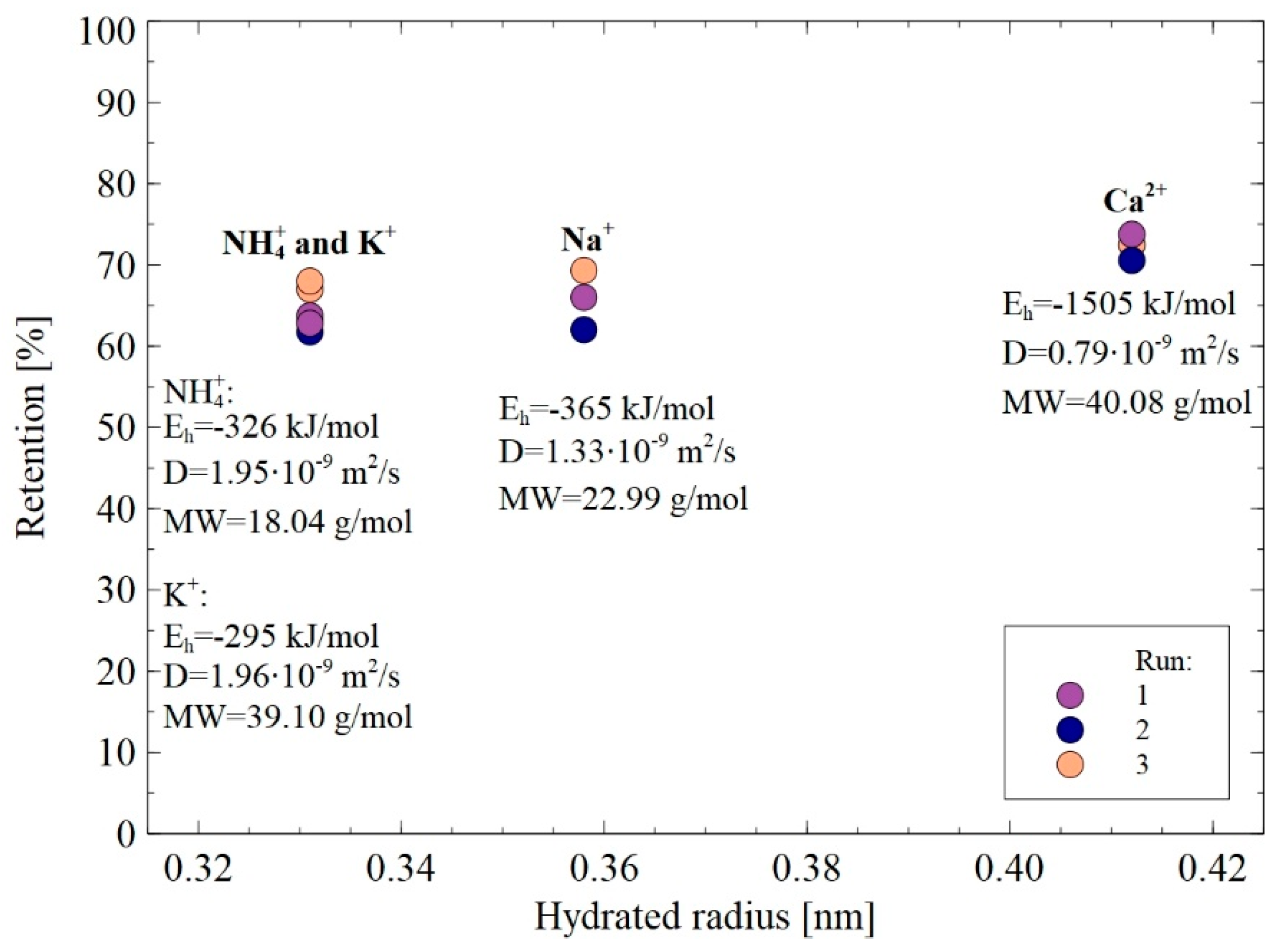
3.3.2. Cations
- (i)
- The rejection of divalent cation Ca2+ was higher than that of monovalent ones NH4+, K+, and Na+, which can be attributed to the fact that Ca2+ is characterized by higher charge density. This result indicates the key role of the Donnan effect during the separation of cations present in 1,3-PD fermentation broths.
- (ii)
- The Stokes radius of cations: NH4+, K+, Na+, and Ca2+ are 0.331 nm, 0.331 nm, 0.358 nm, and 0.412 nm, respectively. It indicates that the highest retention was obtained for cations characterized by the highest hydrated radius. Therefore, it can be concluded that the steric hindrance effects also played a significant role in the cations separation. It is confirmed by the fact that the same retention degree (59%) has been noted for cations (NH4+ and K+) with the same hydrated radius (0.331 nm). It is necessary to mention that no effect of MW on the retention was recorded, which indicates that with regard to cations separation, the hydrated radius is a more significant parameter.
