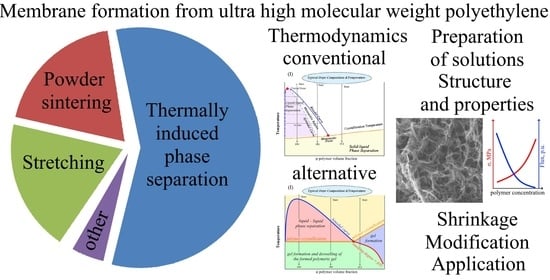
Current State-of-the-Art in Membrane Formation from Ultra-High Molecular Weight Polyethylene
Abstract
:1. Introduction
2. Methods of Preparation of UHMWPE Membranes
2.1. Solvent-Free UHMWPE Membrane Formation Methods
2.1.1. Powder Sintering
- Changing the polymer MW [78], which determines the rheological properties of the polymer melt;
2.1.2. Stretching
2.2. Solvent-Based Methods of UHMWPE Membrane Formation
2.2.1. Conventional Physico-Chemical Basis of the TIPS Method
2.2.2. Alternative Physico-Chemical Basis of the TIPS Method
2.2.3. Problem of Dope Solution Preparation
- Almost half of the published works report using various antioxidants that prevent degradation of polymer macromolecules for successful preparation of homogeneous UHMWPE solutions.
- Although the MW of the polymers used to prepare the solutions covers a wide range of values (including the values that are beyond the range of values corresponding to the UHMWPE definition) from 0.5 × 106 to 9 × 106 g/mol, UHMWPE with MW ~3 × 106 g/mol is the most widely used.
- In most cases, solutions are produced at temperatures of 160–250 °C, which is significantly higher than the melting point of the pure polymer (135–145 °C) under conditions of high shear (twin screw extruders, batch mixers, rheometers). Often, a preliminary step of thorough mixing of the polymer with the diluent at slightly elevated or room temperature is added.
- In more than 60% of the cited works, the authors use liquid paraffin (LP), mineral, white and paraffin oils as the solvent for UHMWPE. Interestingly, these are essentially different names for the same object—a liquid mixture of linear and/or branched saturated hydrocarbons. Moreover, as a rule, the fractional composition of the solvent is not disclosed. Only a few works [33,50,198,199,200,201,202] specify the MW range of the components in LP, usually ranging from 150 to 300 g/mol. In [198], the authors used a mixture of hydrocarbons with an average molecular weight of 500 g/mol, which should probably be classified not as LP but as paraffin wax.
- Only a few studies have employed other substances as solvents for UHMWPE. In particular, it was proposed to use decalin, xylene, naphthenic oil, diphenyl ether, etc. A slightly larger variety of potential solvents have been proposed in patents [204,206,217], for example, dioctyl phthalate, dibutyl phthalate, aromatic oils, etc. However, LP is considered the best choice.
2.2.4. Investigation of Thermal Behavior of UHMWPE Mixtures with Various Solvents
2.2.5. Factors Controlling the Structure and Properties of the UHMWPE Membranes Prepared via TIPS
Polymer Concentration in the Dope Solution
MW of the Polymer
Solvent Nature
Cooling Conditions
2.2.6. Combination of TIPS and Stretching
2.2.7. Other Variations of the TIPS Method
2.3. Advantages and Disadvantages of UHMWPE Membrane Formation Methods
3. The Problem of UHMWPE Membrane Shrinkage
4. Modification of UHMWPE Membranes
5. Application of UHMWPE Membranes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent Development in Modification of Polysulfone Membrane for Water Treatment Application. J. Water Process Eng. 2021, 40, 101835. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-Based Seawater Desalination: Present and Future Prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Koris, A.; Vatai, G. Dry Degumming of Vegetable Oils by Membrane Filtration. Desalination 2002, 148, 149–153. [Google Scholar] [CrossRef]
- Youn, K.S.; Hong, J.H.; Bae, D.H.; Kim, S.J.; Kim, S.D. Effective Clarifying Process of Reconstituted Apple Juice Using Membrane Filtration with Filter-Aid Pretreatment. J. Memb. Sci. 2004, 228, 179–186. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Huang, Y.H.; Lai, C.H.; Ho, W.J.; Ko, W.C. Develop a Novel Method for Removing Fusel Alcohols from Rice Spirits Using Nanofiltration. J. Food Sci. 2010, 75, N25–N29. [Google Scholar] [CrossRef]
- Volkov, A.V.; Korneeva, G.A.; Tereshchenko, G.F. Organic Solvent Nanofiltration: Prospects and Application. Russ. Chem. Rev. 2008, 77, 983. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.H. Review of CO2 Absorption Using Chemical Solvents in Hollow Fiber Membrane Contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in Lithium Ion Batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros-Méndez, S. Recent Advances on Separator Membranes for Lithium-Ion Battery Applications: From Porous Membranes to Solid Electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Artzi-Gerlitz, R.; Benkstein, K.D.; Lahr, D.L.; Hertz, J.L.; Montgomery, C.B.; Bonevich, J.E.; Semancik, S.; Tarlov, M.J. Fabrication and Gas Sensing Performance of Parallel Assemblies of Metal Oxide Nanotubes Supported by Porous Aluminum Oxide Membranes. Sens. Actuators B Chem. 2009, 136, 257–264. [Google Scholar] [CrossRef]
- Nomura, M.; Ono, K.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S.I. Preparation of a Stable Silica Membrane by a Counter Diffusion Chemical Vapor Deposition Method. J. Memb. Sci. 2005, 251, 151–158. [Google Scholar] [CrossRef]
- Wee, S.L.; Tye, C.T.; Bhatia, S. Membrane Separation Process—Pervaporation through Zeolite Membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A.F. Fabrication of Carbon Membranes for Gas Separation––A Review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A Review of Polymeric Membranes and Processes for Potable Water Reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y. ming Application and Modification of Poly(Vinylidene Fluoride) (PVDF) Membranes—A Review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ma, W.; Wang, X. A Review on Microporous Polyvinylidene Fluoride Membranes Fabricated via Thermally Induced Phase Separation for MF/UF Application. J. Memb. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef] [Green Version]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in Preparation, Modification, and Application of Polypropylene Membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of Polyethersulfone Membranes—A Review of Methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Habib, S.; Weinman, S.T. A Review on the Synthesis of Fully Aromatic Polyamide Reverse Osmosis Membranes. Desalination 2021, 502, 114939. [Google Scholar] [CrossRef]
- Yuan, S.; Strobbe, D.; Kruth, J.P.; Van Puyvelde, P.; Van der Bruggen, B. Production of Polyamide-12 Membranes for Microfiltration through Selective Laser Sintering. J. Memb. Sci. 2017, 525, 157–162. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose Acetate in Fabrication of Polymeric Membranes: A Review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Yuan, W.; Zhou, F.; Guo, W.; Li, J.; Zhuang, Z.; Su, X.; Lu, B.; Zhao, Y.; Tang, Y.; et al. A Critical Review on Surface-Pattern Engineering of Nafion Membrane for Fuel Cell Applications. Renew. Sustain. Energy Rev. 2021, 145, 110860. [Google Scholar] [CrossRef]
- Shen, L.Q.; Xu, Z.K.; Xu, Y.Y. Preparation and Characterization of Microporous Polyethylene Hollow Fiber Membranes. J. Appl. Polym. Sci. 2002, 84, 203–210. [Google Scholar] [CrossRef]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh Molecular Weight Polyethylene: Catalysis, Structure, Properties, Processing and Applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-High Molecular Weight Polyethylene: Influence of the Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017, 10, 791. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Yu, W. Phase Separation and Structure Control in Ultra-High Molecular Weight Polyethylene Microporous Membrane. J. Memb. Sci. 2011, 379, 268–278. [Google Scholar] [CrossRef]