- (iii)
- The diffusion coefficients of cations: NH4+, K+, Na+, and Ca2+ are equal to 1.95·10−9, 1.96·10−9, 1.33·10−9, and 0.97·10−9 m2/s respectively, thus, they are inversely reflected in the rejection sequence. It confirms the thesis that cations with higher diffusion coefficients easily passed through the membrane.
- (iv)
- (The lowest energy hydration, equal to −326 and −295 kJ/mol, have been reported for NH4+ and K+, respectively, hence, they can easily reduce the number of water molecules in the hydration shell (Equation (14)) and consequently, flow easily through the membrane. In turn, multivalent cations such as Ca2+ require more energy to lose their hydration shells, so their flow through the membrane was difficult [38].
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Li, Z.; Zhao, L.; Sun, M.; Zhang, X.; Yang, D.; Xu, G. Synthesis of 1,3-Propanediol by Cross Aldol Reaction and Hydrogenation of Formaldehyde with Acetaldehyde. ACS Sustain. Chem. Eng. 2022, 10, 2355–2367. [Google Scholar] [CrossRef]
- Md Radzi, M.R.; Manogaran, M.D.; Yusoff, M.H.M.; Zulqarnain; Anuar, M.R.; Shoparwe, N.F.; Rahman, M.F.A. Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catalysts 2022, 12, 945. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Q.; Zhou, Z.; Li, Z.; Meng, K.; Fang, T.; You, Z.; Zhang, G.; Yin, B.; Shen, J.; et al. Hydrogenolysis of Glycerol to 1,3-Propanediol: Are Spatial and Electronic Configuration of “Metal-Solid Acid” Interface Key for Active and Durable Catalysts? ChemCatChem 2022, 14, e202101316. [Google Scholar] [CrossRef]
- Bhowmik, S.; Darbha, S. Advances in Solid Catalysts for Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. Catal. Rev. 2020, 63, 639–703. [Google Scholar] [CrossRef]
- Zhang, C.; Sharma, S.; Wang, W.; Zeng, A. A Novel Downstream Process for Highly Pure 1,3-propanediol from an Efficient Fed-batch Fermentation of Raw Glycerol by Clostridium Pasteurianum. Eng. Life Sci. 2021, 21, 351–363. [Google Scholar] [CrossRef]
- Andrzejewski, A.; Szczygiełda, M.; Prochaska, K. Optimisation of Lactic Bio-Acid Separation from Actual Post-Fermentation Broth Using Nanofiltration Process and Fouling Analysis. Desalination Water Treat. 2021, 214, 203–213. [Google Scholar] [CrossRef]
- Antczak, J.; Szczygielda, M.; Prochaska, K. An Environment-Friendly Multi-Step Membrane-Based System to Succinic Acid Recovery from the Fermentation Broth. Desalination Water Treat. 2018, 128, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, D.; Kumar, M.; Pugazhenthi, G.; Vasanth, D. Separation of Bacteria Kocuria Rhizophila from Fermentation Broth by Cross-Flow Microfiltration Using Inexpensive Tubular Ceramic Membrane. Arab. J. Sci. Eng. 2022, 47, 5767–5776. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Gryta, M.; Tomczak, W. Microfiltration of Post-Fermentation Broth with Backflushing Membrane Cleaning. Chem. Pap. 2015, 69, 544–552. [Google Scholar] [CrossRef]
- Tran, M.L.; Chen, Y.-S.; Juang, R.-S. Fouling Analysis in One-Stage Ultrafiltration of Precipitation-Treated Bacillus Subtilis Fermentation Liquors for Biosurfactant Recovery. Membranes 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. The Application of Ultrafiltration for Separation of Glycerol Solution Fermented by Bacteria. Pol. J. Chem. Technol. 2013, 15, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, G.; Wan, Y.; Luo, J. Nanofiltration Membrane for Bio-separation: Process-oriented Materials Innovation. Eng. Life Sci. 2021, 21, 405–416. [Google Scholar] [CrossRef]
- Doménech, N.G.; Purcell-Milton, F.; Gun’ko, Y.K. Recent Progress and Future Prospects in Development of Advanced Materials for Nanofiltration. Mater. Today Commun. 2020, 23, 100888. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bayati, B.; Poerio, T.; Argurio, P.; Kikhavani, T.; Namdari, M.; Ferreira, L.M. Application of NF Polymeric Membranes for Removal of Multicomponent Heat-Stable Salts (HSS) Ions from Methyl Diethanolamine (MDEA) Solutions. Molecules 2020, 25, 4911. [Google Scholar] [CrossRef]