- Quan, J.; Wang, H.; Li, T.; Fan, L.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Air and Water Vapor Permeable UHMWPE Composite Membranes for X-Ray Shielding. Ind. Eng. Chem. Res. 2020, 59, 9136–9142. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, F.; Cao, Y.; Xiang, M.; Kang, J.; Wu, T.; Fu, Q. Investigation on Cavitation Behavior of Ultrahigh Molecular Weight Polyethylene during Stretching in Wet Process and Dry Process. Polymer 2021, 230, 124081. [Google Scholar] [CrossRef]
- Wandenberg, E.J. Process for Polymerizing Olefins Wherein Hydrogen Is Utilized as a Molecular Weight Control Agent. U.S. Patent 3,051,690, 28 August 1962. [Google Scholar]
- Kurtz, S.M.; Muratoglu, O.K.; Evans, M.; Edidin, A.A. Advances in the Processing, Sterilization, and Crosslinking of Ultra-High Molecular Weight Polyethylene for Total Joint Arthroplasty. Biomaterials 1999, 20, 1659–1688. [Google Scholar] [CrossRef]
- Arora, S.; Majumdar, A.; Butola, B.S. Structure Induced Effectiveness of Shear Thickening Fluid for Modulating Impact Resistance of UHMWPE Fabrics. Compos. Struct. 2019, 210, 41–48. [Google Scholar] [CrossRef]
- Quan, J.; Wang, H.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. UHMWPE/Nanoparticle Composite Membrane for Personal Radiation Shielding. Compos. Sci. Technol. 2021, 201, 108500. [Google Scholar] [CrossRef]
- Kartikeya, K.; Chouhan, H.; Ahmed, A.; Bhatnagar, N. Determination of Tensile Strength of UHMWPE Fiber-Reinforced Polymer Composites. Polym. Test. 2020, 82, 106293. [Google Scholar] [CrossRef]
- Han, L.; Cai, H.; Chen, X.; Zheng, C.; Guo, W. Study of UHMWPE Fiber Surface Modification and the Properties of UHMWPE/Epoxy Composite. Polymers 2020, 12, 521. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Cheng, S. Properties of UHMWPE Fiber-Reinforced Composites. Polym. Bull. 2013, 70, 821–835. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Sipyagina, N.A.; Straumal, E.A.; Maksimkin, A.V.; Kolesnikov, E.A.; Senatov, F.S. Properties of Highly Porous Aerogels Prepared from Ultra-High Molecular Weight Polyethylene. Polymer 2019, 182, 121824. [Google Scholar] [CrossRef]
- Su, M.; Pan, Y.; Zheng, G.; Liu, C.; Shen, C.; Liu, X. An Ultra-Light, Superhydrophobic and Thermal Insulation Ultra-High Molecular Weight Polyethylene Foam. Polymer 2021, 218, 123528. [Google Scholar] [CrossRef]
- Leven, F.; Ulbricht, M.; Limberg, J.; Ostermann, R. Novel Finely Structured Polymer Aerogels Using Organogelators as a Structure-Directing Component. J. Mater. Chem. A 2021, 9, 20695–20702. [Google Scholar] [CrossRef]
- Li, R.; Gao, P.; Li, R.; Gao, P. Nanoporous UHMWPE Membrane Separators for Safer and High-Power-Density Rechargeable Batteries. Glob. Chall. 2017, 1, 1700020. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Wan, C.; Mansoor, B.; Usha, Z.R.; Yu, R.; Habumugisha, J.C.; Chen, W.; Chen, X.; Li, L. Superior Lithium Battery Separator with Extraordinary Electrochemical Performance and Thermal Stability Based on Hybrid UHMWPE/SiO2 Nanocomposites via the Scalable Biaxial Stretching Process. Compos. Part B Eng. 2021, 211, 108658. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Han, Z.; Wu, J.; Zhao, R.; An, X.; Zhao, X.; Li, Y.; Zhang, L. Preparation of a Novel UHMWPE Lithium Battery Separator by Electrospraying Method. J. Phys. Conf. Ser. 2021, 2011, 012087. [Google Scholar] [CrossRef]
- Huang, X. Separator Technologies for Lithium-Ion Batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Y.; Xie, X.; Yang, L.; Bai, Y.; Liu, G.; Dong, H.; Wang, T.; Huang, X.; He, J. Morphology of Porous UHMWPE Originating from the S–L Phase Separation in UHMWPE/Liquid Paraffin (LP) Blends. Iran. Polym. J. (English Ed.) 2022, 31, 1047–1056. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Yu, R.; Usha, Z.R.; Chen, W.; Chen, X.; Li, L. High Performance Ultra-High Molecular Weight Polyethylene Nanocomposite Separators with Excellent Rate Capabilities Designed for next-Generation Lithium-Ion Batteries. Mater. Today Phys. 2022, 23, 100626. [Google Scholar] [CrossRef]
- Wu, Y.C.; Cui, Y.H.; Jin, H.L.; Ning, C.C. Study on the Preparation and Thermal Shrinkage Properties of Nano-SiO2/UHMWPE/HDPE Blend Microporous Membranes. J. Appl. Polym. Sci. 2015, 132, 41321. [Google Scholar] [CrossRef]
- Remanan, S.; Sharma, M.; Bose, S.; Das, N.C. Recent Advances in Preparation of Porous Polymeric Membranes by Unique Techniques and Mitigation of Fouling through Surface Modification. ChemistrySelect 2018, 3, 609–633. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Gorin, Y.G.; Yeszhanov, A.B.; Kozlovskiy, A.L.; Zdorovets, M.V. Preparation of PET Track-Etched Membranes for Membrane Distillation by Photo-Induced Graft Polymerization. Mater. Chem. Phys. 2018, 205, 55–63. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.S.; Park, M.; Jang, M. Effects of Precursor Properties on the Preparation of Polyethylene Hollow Fiber Membranes by Stretching. J. Memb. Sci. 2008, 318, 201–209. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Arzhakova, O.V.; Yarysheva, L.M.; Volynskii, A.L. Biaxial Tensile Drawing of Poly(Ethylene Terephthalate) via Environmental Crazing as a Method for Creating a Porous Structure. Polymer 2018, 158, 243–253. [Google Scholar] [CrossRef]
- Kravets, L.I.; Yarmolenko, M.A.; Rogachev, A.A.; Gainutdinov, R.V.; Gilman, A.B.; Altynov, V.A.; Lizunov, N.E. Formation of Superhydrophobic Coatings on the Track-Etched Membrane Surface by the Method of Electron-Beam Deposition of Polymers in Vacuum. Inorg. Mater. Appl. Res. 2020, 11, 476–487. [Google Scholar] [CrossRef]
- Kravets, L.I.; Yarmolenko, M.A.; Gainutdinov, R.V.; Satulu, V.; Mitu, B.; Dinescu, G. Formation of Hydrophobic Polymer Coatings on the Track-Etched Membrane Surface. J. Phys. Conf. Ser. 2021, 1954, 012022. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Feng, X. Separation of Acetone–Butanol–Ethanol (ABE) from Dilute Aqueous Solutions by Pervaporation. Sep. Purif. Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Reuvers, A.J.; Smolders, C.A. Formation of Membranes by Means of Immersion Precipitation: Part II. the Mechanism of Formation of Membranes Prepared from the System Cellulose Acetate-Acetone-Water. J. Memb. Sci. 1987, 34, 67–86. [Google Scholar] [CrossRef] [Green Version]
- Reuvers, A.J.; van den Berg, J.W.A.; Smolders, C.A. Formation of Membranes by Means of Immersion Precipitation: Part, I. A Model to Describe Mass Transfer during Immersion Precipitation. J. Memb. Sci. 1987, 34, 45–65. [Google Scholar] [CrossRef]
- Young, T.H.; Cheng, L.P.; Lin, D.J.; Fane, L.; Chuang, W.Y. Mechanisms of PVDF Membrane Formation by Immersion-Precipitation in Soft (1-Octanol) and Harsh (Water) Nonsolvents. Polymer 1999, 40, 5315–5323. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A Novel Green Solvent Alternative for Polymeric Membrane Preparation via Nonsolvent-Induced Phase Separation (NIPS). J. Memb. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kinzer, K.E.; Tseng, H.S. Microporous Membrane Formation via Thermally Induced Phase Separation. I. Solid-Liquid Phase Separation. J. Memb. Sci. 1990, 52, 239–261. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous Membrane Formation via Thermally-Induced Phase Separation. II. Liquid-Liquid Phase Separation. J. Memb. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of Combined Nonsolvent and Thermally Induced Phase Separation (N-TIPS) Method for Fabricating Novel PVDF Hollow Fiber Membranes Using Mixed Diluents. J. Memb. Sci. 2019, 572, 210–222. [Google Scholar] [CrossRef]
- Jung, J.T.; Wang, H.H.; Kim, J.F.; Lee, J.; Kim, J.S.; Drioli, E.; Lee, Y.M. Tailoring Nonsolvent-Thermally Induced Phase Separation (N-TIPS) Effect Using Triple Spinneret to Fabricate High Performance PVDF Hollow Fiber Membranes. J. Memb. Sci. 2018, 559, 117–126. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.P.; Bouyer, D.; Drioli, E.; Tavajohi Hassan Kiadeh, N. Investigating the Potential of Membranes Formed by the Vapor Induced Phase Separation Process. J. Memb. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Yushkin, A.; Balynin, A.; Efimov, M.; Pochivalov, K.; Petrova, I.; Volkov, A. Fabrication of Polyacrylonitrile UF Membranes by VIPS Method with Acetone as Co-Solvent. Membranes 2022, 12, 523. [Google Scholar] [CrossRef]
- Pervin, R.; Ghosh, P.; Basavaraj, M.G. Tailoring Pore Distribution in Polymer Films via Evaporation Induced Phase Separation. RSC Adv. 2019, 9, 15593–15605. [Google Scholar] [CrossRef] [Green Version]
- Plisko, T.V.; Penkova, A.V.; Burts, K.S.; Bildyukevich, A.V.; Dmitrenko, M.E.; Melnikova, G.B.; Atta, R.R.; Mazur, A.S.; Zolotarev, A.A.; Missyul, A.B. Effect of Pluronic F127 on Porous and Dense Membrane Structure Formation via Non-Solvent Induced and Evaporation Induced Phase Separation. J. Memb. Sci. 2019, 580, 336–349. [Google Scholar] [CrossRef]
- Yu, B.; Huang, Z.; Fang, D.; Yu, S.; Fu, T.; Tang, Y.; Li, Z. Biomimetic Porous Fluoropolymer Films with Brilliant Whiteness by Using Polymerization-Induced Phase Separation. Adv. Mater. Interfaces 2022, 9, 2101485. [Google Scholar] [CrossRef]
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D Printing of Inherently Nanoporous Polymers via Polymerization-Induced Phase Separation. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.F.; Jung, J.T.; Wang, H.H.; Lee, S.Y.; Moore, T.; Sanguineti, A.; Drioli, E.; Lee, Y.M. Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS) and Stretching Methods. J. Memb. Sci. 2016, 509, 94–104. [Google Scholar] [CrossRef]
- Senatov, F.S.; Baranov, A.A.; Muratov, D.S.; Gorshenkov, M.V.; Kaloshkin, S.D.; Tcherdyntsev, V.V. Microstructure and Properties of Composite Materials Based on UHMWPE after Mechanical Activation. J. Alloys Compd. 2014, 615, S573–S577. [Google Scholar] [CrossRef]
- Fei, Z.; Ying, S.; Fan, P.; Chen, F.; Haque, E.; Zhong, M. Facile Preparation of a Crosslinked Hydrophilic UHMWPE Membrane. J. Appl. Polym. Sci. 2020, 137, 1–7. [Google Scholar] [CrossRef]
- Dittmann, J.; Maurath, J.; Bitsch, B.; Willenbacher, N. Highly Porous Materials with Unique Mechanical Properties from Smart Capillary Suspensions. Adv. Mater. 2016, 28, 1689–1696. [Google Scholar] [CrossRef]
- Leal, T.L.; Souto, K.M.; Carvalho, L.H.; Lira, L.H.; Junior, C.A.; d’Almeida, J.R.M. Cold Plasma Modification Effects on the Water Flow through Sintered UHMWPE Membranes. Polym.—Plast. Technol. Eng. 2009, 48, 136–140. [Google Scholar] [CrossRef]
- Joshi, R.; Friedrich, J.; Krishna-Subramanian, S. Surface Modification of Ultra-High Molecular Weight Polyethylene Membranes Using Underwater Plasma Polymerization. Plasma Chem. Plasma Process. 2013, 33, 921–940. [Google Scholar] [CrossRef]
- Otto, C.; Handge, U.A.; Georgopanos, P.; Aschenbrenner, O.; Kerwitz, J.; Abetz, C.; Metze, A.L.; Abetz, V. Porous UHMWPE Membranes and Composites Filled with Carbon Nanotubes: Permeability, Mechanical, and Electrical Properties. Macromol. Mater. Eng. 2017, 302, 1–14. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; He, G. Fabrication of Self-Lubricating Porous UHMWPE with Excellent Mechanical Properties and Friction Performance via Rotary Sintering. Polymers 2020, 12, 1335. [Google Scholar] [CrossRef]
- Gomes, N.D.S.; Pontes Junior, A.L.; Leite, R.C.N.; Carvalho, L.H. Evaluation of Organoclay Addition in Surface Modified Sintered Uhmwpe Membranes for Oil Separation. Mater. Sci. Forum 2015, 820, 349–354. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Senatov, F.S.; Anisimova, N.Y.; Kiselevskiy, M.V.; Zalepugin, D.Y.; Chernyshova, I.V.; Tilkunova, N.A.; Kaloshkin, S.D. Multilayer Porous UHMWPE Scaffolds for Bone Defects Replacement. Mater. Sci. Eng. C 2017, 73, 366–372. [Google Scholar] [CrossRef]
- Tan, W.S.; Chua, C.K.; Chong, T.H.; Fane, A.G.; Jia, A. 3D Printing by Selective Laser Sintering of Polypropylene Feed Channel Spacers for Spiral Wound Membrane Modules for the Water Industry. Virtual Phys. Prototyp. 2016, 11, 151–158. [Google Scholar] [CrossRef]
- Georlette, P.; Leva, J. Composition Comprising a Vinylidene Fluoride Polymer and a Blowing Agent. U.S. Patent 4,425,443, 10 January 1984. [Google Scholar]
- Dickey, C.A.; Mcdaniel, J.E. Method of producing spherical thermoplastic particles. U.S. Patent 3,896,196, 22 July 1975. [Google Scholar]
- Deplancke, T.; Lame, O.; Rousset, F.; Aguili, I.; Seguela, R.; Vigier, G. Diffusion versus Cocrystallization of Very Long Polymer Chains at Interfaces: Experimental Study of Sintering of UHMWPE Nascent Powder. Macromolecules 2014, 47, 197–207. [Google Scholar] [CrossRef]
- Salimon, A.I.; Statnik, E.S.; Zadorozhnyy, M.Y.; Senatov, F.S.; Zherebtsov, D.D.; Safonov, A.A.; Korsunsky, A.M. Porous Open-Cell UHMWPE: Experimental Study of Structure and Mechanical Properties. Materials 2019, 12, 2195. [Google Scholar] [CrossRef] [Green Version]
- Deplancke, T.; Lame, O.; Rousset, F.; Seguela, R.; Vigier, G. Mechanisms of Chain Reentanglement during the Sintering of UHMWPE Nascent Powder: Effect of Molecular Weight. Macromolecules 2015, 48, 5328–5338. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Tang, X.; Ma, Y.; Fan, X.; Li, W.; Yu, W.; Wang, J.; Yang, Y. Contribution of the Initially Entangled State and Particle Size to the Sintering Kinetics of UHMWPE. Macromolecules 2022, 55, 1310–1320. [Google Scholar] [CrossRef]
- Ren, P.G.; Di, Y.Y.; Zhang, Q.; Li, L.; Pang, H.; Li, Z.M. Composites of Ultrahigh-Molecular-Weight Polyethylene with Graphene Sheets and/or MWCNTs with Segregated Network Structure: Preparation and Properties. Macromol. Mater. Eng. 2012, 297, 437–443. [Google Scholar] [CrossRef]
- Rastogi, S.; Kurelec, L.; Lemstra, P.J. Chain Mobility in Polymer Systems: On the Borderline between Solid and Melt. 2. Crystal Size Influence in Phase Transition and Sintering of Ultrahigh Molecular Weight Polyethylene via the Mobile Hexagonal Phase. Macromolecules 1998, 31, 5022–5031. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Q.; Wang, K.; Zhu, B.H.; Su, W.; Li, J.F.; Fu, Q. Pursuit of the Correlation between Yield Strength and Crystallinity in Sintering-Molded UHMWPE. Polymer 2021, 215, 123352. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Qiu, Z.; Cui, Y.; Cong, C.; Meng, X.; Ye, H.; Zhou, Q. Effects of the Sintering Temperature on the Superior Cryogenic Toughness of Ultra-High Molecular Weight Polyethylene (UHMWPE). Chem. Eng. J. 2022, 444, 136366. [Google Scholar] [CrossRef]