- Garcia, P.; Martin, P.; Carlot, G.; Castelier, E.; Ripert, M.; Sabathier, C.; Valot, C.; D’Acapito, F.; Hazemann, J.-L.; Proux, O.; et al. A Study of Xenon Aggregates in Uranium Dioxide Using X-Ray Absorption Spectroscopy. J. Nucl. Mater. 2006, 352, 136–143. [Google Scholar] [CrossRef]
- Nath, K.; Dave, H.K.; Patel, T.M. Revisiting the Recent Applications of Nanofiltration in Food Processing Industries: Progress and Prognosis. Trends Food Sci. Technol. 2018, 73, 12–24. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration Systems and Applications in Wastewater Treatment: Review Article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Du, Y.; Pramanik, B.K.; Zhang, Y.; Dumée, L.; Jegatheesan, V. Recent Advances in the Theory and Application of Nanofiltration: A Review. Curr. Pollut. Rep. 2022, 8, 51–80. [Google Scholar] [CrossRef]
- Kotrappanavar, N.S.; Hussain, A.A.; Abashar, M.E.E.; Al-Mutaz, I.S.; Aminabhavi, T.M.; Nadagouda, M.N. Prediction of Physical Properties of Nanofiltration Membranes for Neutral and Charged Solutes. Desalination 2011, 280, 174–182. [Google Scholar] [CrossRef]
- González, M.I.; Alvarez, S.; Riera, F.A.; Álvarez, R. Lactic Acid Recovery from Whey Ultrafiltrate Fermentation Broths and Artificial Solutions by Nanofiltration. Desalination 2008, 228, 84–96. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Hawkes, S. Effects of Membrane Fouling on the Nanofiltration of Pharmaceutically Active Compounds (PhACs): Mechanisms and Role of Membrane Pore Size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Kimani, E.M. The Influence of Feedwater PH on Membrane Charge Ionization and Ion Rejection by Reverse Osmosis: An Experimental and Theoretical Study. J. Membr. Sci. 2022, 660, 120800. [Google Scholar] [CrossRef]
- Sigurdardottir, S.B.; DuChanois, R.M.; Epsztein, R.; Pinelo, M.; Elimelech, M. Energy Barriers to Anion Transport in Polyelectrolyte Multilayer Nanofiltration Membranes: Role of Intra-Pore Diffusion. J. Membr. Sci. 2020, 603, 117921. [Google Scholar] [CrossRef]
- Zhu, Y.; Galier, S.; Roux-de Balmann, H. Description of the Variation of Retention versus PH in Nanofiltration of Organic Acids. J. Membr. Sci. 2021, 637, 119588. [Google Scholar] [CrossRef]
- Dey, P.; Linnanen, L.; Pal, P. Separation of Lactic Acid from Fermentation Broth by Cross Flow Nanofiltration: Membrane Characterization and Transport Modelling. Desalination 2012, 288, 47–57. [Google Scholar] [CrossRef]
- Freger, V. Swelling and Morphology of the Skin Layer of Polyamide Composite Membranes: An Atomic Force Microscopy Study. Environ. Sci. Technol. 2004, 38, 3168–3175. [Google Scholar] [CrossRef]
- Dang, H.Q.; Price, W.E.; Nghiem, L.D. The Effects of Feed Solution Temperature on Pore Size and Trace Organic Contaminant Rejection by the Nanofiltration Membrane NF270. Sep. Purif. Technol. 2014, 125, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Roy, Y.; Warsinger, D.M.; Lienhard, J.H. Effect of Temperature on Ion Transport in Nanofiltration Membranes: Diffusion, Convection and Electromigration. Desalination 2017, 420, 241–257. [Google Scholar] [CrossRef]
- Zaman, N.K.; Law, J.Y.; Chai, P.V.; Rohani, R.; Mohammad, A.W. Recovery of Organic Acids from Fermentation Broth Using Nanofiltration Technologies: A Review. JPS 2017, 28, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Yao, Z.; Jiao, L.; Waheed, M.; Sun, Z.; Zhang, L. Separation Mechanism, Selectivity Enhancement Strategies and Advanced Materials for Mono-/Multivalent Ion-Selective Nanofiltration Membrane. Adv. Membr. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, Y.-L.; Dai, R.; Li, X.; Wang, Z. Roles of Anion–Cation Coupling Transport and Dehydration-Induced Ion–Membrane Interaction in Precise Separation of Ions by Nanofiltration Membranes. Environ. Sci. Technol. 2022, 56, 14069–14079. [Google Scholar] [CrossRef]