- Han, K.S.; Wallace, J.F.; Truss, R.W.; Geil, P.H. Powder Compaction, Sintering, and Rolling of Ultra High Molecular Weight Polyethylene and Its Composites. J. Macromol. Sci. Part B Phys. 2006, 19, 313–349. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Rukhlya, E.G.; Yarysheva, L.M.; Bagrov, D.V.; Volynskii, A.L.; Bakeev, N.F. The Structural Evolution of High-Density Polyethylene during Crazing in Liquid Medium. Eur. Polym. J. 2015, 66, 458–469. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, F.; Meng, L.; Chen, X.; Lv, F.; Zhang, Q.; Li, L. Microbuckling: A Possible Mechanism to Trigger Nonlinear Instability of Semicrystalline Polymer. Polymer 2018, 154, 48–54. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Meng, L.; Chen, X.; Lv, F.; Zhang, Q.; Li, L. Stress-Induced Microphase Separation of Interlamellar Amorphous Phase in Hard-Elastic Isotactic Polypropylene Film. Polymer 2018, 148, 79–92. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Shan, G.; Bao, Y.; Wang, W.; Pan, P. Stretch-Induced α-to-β Crystal Transition and Lamellae Structural Evolution of Poly(Butylene Adipate- Ran-Terephthalate) Aliphatic-Aromatic Copolyester. Macromolecules 2019, 52, 1334–1347. [Google Scholar] [CrossRef]
- Habumugisha, J.C.; Feng, S.; Iqbal, O.; Lin, Y.; An, M.; Meng, L.; Wang, D.; Chen, W.; Li, L. Stretch-Induced Structural Evolution of Pre-Oriented Isotactic Polypropylene Films: An in-Situ Synchrotron Radiation SAXS/WAXS Study. Polymer 2021, 214, 123234. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, Y.; Wang, Y.; Zhang, C.; Wang, D. Tensile Deformation Mechanism of Propylene-1-Butene Random Copolymer: The Role of Initial Crystalline Morphology. Polymer 2021, 236, 124310. [Google Scholar] [CrossRef]
- Kida, T.; Hiejima, Y.; Nitta, K.H.; Yamaguchi, M. Evaluation of Microscopic Structural Changes during Strain Hardening of Polyethylene Solids Using In Situ Raman, SAXS, and WAXD Measurements under Step-Cycle Test. Polymer 2022, 250, 124869. [Google Scholar] [CrossRef]
- Iqbal, O.; Guo, H.; Chen, W. Structural Origin of Double Yielding: The Critical Role of Crystallite Aggregate Heterogeneity. Macromolecules 2021, 54, 8381–8392. [Google Scholar] [CrossRef]
- Bartczak, Z.; Vozniak, A. Deformation Instabilities and Lamellae Fragmentation during Deformation of Cross-Linked Polyethylene. Polymers 2019, 11, 1954. [Google Scholar] [CrossRef] [Green Version]
- Bartczak, Z.; Vozniak, A. WAXS/SAXS Study of Plastic Deformation Instabilities and Lamellae Fragmentation in Polyethylene. Polymer 2019, 177, 160–177. [Google Scholar] [CrossRef]
- Cai, Z.; Bao, H.; Zhu, C.; Zhu, S.; Huang, F.; Shi, J.; Hu, J.; Zhou, Q. Structure Evolution of Polyamide 1212 during the Uniaxial Stretching Process: In Situ Synchrotron Wide-Angle X-Ray Diffraction and Small-Angle X-Ray Scattering Analysis. Ind. Eng. Chem. Res. 2016, 55, 7621–7627. [Google Scholar] [CrossRef]
- Chang, B.; Schneider, K.; Xiang, F.; Vogel, R.; Roth, S.; Heinrich, G. Critical Strains for Lamellae Deformation and Cavitation during Uniaxial Stretching of Annealed Isotactic Polypropylene. Macromolecules 2018, 51, 6276–6290. [Google Scholar] [CrossRef]
- Fu, L.; Lu, Y.; Jiang, Z.; Chen, R.; Men, Y. Towards a Better Understanding of the Crystallization and Melting Behaviors of High-Density Polyethylene Samples Prepared from Quasi-Isothermal and Stretching Oriented Localized Melts. Polymer 2021, 218, 123485. [Google Scholar] [CrossRef]
- Zhang, Q.; Lan, L.; Zheng, Z.; Liu, P.; Wu, H.; Guo, S.; Lin, C.; He, G. Constructing Highly Oriented and Condensed Shish-Kebab Crystalline Structure of HDPE/UHMWPE Blends via Intense Stretching Process: Achieving High Mechanical Properties and in-Plane Thermal Conductivity. Polymer 2022, 241, 124532. [Google Scholar] [CrossRef]
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of Polymerization Conditions on Melting Kinetics of Low Entangled Uhmwpe and Its Implications on Mechanical Properties. Macromolecules 2014, 47, 4750–4760. [Google Scholar] [CrossRef]
- Chen, L.; Deng, B.; Li, X.; Wang, Z. Structural Evolution of UHMWPE Gel Fibers as High Degree Plasticized System during Stretching: An in-Situ Wide and Small Angle X-Ray Scattering Study. Polymer 2022, 255, 125149. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Nishitsuji, S.; Kurose, T.; Ito, H. Structural Formation of UHMWPE Film Tracked by Real-Time Retardation Measurements during Uniaxial/Biaxial Stretching. Mater. 2018, 11, 2292. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Mao, Y.; Ma, H.; Zuo, F.; Hsiao, B.S.; Chu, B. An In-Situ X-Ray Scattering Study during Uniaxial Stretching of Ionic Liquid/Ultra-High Molecular Weight Polyethylene Blends. Polymer 2011, 52, 4610–4618. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, C.; Gong, J.; Ma, J.; Xu, J. Transition from Shish-Kebab to Fibrillar Crystals during Ultra-High Hot Stretching of Ultra-High Molecular Weight Polyethylene Fibers: In Situ Small and Wide Angle X-Ray Scattering Studies. Eur. Polym. J. 2015, 73, 127–136. [Google Scholar] [CrossRef]
- Deng, B.; Chen, L.; Li, X.; Wang, Z. Influence of Prereserved Shish Crystals on the Structural Evolution of Ultrahigh-Molecular Weight Polyethylene Films during the Hot Stretching Process. Macromolecules 2022, 55, 4600–4613. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, C.; Gong, J.; Yang, S.; Ma, J.; Xu, J. Lamellae Break Induced Formation of Shish-Kebab during Hot Stretching of Ultra-High Molecular Weight Polyethylene Precursor Fibers Investigated by in Situ Small Angle X-Ray Scattering. Polymer 2014, 55, 4299–4306. [Google Scholar] [CrossRef]
- Steeman, R.J.M.; Jongedijk, M. Process for Making a Polymeric Film. WO2013004640A1, 29 June 2012. [Google Scholar]
- De Weijer, A.P.; Van De Hee, H.; Peters, M.W.M.G.; Rastogi, S.; Wang, B. Polyethylene Film with High Tensile Strength and High Tensile Energy to Break. WO2009007045A1, 2 June 2008. [Google Scholar]
- Zhang, Z.X.; Zhang, T.; Zhang, X.; Xin, Z.; Deng, X.; Prakashan, K. Mechanically Stable Superhydrophobic Polymer Films by a Simple Hot Press Lamination and Peeling Process. RSC Adv. 2016, 6, 12530–12536. [Google Scholar] [CrossRef]
- Michler, G.H.; Seydewitz, V.; Buschnakowski, M.; Myasnikowa, L.P.; Ivan’Kova, E.M.; Marikhin, V.A.; Boiko, Y.M.; Goerlitz, S. Correlation among Powder Morphology, Compactability, and Mechanical Properties of Consolidated Nascent UHMWPE. J. Appl. Polym. Sci. 2010, 118, 866–875. [Google Scholar] [CrossRef]
- Harding, K.C.; Weedon, G.C.; Owen, L. Wide Ultra High Molecular Weight Polyethylene Sheet and Method of Manufacture. U.S. Patent US7964266B2, 13 April 2007. [Google Scholar]
- Dam Backer, J.A.; Kranz, B.C.; Slager, B. Process for Producing High Strength Polyethylene Film. U.S. Patent US8188207B2, 21 April 2009. [Google Scholar]
- Arzhakova, O.V.; Nazarov, A.I.; Solovei, A.R.; Dolgova, A.A.; Kopnov, A.Y.; Chaplygin, D.K.; Tyubaeva, P.M.; Yarysheva, A.Y. Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects. Membranes 2021, 11, 834. [Google Scholar] [CrossRef]
- Uehara, H.; Tamura, T.; Hashidume, K.; Tanaka, H.; Yamanobe, T. Non-Solvent Processing for Robust but Thin Membranes of Ultra-High Molecular Weight Polyethylene. J. Mater. Chem. A 2014, 2, 5252–5257. [Google Scholar] [CrossRef]
- Castro, A.J. Method for making microporous products. US Patent US4247498A, 27 January 1981. [Google Scholar]
- Hiatt, W.C.; Vitzthum, G.H.; Wagener, K.B.; Gerlach, K.; Josefiak, C. Microporous membranes via upper critical temperature phase separation. ACS Symp. Ser. 1985, 269, 229–244. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D.R. Microporous Membrane Formation via Thermally-Induced Phase Separation. III. Effect of Thermodynamic Interactions on the Structure of Isotactic Polypropylene Membranes. J. Memb. Sci. 1991, 64, 13–29. [Google Scholar] [CrossRef]
- Lim, G.B.A.; Kim, S.S.; Ye, Q.; Wang, Y.F.; Lloyd, D.R. Microporous Membrane Formation via Thermally-Induced Phase Separation. IV. Effect of Isotactic Polypropylene Crystallization Kinetics on Membrane Structure. J. Memb. Sci. 1991, 64, 31–40. [Google Scholar] [CrossRef]
- Kim, S.S.; Lim, G.B.A.; Alwattari, A.A.; Wang, Y.F.; Lloyd, D.R. Microporous Membrane Formation via Thermally-Induced Phase Separation. V. Effect of Diluent Mobility and Crystallization on the Structure of Isotactic Polypropylene Membranes. J. Memb. Sci. 1991, 64, 41–53. [Google Scholar] [CrossRef]
- Alwattari, A.A.; Lloyd, D.R. Microporous Membrane Formation via Thermally-Induced Phase Separation. VI. Effect of Diluent Morphology and Relative Crystallization Kinetics on Polypropylene Membrane Structure. J. Memb. Sci. 1991, 64, 55–67. [Google Scholar] [CrossRef]
- McGuire, K.S.; Lloyd, D.R.; Lim, G.B.A. Microporous Membrane Formation via Thermally-Induced Phase Separation. VII. Effect of Dilution, Cooling Rate, and Nucleating Agent Addition on Morphology. J. Memb. Sci. 1993, 79, 27–34. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhou, B.; Wang, L.; Lin, Y.; Zhang, C.; Wang, X. A Criterion of Diluent Selection for the Polymeric Membrane Formation via Thermally Induced Phase Separation Process Based on Hansen Solubility Parameter Theory. Adv. Membr. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Kim, J.F.; Kim, J.H.; Lee, Y.M.; Drioli, E. Thermally Induced Phase Separation and Electrospinning Methods for Emerging Membrane Applications: A Review. AIChE J. 2016, 62, 461–490. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Xu, Z.; Wei, Y.; Yang, H. Formation of Microporous Polymeric Membranes via Thermally Induced Phase Separation: A Review. Front. Chem. Sci. Eng. 2016, 10, 57–75. [Google Scholar] [CrossRef]
- Tsai, F.J.; Torkelson, J.M. Roles of Phase Separation Mechanism and Coarsening in the Formation of Poly(Methyl Methacrylate) Asymmetric Membranes. Macromolecules 1990, 23, 775–784. [Google Scholar] [CrossRef]
- Pan, J.; Xiao, C.; Huang, Q.; Wang, C.; Liu, H. Fabrication and Properties of Poly(Ethylene Chlorotrifluoroethylene) Membranes via Thermally Induced Phase Separation (TIPS). RSC Adv. 2015, 5, 45249–45257. [Google Scholar] [CrossRef]
- Tang, Y.H.; He, Y.D.; Wang, X.L. Effect of Adding a Second Diluent on the Membrane Formation of Polymer/Diluent System via Thermally Induced Phase Separation: Dissipative Particle Dynamics Simulation and Its Experimental Verification. J. Memb. Sci. 2012, 409–410, 164–172. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Li, Y.; Li, J. PVDF Membrane Formation via Thermally Induced Phase Separation. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 99–104. [Google Scholar] [CrossRef]
- Sun, H.; Rhee, K.B.; Kitano, T.; Mah, S.I. High-Density Polyethylene (HDPE) Hollow Fiber Membrane via Thermally Induced Phase Separation. I. Phase Separation Behaviors of HDPE-Liquid Paraffin (LP) Blends and Its Influence on the Morphology of the Membrane. J. Appl. Polym. Sci. 1999, 73, 2135–2142. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the Non-Solvent Induced Phase Separation (NIPS) Effect during the Fabrication of Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Papkov, S.P. Physico-Chemical Basis of the Polymer Solutions Processing; Khimiya: Moscow, Russia, 1971; pp. 89–99. (In Russian) [Google Scholar]
- Zhao, Q.; Xie, R.; Luo, F.; Faraj, Y.; Liu, Z.; Ju, X.J.; Wang, W.; Chu, L.Y. Preparation of High Strength Poly(Vinylidene Fluoride) Porous Membranes with Cellular Structure via Vapor-Induced Phase Separation. J. Memb. Sci. 2018, 549, 151–164. [Google Scholar] [CrossRef]
- Pan, J.; Xiao, C.; Huang, Q.; Liu, H.; Hu, J. ECTFE Porous Membranes with Conveniently Controlled Microstructures for Vacuum Membrane Distillation. J. Mater. Chem. A 2015, 3, 23549–23559. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S. Molecular Elucidation of Morphology and Mechanical Properties of PVDF Hollow Fiber Membranes from Aspects of Phase Inversion, Crystallization, and Rheology. In Hollow Fiber Membranes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 333–360. [Google Scholar] [CrossRef]
- Mehta, R.H.; Kalika, D.S. Characteristics of Poly (Ether Ether Ketone) Microporous Membranes Prepared via Thermally Induced Phase Separation (TIPS). J. Appl. Polym. Sci. 1997, 66, 2347–2355. [Google Scholar] [CrossRef]
- Tang, N.; Jia, Q.; Zhang, H.; Li, J.; Cao, S. Preparation and Morphological Characterization of Narrow Pore Size Distributed Polypropylene Hydrophobic Membranes for Vacuum Membrane Distillation via Thermally Induced Phase Separation. Desalination 2010, 256, 27–36. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Lebedeva, T.N.; Ilyasova, A.N.; Yurov, M.Y.; Golovanov, R.Y.; Artemov, V.V.; Volkov, V.V.; Ezhov, A.A.; Volkov, A.V.; et al. Thermally Induced Phase Separation in Semicrystalline Polymer Solutions: How Does the Porous Structure Actually Arise? Mater. Today Commun. 2021, 28, 102558. [Google Scholar] [CrossRef]
- Smith, P.; Pennings, A.J. Eutectic Crystallization of Pseudo Binary Systems of Polyethylene and High Melting Diluents. Polymer 1974, 15, 413–419. [Google Scholar] [CrossRef]
- Wittmann, J.C.; Manley, R.S.J. Polymer–Monomer Binary Mixtures. Eutectic Crystallization of Poly(Ethylene Oxide)–Trioxane Mixtures. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 2277–2280. [Google Scholar] [CrossRef]
- Hagström, B. Mechanical Properties and Phase Diagrams of Alloys of High Density Polyethylene with Some Low Molecular Weight Organic Compounds. J. Mater. Sci. 1985, 20, 3906–3916. [Google Scholar] [CrossRef]
- Vippagunta, S.R.; Wang, Z.; Hornung, S.; Krill, S.L. Factors Affecting the Formation of Eutectic Solid Dispersions and Their Dissolution Behavior. J. Pharm. Sci. 2007, 96, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Kuttich, B.; Matt, A.; Appel, C.; Stühn, B. X-ray Scattering Study on the Crystalline and Semi-Crystalline Structure of Water/PEG Mixtures in Their Eutectic Phase Diagram. Soft Matter 2020, 16, 10260–10267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lesser, A.J.; McCarthy, T.J. Locally Anisotropic Porous Materials from Polyethylene and Crystallizable Diluents. Macromolecules 2009, 42, 8827–8834. [Google Scholar] [CrossRef]
- Clark, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of Terms Relating to Phase Transitions of the Solid State (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef] [Green Version]
- Pochivalov, K.V.; Basko, A.V.; Denisova, Y.I.; Shandryuk, G.A.; Ezhov, A.A.; Artemov, V.V.; Kudryavtsev, Y.V. Isotactic Polypropylene–1,2,4,5-Tetrachlorobenzene: Porous Bodies via Thermally Induced Phase Separation. J. Therm. Anal. Calorim. 2019, 138, 2481–2489. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Lebedeva, T.N.; Ilyasova, A.N.; Golovanov, R.Y.; Yurov, M.Y.; Shandryuk, G.A.; Artemov, V.V.; Ezhov, A.A.; Kudryavtsev, Y.V. Analysis of the Thermal Behavior of Polypropylene-Camphor Mixtures for Understanding the Pathways to Polymeric Membranes via Thermally Induced Phase Separation. J. Phys. Chem. B 2019, 123, 10533–10546. [Google Scholar] [CrossRef]