- Merdaw, A.A.; Sharif, A.O.; Derwish, G.A.W. Mass Transfer in Pressure-Driven Membrane Separation Processes, Part II. Chem. Eng. J. 2011, 168, 229–240. [Google Scholar] [CrossRef]
- Merkel, A.; Voropaeva, D.; Ondrušek, M. The Impact of Integrated Nanofiltration and Electrodialytic Processes on the Chemical Composition of Sweet and Acid Whey Streams. J. Food Eng. 2021, 298, 110500. [Google Scholar] [CrossRef]
- Tansel, B. Significance of Thermodynamic and Physical Characteristics on Permeation of Ions during Membrane Separation: Hydrated Radius, Hydration Free Energy and Viscous Effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Non-Steric Mechanisms of Nanofiltration: Superposition of Donnan and Dielectric Exclusion. Sep. Purif. Technol. 2001, 22–23, 143–158. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Castro-Muñoz, R. Current and Future Applications of Nanofiltration in Food Processing. In Separation of Functional Molecules in Food by Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–348. ISBN 978-0-12-815056-6. [Google Scholar]
- Zhang, T.; He, Z.; Wang, K.; Wang, X.; Xie, Y.F.; Hou, L.A. Loose Nanofiltration Membranes for Selective Rejection of Natural Organic Matter and Mineral Salts in Drinking Water Treatment. J. Membr. Sci. 2022, 662, 120970. [Google Scholar] [CrossRef]
- Adeniyi, A.; Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Mbakop, S.; Onyango, M.S. Preparation of Nanofiltration Membrane Modified with Sawdust-Derived Cellulose Nanocrystals for Removal of Nitrate from Drinking Water. Membranes 2022, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Ngo, M.T.T.; Makabe, R.; Ueyama, T.; Takeuchi, H.; Nga, T.T.V.; Bui, X.-T.; Tanaka, H. Submerged Nanofiltration without Pre-Treatment for Direct Advanced Drinking Water Treatment. Chemosphere 2021, 265, 129056. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medrano, V.; Cuartas-Uribe, B.; Bes-Piá, M.-A.; Mendoza-Roca, J.-A. Application of Nanofiltration and Reverse Osmosis Membranes for Tannery Wastewater Reuse. Water 2022, 14, 2035. [Google Scholar] [CrossRef]
- Yacouba, Z.A.; Lesage, G.; Mendret, J.; Zaviska, F.; Petit, E.; Brosillon, S. Fate and Toxicity of Carbamazepine and Its Degradation By-Products During Coupling of Ozonation and Nanofiltration for Urban Wastewater Reuse. Front. Environ. Chem. 2021, 2, 798785. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Gutiérrez Ruiz, S.; Quiroga Alonso, J.M. Removal of Emerging Contaminants from Wastewater Using Nanofiltration for Its Subsequent Reuse: Full–Scale Pilot Plant. J. Clean. Prod. 2019, 214, 514–523. [Google Scholar] [CrossRef]
- McCarthy, W.P.; Blais, H.N.; O’Callaghan, T.F.; Hossain, M.; Moloney, M.; Danaher, M.; O’Connor, C.; Tobin, J.T. Application of Nanofiltration for the Removal of Chlorate from Skim Milk. Int. Dairy J. 2022, 128, 105321. [Google Scholar] [CrossRef]
- Gaglianò, M.; Conidi, C.; De Luca, G.; Cassano, A. Partial Removal of Sugar from Apple Juice by Nanofiltration and Discontinuous Diafiltration. Membranes 2022, 12, 712. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Wan, Y. Biofouling in Sugarcane Juice Refining by Nanofiltration Membrane: Fouling Mechanism and Cleaning. J. Membr. Sci. 2020, 612, 118432. [Google Scholar] [CrossRef]
- Antczak, J.; Szczygiełda, M.; Prochaska, K. Nanofiltration Separation of Succinic Acid from Post-Fermentation Broth: Impact of Process Conditions and Fouling Analysis. J. Ind. Eng. Chem. 2019, 77, 253–261. [Google Scholar] [CrossRef]
- Davey, C.J.; Havill, A.; Leak, D.; Patterson, D.A. Nanofiltration and Reverse Osmosis Membranes for Purification and Concentration of a 2,3-Butanediol Producing Gas Fermentation Broth. J. Membr. Sci. 2016, 518, 150–158. [Google Scholar] [CrossRef]