- Basko, A.V.; Pochivalov, K.V.; Bazanov, A.V.; Shandryuk, G.A.; Ezhov, A.A.; Artemov, V.V.; Kudryavtsev, Y.V. Phase Diagram of the Low-Density Polyethylene—Dimethyl Terephthalate System: A New Topology. Thermochim. Acta 2020, 684, 178499. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Kudryavtsev, Y.V. Binary Mixtures of Semicrystalline Polymers with Low-Molecular-Mass Compounds: Thermal Behaviour and Phase Structure. Russ. Chem. Rev. 2020, 89, 311–338. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Lebedeva, T.N.; Ilyasova, A.N.; Guseinov, S.S.; Kudryavtsev, Y.V. Thermodynamically-Informed Approach to the Synthesis of 3D Printing Powders from the Mixtures of Polyamide 12 with Benzyl Alcohol. Powder Technol. 2022, 408, 117685. [Google Scholar] [CrossRef]
- Richards, R.B. The phase equilibria between a crystalline polymer and solvents. Trans. Farad. Soc. 1946, 42, 10–28. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Lebedeva, T.N.; Antina, L.A.; Golovanov, R.Y.; Artemov, V.V.; Ezhov, A.A.; Kudryavtsev, Y.V. Low-Density Polyethylene-Thymol: Thermal Behavior and Phase Diagram. Thermochim. Acta 2018, 659, 113–120. [Google Scholar] [CrossRef]
- Basko, A.V.; Lebedeva, T.N.; Yurov, M.Y.; Pochivalov, K.V. The Effect of Physical State of Thymol on the Duration of Its Release from the Mixture with a Semicrystalline Polymer: Thermodynamic Aspects and Kinetics of the Process. Polym. Sci.—Ser. A 2021, 64, 10–18. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Lebedeva, T.N.; Ilyasova, A.N.; Basko, A.V.; Kudryavtsev, Y.V. A New Look at the Semicrystalline Polymer—Liquid Systems: Phase Diagrams Low-Density Polyethylene—n-Alkanes. Fluid Phase Equilib. 2018, 471, 1–7. [Google Scholar] [CrossRef]
- Il’yasova, A.N.; Lebedeva, T.N.; Shilov, A.N.; Pochivalov, K.V. Estimation of the Thermodynamic Quality of Alkylbenzenes with Respect to Low Density Polyethylene. Polym. Sci. Ser. A 2018, 59, 839–843. [Google Scholar] [CrossRef]
- Staub, M.C.; Li, C.Y. Polymer Crystallization at Liquid-Liquid Interface. Polym. Cryst. 2018, 1, e10045. [Google Scholar] [CrossRef]
- McGuire, K.S.; Laxminarayan, A.; Lloyd, D.R. A Simple Method of Extrapolating the Coexistence Curve and Predicting the Melting Point Depression Curve from Cloud Point Data for Polymer-Diluent Systems. Polymer 1994, 35, 4404–4407. [Google Scholar] [CrossRef]
- Song, Z.; Xing, M.; Zhang, J.; Li, B.; Wang, S. Determination of Phase Diagram of a Ternary PVDF/γ-BL/DOP System in TIPS Process and Its Application in Preparing Hollow Fiber Membranes for Membrane Distillation. Sep. Purif. Technol. 2012, 90, 221–230. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, P. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yin, J.; Lv, R.; Du, Q.; Zhong, W. Preparation and properties of MPEG-grafted EAA membranes via thermally induced phase separation. J. Membr. Sci. 2005, 267, 90–98. [Google Scholar] [CrossRef]
- Hu, W.; Frenkel, D. Polymer Crystallization Driven by Anisotropic Interactions. Adv. Polym. Sci. 2005, 191, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Doong, S.J.; Ho, W.S.W. Sorption of Organic Vapors in Polyethylene. Ind. Eng. Chem. Res. 1991, 30, 1351–1361. [Google Scholar] [CrossRef]
- Krajakova, L.; Laskova, M.; Chmelar, J.; Jindrova, K.; Kosek, J. Sorption of Liquid Diluents in Polyethylene: Comprehensive Experimental Data for Slurry Polymerization. Ind. Eng. Chem. Res. 2019, 58, 7037–7043. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Chen, G.; Lin, Y.; Wang, X. Formation of Microporous Membrane of Isotactic Polypropylene in Dibutyl Phthalate-Soybean Oil via Thermally Induced Phase Separation. J. Appl. Polym. Sci. 2007, 105, 2000–2007. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mat. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, J.; Wang, X. Effect of diluents on the crystallization behavior of poly(4-methyl-1-pentene) and membrane morphology via thermally induced phase separation. J. Appl. Polym. Sci. 2008, 108, 1348–1355. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, J.; Wang, X.; Tao, H.; Ge, L. Formation of Poly(Vinylidene Fluoride) (PVDF) Membranes via Thermally Induced Phase Separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Li, X.; Lu, X. Morphology of Polyvinylidene Fluoride and Its Blend in Thermally Induced Phase Separation Process. J. Appl. Polym. Sci. 2006, 101, 2944–2952. [Google Scholar] [CrossRef]
- Zhou, B.; Tang, Y.; Li, Q.; Lin, Y.; Yu, M.; Xiong, Y.; Wang, X. Preparation of Polypropylene Microfiltration Membranes via Thermally Induced (Solid–Liquid or Liquid–Liquid) Phase Separation Method. J. Appl. Polym. Sci. 2015, 132, 42490. [Google Scholar] [CrossRef]
- Ma, W.; Chen, S.; Zhang, J.; Wang, X. Kinetics of Thermally Induced Phase Separation in the PVDF Blend/Methyl Salicylate System and Its Effect on Membrane Structures. J. Macromol. Sci. Part B. Phys. 2011, 50, 1–15. [Google Scholar] [CrossRef]
- Ma, W.; Chen, S.; Zhang, J.; Wang, X.; Miao, W. Morphology and crystallization behavior of poly(vinylidene fluoride)/poly(methyl methacrylate)/methyl salicylate, and benzophenone systems via thermally induced phase separation. J. Polym. Sci. Part B. Polym. Phys. 2009, 47, 248–260. [Google Scholar] [CrossRef]
- Du, F.; Yener, H.E.; Hillrichs, G.; Boldt, R.; Androsch, R. Crystallization-Induced Polymer Scaffold Formation in the Polymer/Drug Delivery System Poly(l-lactic acid)/Ethyl Butylacetylaminopropionate (PLLA/IR3535). Biomacromolecules 2021, 22, 3950–3959. [Google Scholar] [CrossRef]
- Mehta, R.H.; Madsen, D.A.; Kalika, D.S. Microporous membranes based on poly(ether ether ketone) via thermally-induced phase separation. J. Membr. Sci. 1995, 107, 93–106. [Google Scholar] [CrossRef]
- Kanno, T.; Uyama, H. Unique Leafy Morphology of Poly(Lactic Acid) Monoliths Controlled via Novel Phase Separation Technology. RSC Adv. 2017, 7, 33726–33732. [Google Scholar] [CrossRef] [Green Version]
- Zia, Q.; Androsch, R.; Radusch, H.J.; Piccarolo, S. Morphology, Reorganization and Stability of Mesomorphic Nanocrystals in Isotactic Polypropylene. Polymer 2006, 47, 8163–8172. [Google Scholar] [CrossRef]
- Matsuyama, H.; Maki, T.; Teramoto, M.; Asano, K. Effect of Polypropylene Molecular Weight on Porous Membrane Formation by Thermally Induced Phase Separation. J. Memb. Sci. 2002, 204, 323–328. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; Chang, H.; Wang, S. Effect of Diluent on the Morphology and Performance of IPP Hollow Fiber Microporous Membrane via Thermally Induced Phase Separation. Chinese J. Chem. Eng. 2006, 14, 394–397. [Google Scholar] [CrossRef]
- Matsuyama, H.; Teramoto, M.; Kudari, S.; Kitamura, Y. Effect of Diluents on Membrane Formation via Thermally Induced Phase Separation. J. Appl. Polym. Sci. 2001, 82, 169–177. [Google Scholar] [CrossRef]
- Zhang, C.F.; Zhu, B.K.; Ji, G.L.; Xu, Y.Y. Supercritical Carbon Dioxide Extraction in Membrane Formation by Thermally Induced Phase Separation. J. Appl. Polym. Sci. 2007, 103, 1632–1639. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, Z.; Yu, J.; Tang, G.; Chen, L.; Zhu, J. Preparation of Ultra-High Molecular Weight Polyethylene Microporous Membranes via Thermally Induced Phase Separation. Adv. Mater. Res. 2012, 476–478, 2363–2367. [Google Scholar] [CrossRef]