- Khunnonkwao, P.; Jantama, K.; Kanchanatawee, S.; Galier, S.; Roux-de Balmann, H. A Two Steps Membrane Process for the Recovery of Succinic Acid from Fermentation Broth. Sep. Purif. Technol. 2018, 207, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Laube, H.; Schneider, R.; Venus, J. Investigation of Spiral-Wound Membrane Modules for the Cross-Flow Nanofiltration of Fermentation Broth Obtained from a Pilot Plant Fermentation Reactor for the Continuous Production of Lactic Acid. Bioresour. Bioprocess. 2017, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Sikder, J.; Chakraborty, S.; Pal, P.; Drioli, E.; Bhattacharjee, C. Purification of Lactic Acid from Microfiltrate Fermentation Broth by Cross-Flow Nanofiltration. Biochem. Eng. J. 2012, 69, 130–137. [Google Scholar] [CrossRef]
- Staszak, K.; Karaś, Z.; Jaworska, K. Comparison of Polymeric and Ceramic Membranes Performance in the Process of Micellar Enhanced Ultrafiltration of Cadmium(II) Ions from Aqueous Solutions. Chem. Pap. 2013, 67, 380–388. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Abdul Shukor, M.H.; Mohamad, A.W. Purification of High Value Succinic Acid from Biomass Fermentation Broth Via Nanofiltration. Indian J. Sci. Technol. 2016, 9, 21. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Isloor, A.M.; Jahim, J.M. Investigation of Succinic Acid Recovery from Aqueous Solution and Fermentation Broth Using Polyimide Nanofiltration Membrane. J. Environ. Chem. Eng. 2020, 8, 101895. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Liu, Y.; Wang, X.; Cao, Z. Separation of L-Glutamine from Fermentation Broth by Nanofiltration. J. Membr. Sci. 2003, 222, 191–201. [Google Scholar] [CrossRef]
- Li, Y.; Shahbazi, A. Lactic Acid Recovery from Cheese Whey Fermentation Broth Using Combined Ultrafiltration and Nanofiltration Membranes. Appl. Biochem. Biotechnol. 2006, 132, 985–996. [Google Scholar] [CrossRef]
- Gryta, M.; Markowska-Szczupak, A.; Grzechulska-Damszel, J.; Bastrzyk, J.; Waszak, M. The Study of Glycerol-Based Fermentation and Broth Downstream by Nanofiltration. Pol. J. Chem. Technol. 2014, 16, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Bastrzyk, J.; Gryta, M.; Karakulski, K. Fouling of Nanofiltration Membranes Used for Separation of Fermented Glycerol Solutions. Chem. Pap. 2014, 68, 757–765. [Google Scholar] [CrossRef]
- Alves, Y.P.C.; Antunes, F.A.F.; da Silva, S.S.; Forte, M.B.S. From By- to Bioproducts: Selection of a Nanofiltration Membrane for Biotechnological Xylitol Purification and Process Optimization. Food Bioprod. Process. 2021, 125, 79–90. [Google Scholar] [CrossRef]
- Jeantet, R.; Maubois, J.L.; Boyaval, P. Semicontinuous Production of Lactic Acid in a Bioreactor Coupled with Nanofiltration Membranes. Enzym. Microb. Technol. 1996, 19, 614–619. [Google Scholar] [CrossRef]
- Kang, S.H.; Chang, Y.K. Removal of Organic Acid Salts from Simulated Fermentation Broth Containing Succinate by Nanofiltration. J. Membr. Sci. 2005, 246, 49–57. [Google Scholar] [CrossRef]
- Thuy, N.T.H.; Boontawan, A. Production of Very-High Purity Succinic Acid from Fermentation Broth Using Microfiltration and Nanofiltration-Assisted Crystallization. J. Membr. Sci. 2017, 524, 470–481. [Google Scholar] [CrossRef]
- Omwene, P.I.; Sarihan, Z.B.O.; Karagunduz, A.; Keskinler, B. Bio-Based Succinic Acid Recovery by Ion Exchange Resins Integrated with Nanofiltration/Reverse Osmosis Preceded Crystallization. Food Bioprod. Process. 2021, 129, 1–9. [Google Scholar] [CrossRef]
- Wallace, E.; Cuhorka, J.; Mikulášek, P. Characterization of Nanofiltration Membrane and Its Practical Use for Separation of Zinc from Wastewater. Waste Forum 2018, 3, 314–325. [Google Scholar]
- Oatley, D.L.; Llenas, L.; Aljohani, N.H.M.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M.; de Pablo, J. Investigation of the Dielectric Properties of Nanofiltration Membranes. Desalination 2013, 315, 100–106. [Google Scholar] [CrossRef]
- Mancini, E.; Ramin, P.; Styrbæck, P.; Bjergholt, C.; Soheil Mansouri, S.; Gernaey, K.V.; Luo, J.; Pinelo, M. Separation of Succinic Acid from Fermentation Broth: Dielectric Exclusion, Donnan Effect and Diffusion as the Most Influential Mass Transfer Mechanisms. Sep. Purif. Technol. 2022, 281, 119904. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Gamaj, M.I. Estimation of the Diffusion Coefficient and Hydrodynamic Radius (Stokes Radius) for Inorganic Ions in Solution Depending on Molar Conductivity as Electro-Analytical Technique-A Review. J. Chem. Rev. 2020, 2, 182–188. [Google Scholar] [CrossRef]
- Bouchoux, A.; Roux-de Balmann, H.; Lutin, F. Investigation of Nanofiltration as a Purification Step for Lactic Acid Production Processes Based on Conventional and Bipolar Electrodialysis Operations. Sep. Purif. Technol. 2006, 52, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Samson, E.; Marchand, J.; Snyder, K.A. Calculation of Ionic Diffusion Coefficients on the Basis of Migration Test Results. Mat. Struct. 2003, 36, 156–165. [Google Scholar] [CrossRef]
- Wang, D.-X.; Su, M.; Yu, Z.-Y.; Wang, X.-L.; Ando, M.; Shintani, T. Separation Performance of a Nanofiltration Membrane Influenced by Species and Concentration of Ions. Desalination 2005, 175, 219–225. [Google Scholar] [CrossRef]
- Flury, M.; Gimmi, T.F. 6.2 Solute Diffusion. In SSSA Book Series; Dane, J.H., Clarke Topp, G., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 1323–1351. ISBN 978-0-89118-893-3. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Andersson, M.P.; Stipp, S.L.S. Predicting Hydration Energies for Multivalent Ions. J. Comput. Chem. 2014, 35, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Kiriukhin, M.Y.; Collins, K.D. Dynamic Hydration Numbers for Biologically Important Ions. Biophys. Chem. 2002, 99, 155–168. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Cisneros, B.J.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-González, M.; Ahmed, A.; Maamo, K.; Salem, M.; Jordan, C.; Harasek, M. Evaluation of Nanofiltration Membranes for Pure Lactic Acid Permeability. Membranes 2022, 12, 302. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y.; Hsu, Y.-J.; Dong, C.-D.; Chen, C.-W.; Wu, C.-H. In-Situ Radical Graft Modification of NF270 to Improve Membrane Separation: Effects of Water Salinity and Fouling Types. Environ. Technol. Innov. 2022, 27, 102758. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Zhang, H.; Luo, J.; Woodley, J.M.; Wan, Y. Targeted Modification of Polyamide Nanofiltration Membrane for Efficient Separation of Monosaccharides and Monovalent Salt. J. Membr. Sci. 2021, 628, 119250. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Chen, X.; Sheng, M.; Cao, G.; Cai, L.; Meng, S.; Tang, C.Y. Degradation of Polyamide Nanofiltration Membranes by Bromine: Changes of Physiochemical Properties and Filtration Performance. Environ. Sci. Technol. 2021, 55, 6329–6339. [Google Scholar] [CrossRef]
- Ramdani, A.; Deratani, A.; Taleb, S.; Drouiche, N.; Lounici, H. Performance of NF90 and NF270 Commercial Nanofiltration Membranes in the Defluoridation of Algerian Brackish Water. Desalination Water Treat. 2021, 212, 286–296. [Google Scholar] [CrossRef]
- Knežević, K.; Saracevic, E.; Krampe, J.; Kreuzinger, N. Comparison of Ion Removal from Waste Fermentation Effluent by Nanofiltration, Electrodialysis and Ion Exchange for a Subsequent Sulfuric Acid Recovery. J. Environ. Chem. Eng. 2022, 10, 108423. [Google Scholar] [CrossRef]
- Boussu, K.; De Baerdemaeker, J.; Dauwe, C.; Weber, M.; Lynn, K.G.; Depla, D.; Aldea, S.; Vankelecom, I.F.J.; Vandecasteele, C.; Van der Bruggen, B. Physico-Chemical Characterization of Nanofiltration Membranes. ChemPhysChem 2007, 8, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Ouali, S.; Loulergue, P.; Biard, P.-F.; Nasrallah, N.; Szymczyk, A. Ozone Compatibility with Polymer Nanofiltration Membranes. J. Membr. Sci. 2021, 618, 118656. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Cross-Flow Microfiltration of Glycerol Fermentation Broths with Citrobacter Freundii. Membranes 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of Heavy Metal Ions by Nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Postel, S.; Spalding, G.; Chirnside, M.; Wessling, M. On Negative Retentions in Organic Solvent Nanofiltration. J. Membr. Sci. 2013, 447, 57–65. [Google Scholar] [CrossRef]