- Liu, S.; Yu, W.; Zhou, C. Molecular Self-Assembly Assisted Liquid-Liquid Phase Separation in Ultrahigh Molecular Weight Polyethylene/Liquid Paraffin/Dibenzylidene Sorbitol Ternary Blends. Macromolecules 2013, 46, 6309–6318. [Google Scholar] [CrossRef]
- Zhao, C.; He, J.; Li, J.; Tong, J.; Xiong, J. Preparation and Properties of UHMWPE Microporous Membrane for Lithium Ion Battery Diaphragm. IOP Conf. Ser. Mater. Sci. Eng. 2018, 324, 012089. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wang, X.; Yu, J.; Wang, Y.; Zhu, J.; Hu Liu, Z.R.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z.; et al. A Novel Approach to Design Nanoporous Polyethylene/Polyester Composite Fabric via TIPS for Human Body Cooling. Macromol. Mater. Eng. 2018, 303, 1700456. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Surface Modification of UHMWPE/Fabric Composite Membrane via Self-Polymerized Polydopamine Followed by MPEG-NH2 Immobilization. J. Appl. Polym. Sci. 2018, 135, 46428. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Lin, Y.; Chen, X.; Qian, Q.; Chen, Q.; Yu, D.; Chen, X. Sensitive Phase Separation Behavior of Ultra-High Molecular Weight Polyethylene in Polybutene. Polym. Test. 2020, 81, 106243. [Google Scholar] [CrossRef]
- Wang, X.; Mu, Y.; Zhao, G.; Gao, J.; Zhou, Y.; Cheng, Z. The Effect of Stretching on UHWMPE Microporous Film by Thermally Induced Phase Separation Method. Mater. Sci. Forum 2020, 993, 906–914. [Google Scholar] [CrossRef]
- Liu, R.; Liu, S.; Yu, J.; Zhang, W.; Dai, J.; Zhang, Y.; Zhang, G. The Construction of a Hydrophilic Inorganic Layer Enables Mechanochemically Robust Super Antifouling UHMWPE Composite Membrane Surfaces. Polymers 2020, 12, 569. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Peng, M.; Qiao, F.; Zhang, J.L. Preparation of Microporous Ultra High Molecular Weight Polyethylene (UHMWPE) by Thermally Induced Phase Separation of a UHMWPE/Liquid Paraffin Mixture. Chinese J. Polym. Sci. (English Ed.) 2008, 26, 653–657. [Google Scholar] [CrossRef]
- Liu, S.; Yu, W.; Zhou, C. Tuning the Water Permeability of Ultra-High Molecular Weight Polyethylene Microporous Membrane by Molecular Self-Assembly and Flow Field. Polymer 2014, 55, 2113–2124. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, B.; He, D. Crystallization and Microporous Membrane Properties of Ultrahigh Molecular Weight Polyethylene with Dibenzylidene Sorbitol. J. Appl. Polym. Sci. 2014, 131, 8714–8721. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Development and Evaluation of UHMWPE/Woven Fabric Composite Microfiltration Membranes via Thermally Induced Phase Separation. RSC Adv. 2016, 6, 90701–90710. [Google Scholar] [CrossRef]
- Sheng, L.; Du, Y.; Zhang, H.; Chen, Z.; Pan, J.; Wang, T.; Huang, X.; He, J. Effects of Cooling Process on the Solid–Liquid Phase Separation Process in Ultra-High-Molecular-Weight Polyethylene/Liquid Paraffin Blends. Polym. Bull. 2020, 77, 165–181. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, L.; Zhang, H.; Li, M.L.; Xu, R.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; et al. Investigation on the Solid–Liquid (S–L) Phase Separation of the PE/LP Blend with Different Molecular Weight Polyethylene. Polym. Bull. 2022, 79, 1521–1534. [Google Scholar] [CrossRef]
- Sbriglia, G.A. Method for Producing Porous Articles from Ultra High Molecular Weight Polyethylene. U.S. Patent US9926416B2, 30 January 2014. [Google Scholar]
- Lopatin, G.; Yen, L.Y. Microporous Membranes of Ultrahigh Molecular Weight Polyethylene. U.S. Patent US4778601A, 18 October 1984. [Google Scholar]
- Pluyter, P.B.; Smith, P.; Barbara, S.; Van Unen, L.H.T.; Rutten, H.J. J Process of Making Microporous Films of UHMWPE. U.S. Patent US5248461A, 28 September 1991. [Google Scholar]
- Li, N.; Xiao, C. Preparation and Properties of UHMWPE/SiO2 Hybrid Hollow Fibre Membranes via Thermally Induced Phase Separation-Stretching Method. Iran. Polym. J. 2009, 18, 479–489. [Google Scholar]
- Li, N.; Liu, F.; Lu, Q.; Shi, Y.; Xiao, C.; Cheng, B. The Preparation and Study of Poly(Vinylidene Fluoride)/Ultrahigh-Molecular-Weight Polyethylene/SiO2 Hollow Fiber Membrane with Network Enhanced Structure. React. Funct. Polym. 2016, 109, 64–69. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Chen, M.; Li, C.; Wu, H.; Guo, S. Constructing Tunable Bimodal Porous Structure in Ultrahigh Molecular Weight Polyethylene Membranes with Enhanced Water Permeance and Retained Rejection Performance. J. Memb. Sci. 2021, 619, 118778. [Google Scholar] [CrossRef]
- Li, N.; Xiao, C.; Zhang, Z. Effect of Polyethylene Glycol on the Performance of Ultrahigh-Molecular-Weight Polyethylene Membranes. J. Appl. Polym. Sci. 2010, 117, 720–728. [Google Scholar] [CrossRef]
- Li, N.; Xiao, C.; Mei, S.; Zhang, S. The Multi-Pore-Structure of Polymer–Silicon Hollow Fiber Membranes Fabricated via Thermally Induced Phase Separation Combining with Stretching. Desalination 2011, 274, 284–291. [Google Scholar] [CrossRef]
- Li, N.; Xiao, C.; Wang, R.; Zhang, S. The Effect of Binary Diluents on the Performance of Ultrahigh Molecular Weight Polyethylene/SiO2 Hybrid Hollow Fiber Membrane. J. Appl. Polym. Sci. 2012, 124, E169–E176. [Google Scholar] [CrossRef]
- Xu, J.; Li, N.; Zhu, Y. Preparation of Fiber Core Support UHMWPE/SiO2 Composite Hollow Fiber Membrane toward Enhancing Structure Stability and Antifouling. Polym. Eng. Sci. 2022, 62, 472–485. [Google Scholar] [CrossRef]
- Saleem, J.; Bazargan, A.; Barford, J.; Mckay, G. Application of Strong Porous Polymer Sheets for Superior Oil Spill Recovery. Chem. Eng. Technol. 2015, 38, 482–488. [Google Scholar] [CrossRef]
- Li, N.; Lu, Q.; Yin, W.; Xiao, C.; Li, J. The Structure and Properties of Poly(Vinylidene Fluoride)/Ultrahigh-Molecular -Weight Polyethylene Blend Hollow Fiber Membranes via TIPS with Mixed Diluents. J. Memb. Sci. 2020, 595, 117527. [Google Scholar] [CrossRef]
- Toquet, F.; Guy, L.; Schlegel, B.; Cassagnau, P.; Fulchiron, R. Effect of the Naphthenic Oil and Precipitated Silica on the Crystallization of Ultrahigh-Molecular-Weight Polyethylene. Polymer 2016, 97, 63–68. [Google Scholar] [CrossRef]
- Simmons, D.K.; Yaritz, J.G. Membrane Made of a Blend of UHMW Polyolefins. U.S. Patent US10615388B2, 22 March 2006. [Google Scholar]
- Shi, X.; Bin, Y.; Hou, D.; Men, Y.; Matsuo, M. Gelation/Crystallization Mechanisms of UHMWPE Solutions and Structures of Ultradrawn Gel Films. Polym. J. 2013, 46, 21–35. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, K.; Zhao, H.; Wu, Z. Rheological Behavior of Ultrahigh Molecular Weight Polyethylene Semidilute Solutions. I. Solvent Effect. J. Appl. Polym. Sci. 1989, 38, 1369–1375. [Google Scholar] [CrossRef]
- Babiker, M.; Xi, Z.; Fan, S.; Wang, G. The Effect of Gelation Time on UHMWPE Sheet Prepared by Gel and Pressure-Induced Flow Processes. Citeseer 2011, 12, 170–179. [Google Scholar]
- Fang, X.; Wyatt, T.; Hong, Y.; Yao, D. Gel Spinning of UHMWPE Fibers with Polybutene as a New Spin Solvent. Polym. Eng. Sci. 2016, 56, 697–706. [Google Scholar] [CrossRef]
- Quan, J.; Song, Q.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Atmospheric Drying UHMWPE Membranes via Multiple Stage Extractant Exchange Drying Technique. Adv. Fiber Mater. 2022, 4, 235–245. [Google Scholar] [CrossRef]