- Labban, O.; Liu, C.; Chong, T.H.; Lienhard, V.J.H. Fundamentals of Low-Pressure Nanofiltration: Membrane Characterization, Modeling, and Understanding the Multi-Ionic Interactions in Water Softening. J. Membr. Sci. 2017, 521, 18–32. [Google Scholar] [CrossRef]
- Xiong, B.; Richard, T.L.; Kumar, M. Integrated Acidogenic Digestion and Carboxylic Acid Separation by Nanofiltration Membranes for the Lignocellulosic Carboxylate Platform. J. Membr. Sci. 2015, 489, 275–283. [Google Scholar] [CrossRef]
- Bouchoux, A.; Balmann, H.; Lutin, F. Nanofiltration of Glucose and Sodium Lactate SolutionsVariations of Retention between Single- and Mixed-Solute Solutions. J. Membr. Sci. 2005, 258, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Lin, M.; Fang, B. Inhibition and in Situ Removal of Organic Acids during Glucose/Glycerol Co-Fermentation by Lactobacillus Reuteri. Biochem. Eng. J. 2015, 99, 93–98. [Google Scholar] [CrossRef]
- Szymanowska-Powałowska, D.; Kubiak, P. Effect of 1,3-Propanediol, Organic Acids, and Ethanol on Growth and Metabolism of Clostridium Butyricum DSP1. Appl. Microbiol. Biotechnol. 2015, 99, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Fukushi, K.; Yamamoto, K. A Study on the Removal of Organic Acids from Wastewaters Using Nanofiltration Membranes. Sep. Purif. Technol. 2008, 59, 17–25. [Google Scholar] [CrossRef]
- Kettler, R.M.; Palmer, D.A.; Wesolowski, D.J. Dissociation Quotients of Succinic Acid in Aqueous Sodium Chloride Media to 225 C. J. Solution. Chem. 1995, 24, 65–87. [Google Scholar] [CrossRef]
- Pérez-González, A.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I.; Irabien, J.A. Nanofiltration Separation of Polyvalent and Monovalent Anions in Desalination Brines. J. Membr. Sci. 2015, 473, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Diaz, P.A.B.; Kronemberger, F.D.A.; Habert, A.C. Effect of Feed Conditions and Added Solutes on the Performance of Membrane Nanofiltration of Succinic Acid Solutions. Braz. J. Chem. Eng. 2020, 37, 283–295. [Google Scholar] [CrossRef]
- Sosa, P.A.; Roca, C.; Velizarov, S. Membrane Assisted Recovery and Purification of Bio-Based Succinic Acid for Improved Process Sustainability. J. Membr. Sci. 2016, 501, 236–247. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, J.-G.; Shim, H.J.; Chang, Y.K. Modeling of Ammonium Lactate Recovery and Impurity Removal from Simulated Fermentation Broth by Nanofiltration. J. Membr. Sci. 2012, 396, 110–118. [Google Scholar] [CrossRef]
- Lee, E.G.; Kang, S.H.; Kim, H.H.; Chang, Y.K. Recovery of Lactic Acid from Fermentation Broth by the Two-Stage Process of Nanofiltration and Water-Splitting Electrodialysis. Biotechnol. Bioprocess Eng. 2006, 11, 313–318. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Chen, W.-H.; Wei, T.-Y.; Hwang, W.-S.; Wang, C.-P.; Huang, C.-P. Separation of Acetic Acid from Xylose by Nanofiltration. Sep. Purif. Technol. 2009, 67, 95–102. [Google Scholar] [CrossRef]
- Woźniak, M.J.; Prochaska, K. Fumaric Acid Separation from Fermentation Broth Using Nanofiltration (NF) and Bipolar Electrodialysis (EDBM). Sep. Purif. Technol. 2014, 125, 179–186. [Google Scholar] [CrossRef]
- Szoke, S.; Patzay, G.; Weiser, L. Characteristics of Thin-Film Nanofiltration Membranes at Various PH-Values. Desalination 2003, 151, 123–129. [Google Scholar] [CrossRef]
- Yunoki, H.; Nagata, K.; Kokubo, K.-I.; Ito, A.; Watanabe, A. Effects of the Mixture Ratio of Amino Acid and Sodium Chloride on the Rejection of Nanofiltration Membranes under Various Operating Conditions. J. Chem. Eng. Japan/JCEJ 2002, 35, 76–82. [Google Scholar] [CrossRef]
- Tanninen, J.; Mänttäri, M.; Nyström, M. Effect of Salt Mixture Concentration on Fractionation with NF Membranes. J. Membr. Sci. 2006, 283, 57–64. [Google Scholar] [CrossRef]
- Gozálvez-Zafrilla, J.M.; Santafé-Moros, A.; García-Díaz, J.C. Crossed Mixture–Process Design Approach to Model Nanofiltration Rejection for Non-Dilute Multi-Ionic Solutions in a given Range of Solution Compositions. Desalination 2013, 315, 61–69. [Google Scholar] [CrossRef]
- Krieg, H.M.; Modise, S.J.; Keizer, K.; Neomagus, H.W.J.P. Salt Rejection in Nanofiltration for Single and Binary Salt Mixtures in View of Sulphate Removal. Desalination 2005, 171, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Garcia, F.; Ciceron, D.; Saboni, A.; Alexandrova, S. Nitrate Ions Elimination from Drinking Water by Nanofiltration: Membrane Choice. Sep. Purif. Technol. 2006, 52, 196–200. [Google Scholar] [CrossRef]




| Name | Formula | Molecular Weight MW [Da] | Concentration [g/L] | Dissociation Constant pKa [-] | Charge at pH = 7 | Diffusion Coefficient D [10−9 m2/s] | Stokes Radius rS/Hydrated Radius rH [nm] | Hydration Free Energy Eh [kJ/mol] | Shape |
|---|---|---|---|---|---|---|---|---|---|
| glycerol | C3H8O3 | 92.09 | 0.25–0.28 | 14.40 | neutral | 0.95 [67,68] | rS = 0.258 [67,68] | NI | tetrahedral |
| 1,3-propanediol | C3H8O2 | 76.09 | 12.21–13.45 | 14.46 | neutral | NI | NI | NI | NI |
| formic acid | CH2O2 | 46.05 | 2.10–2.31 | 3.84 | negative | formate: 1.84 [69] | formate: rS = 0.200 [69] | formate: −347.95 [17] | trigonal and tetrahedral |
| acetic acid | C2H4O2 | 60.05 | 4.25–4.67 | 4.76 | negative | acetate: 1.38 [69] | acetate: rS = 0.225 [70] | acetate: −328.94 [17] | tetrahedral |
| lactic acid | C3H6O3 | 90.08 | 0.59–1.41 | 3.08 | negative | lactate: 1.06 [71] | lactate: rS = 0.230 [71] | lactate: NI | tetrahedral |
| succinic acid | C4H6O4 | 118.08 | 1.14–1.39 | 4.21 and 5.64 | negative | succinate: 0.99 [69] | succinate: rS = 0.252 | succinate: NI | NI |
| ethanol | C2H6O | 46.10 | 0.89–1.26 | 15.90 | neutral | 1.24 [67] | rS = 0.198 [67] | NI | tetrahedral |
| chloride | Cl− | 35.45 | 0.026–0.041 | - | negative | 2.03 [72,73,74] | rH = 0.332 [75] | −340 [76] | spherical |
| nitrate | NO3− | 62.00 | 0.002 | - | negative | 1.90 [73,74] | rH = 0.335 [75] | −300 [76] | trigonal |
| phosphate | PO43− | 94.97 | 1.926–2.268 | - | negative | 0.61 [74] | rH = 0.339 [77] | −2765 [76] | tetrahedral |
| sulfate | SO42− | 96.06 | 1.333–1.536 | - | negative | 1.07 [72,73,74] | rH = 0.379 [75] | −1080 [76] | tetrahedral |
| ammonium | NH4+ | 18.04 | 0.448–0.545 | - | positive | 1.95 [73] | rH = 0.331 [75] | −326 [36] | tetrahedral |
| sodium | Na+ | 22.99 | 4.451–4.527 | - | positive | 1.33 [72,73,74] | rH = 0.358 [75] | −365 [76] | spherical |
| magnesium | Mg2+ | 24.31 | 0.003–0.010 | - | positive | 0.71 [72,73,74] | rH = 0.428 [75] | −1830 [76] | spherical |
| potassium | K+ | 39.10 | 1.117–1.499 | - | positive | 1.96 [72,73] | rH = 0.331 [75] | −295 [76] | spherical |
| calcium | Ca2+ | 40.08 | 0.011–0.047 | - | positive | 0.79 [72,74] | rH = 0.412 [75] | −1505 [76] | spherical |
| Parameter | Unit | Value |
|---|---|---|
| Manufacturer | [-] | DOW-Filmtec |
| Skin-layer material | [-] | polyamide |
| Molecular weight cut-off | [Da] | 200–300 |
| Average pore radius | [nm] | 0.43 |
| MgSO4 rejection | [%] | 97 |
| NaCl rejection | [%] | 50 |
| Contact angle | [°] | 54.8 |
| Maximal pressure | [MPa] | 4.1 |
| Maximal temperature | [°C] | 45 |
| pH range | [-] | 2–11 |
| Isoelectric point | [-] | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W. The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths. Membranes 2022, 12, 1263. https://doi.org/10.3390/membranes12121263
Tomczak W. The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths. Membranes. 2022; 12(12):1263. https://doi.org/10.3390/membranes12121263
Chicago/Turabian StyleTomczak, Wirginia. 2022. "The Application of the Nanofiltration Membrane NF270 for Separation of Fermentation Broths" Membranes 12, no. 12: 1263. https://doi.org/10.3390/membranes12121263