- Fan, L.L.; Quan, J.Y.; Zhang, H.; Yu, J.R.; Hu, Z.M.; Wang, Y. Preparation of Hydrophilic UHMWPE Hollow Fiber Membranes by Chemically Bounding Silica Nanoparticles. Chinese J. Polym. Sci. 2020, 39, 189–200. [Google Scholar] [CrossRef]
- Brem, A.; Lhost, O.; Tervoort, T.A. Influence of Solvent Quality and Crystallization Conditions on the Drawability of Ultra-High Molecular Weight Polyethylene Cast from Solution. Macromolecules 2020, 53, 5957–5970. [Google Scholar] [CrossRef]
- Quan, J.; Yu, J.; Wang, Y.; Hu, Z. Construction of Intrinsic Superhydrophobic Ultra-High Molecular Weight Polyethylene Composite Membrane for DCMD. J. Memb. Sci. 2022, 648, 120353. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, L.; Liu, X.; Wu, L.; Dai, K.; Liu, C.; Shen, C.; Guo, X.; Zheng, G.; Guo, Z. Superhydrophobic Shish-Kebab Membrane with Self-Cleaning and Oil/Water Separation Properties. ACS Sustain. Chem. Eng. 2018, 6, 9866–9875. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Kharitonov, A.P.; Nematulloev, S.G.; Kaloshkin, S.D.; Gorshenkov, M.V.; Chukov, D.I.; Shchetinin, I.V. Fabrication of Oriented UHMWPE Films Using Low Solvent Concentration. Mater. Des. 2017, 115, 133–137. [Google Scholar] [CrossRef]
- Smith, P.; Lemstra, P.J.; Pijpers, J.P.L.; Kiel, A.M. Ultra-Drawing of High Molecular Weight Polyethylene Cast from Solution. Colloid Polym. Sci. 1981, 259, 1070–1080. [Google Scholar] [CrossRef] [Green Version]
- Bin, Y.; Ma, L.; Adachi, R.; Kurosu, H.; Matsuo, M. Ultra-Drawing of Low Molecular Weight Polyethylene—Ultra-High Molecular Weight Polyethylene Blend Films Prepared by Gelation/Crystallization from Semi-Dilute Solutions. Polymer 2001, 42, 8125–8135. [Google Scholar] [CrossRef]
- Ding, H.; Tian, Y.; Wang, L.; Liu, B. Preparation of Ultrahigh-Molecular-Weight Polyethylene Membranes via a Thermally Induced Phase-Separation Method. J. Appl. Polym. Sci. 2007, 105, 3355–3362. [Google Scholar] [CrossRef]
- Nayak, P.; Ghosh, A.K.; Bhatnagar, N. Investigation of Solution Rheology in Electrospinning of Ultra High Molecular Weight Polyethylene. Fibers Polym. 2022, 23, 48–57. [Google Scholar] [CrossRef]
- Quan, J.; Yu, J.; Wang, Y.; Hu, Z. Oriented Shish-Kebab like Ultra-High Molecular Weight Polyethylene Membrane for Direct Contact Membrane Distillation. Sep. Purif. Technol. 2022, 290, 120847. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.; Ding, M.; Wang, C.; Lv, C.; Wang, Y.; Yang, Y.; Zhang, N.; Shi, Z.; Qian, J.; et al. Hierarchical Structure and Properties of High-Density Polyethylene (HDPE) Microporous Films Fabricated via Thermally-Induced Phase Separation (TIPS): Effect of Presence of Ultra-High Molecular Weight Polyethylene (UHMWPE). Polym. Adv. Technol. 2022, 33, 3125–3136. [Google Scholar] [CrossRef]
- Xia, L.; Xi, P.; Cheng, B. A Comparative Study of UHMWPE Fibers Prepared by Flash-Spinning and Gel-Spinning. Mater. Lett. 2015, 147, 79–81. [Google Scholar] [CrossRef]
- Young, T.H.; Lin, D.J.; Gau, J.J.; Chuang, W.Y.; Cheng, L.P. Morphology of Crystalline Nylon-610 Membranes Prepared by the Immersion-Precipitation Process: Competition between Crystallization and Liquid–Liquid Phase Separation. Polymer 1999, 40, 5011–5021. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, W.S.; Kwon, Y.K.; Lee, K.H. Liquid–Liquid Phase Separation and Crystallization Behavior of Poly(Ethylene Terephthalate)/Poly(Ether Imide) Blend. Polymer 2002, 43, 2827–2833. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishi, T. Local Phase Separation at the Growth Front of a Polymer Spherulite during Crystallization and Nonlinear Spherulitic Growth in a Polymer Mixture with a Phase Diagram. Phys. Rev. A 1989, 39, 783. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.; Mandelkern, L. Effect of Molecular Weight on the Phase Diagram of Linear Polyethylene in 1-Dodecanol. Macromolecules 1990, 23, 5356–5357. [Google Scholar] [CrossRef]
- Hassankiadeh, N.T.; Cui, Z.; Kim, J.H.; Shin, D.W.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. PVDF Hollow Fiber Membranes Prepared from Green Diluent via Thermally Induced Phase Separation: Effect of PVDF Molecular Weight. J. Memb. Sci. 2014, 471, 237–246. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, D.Q.; Zhu, Z.Q. Thermodynamics of Aqueous Two-Phase Systems—the Effect of Polymer Molecular Weight on Liquid–Liquid Equilibrium Phase Diagrams by the Modified NRTL Model. Fluid Phase Equilib. 1998, 147, 25–43. [Google Scholar] [CrossRef]
- Ji, G.L.; Du, C.H.; Zhu, B.K.; Xu, Y.Y. Preparation of Porous PVDF Membrane via Thermally Induced Phase Separation with Diluent Mixture of DBP and DEHP. J. Appl. Polym. Sci. 2007, 105, 1496–1502. [Google Scholar] [CrossRef]
- Li, N.; Xiao, C. Effect of the Preparation Conditions on the Permeation of Ultrahigh-Molecular-Weight Polyethylene/Silicon Dioxide Hybrid Membranes. J. Appl. Polym. Sci. 2010, 117, 2817–2824. [Google Scholar] [CrossRef]
- Matsuo, M.; Manley, R.S.J. Ultradrawing at Room Temperature of High Molecular Weight Polyethylene. Macromolecules 1982, 15, 985–987. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Guo, Y.; Mi, H.Y.; He, P.; Zheng, G.; Liu, C.; Shen, C. Superefficient and Robust Polymer Coating for Bionic Manufacturing of Superwetting Surfaces with “Rose Petal Effect” and “Lotus Leaf Effect”. Prog. Org. Coatings 2021, 151, 106090. [Google Scholar] [CrossRef]
- Dong, B.; Guo, Y.; Sun, S.; Mi, H.Y.; He, P.; Antwi-Afari, M.F.; Liu, C.; Shen, C. Shish-Kebab-Structured UHMWPE Coating for Efficient and Cost-Effective Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 58252–58262. [Google Scholar] [CrossRef]
- Matsuyama, H.; Kim, M.; Lloyd, D.R. Effect of extraction and drying on the structure of microporous polyethylene membranes prepared via thermally induced phase separation. J. Membr. Sci. 2002, 204, 413–419. [Google Scholar] [CrossRef]
- Yuan, W.; Zeng, L.; Li, Y.; Wang, J.; Wang, X.; Liao, Q.; Li, L.; Wei, Z.; Yuan, W.; Zeng, L.; et al. Ultrathin and Super Strong UHMWPE Supported Composite Anion Exchange Membranes with Outstanding Fuel Cells Performance. Small 2022, 18, 2105499. [Google Scholar] [CrossRef]
- Ponomarev, A.N.; Kritskaya, D.A.; Abdrashitov, E.F.; Bokun, V.C.; Sanginov, E.A.; Novikova, K.S.; Dremova, N.N.; Dobrovolsky, Y.A. A New Synthesis Approach for Proton Exchange Membranes Based on Ultra-High-Molecular-Weight Polyethylene. J. Appl. Polym. Sci. 2020, 137, 49563. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Cao, Y.; Yang, F.; Chen, J.; Kang, J.; Xu, R.; Xiang, M. Construction of a Composite Microporous Polyethylene Membrane with Enhanced Fouling Resistance for Water Treatment. J. Memb. Sci. 2021, 618, 118679. [Google Scholar] [CrossRef]
- Yang, K.; Xu, Z.; Li, R.; Liu, Y.; Sun, W.; Tang, Y.; Liu, X.; Fu, Q. Thickness Effects of Surface Direct Fluorination and Plasma Modification on Ultra-High Molecular Weight Polyethylene Ultrathin Membranes. Macromol. Mater. Eng. 2022, 2200557. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Guo, S.; Qiu, J. Tunable all-in-one bimodal porous membrane of ultrahigh molecular weight polyethylene for solar driven interfacial evaporation. Sep. Pur. Tech. 2022, 302, 122071. [Google Scholar] [CrossRef]
- Wencke, Y.L.; Luinstra, G.A.; Duchateau, R.; Proes, F.; Imgrund, P.; Evenson, J.S.; Emmelmann, C. Disentangled UHMWPE@silica Powders for Potential Use in Power Bed Fusion Based Additive Manufacturing. Eur. Polym. J. 2022, 163, 110936. [Google Scholar] [CrossRef]
- Panin, S.V.; Buslovich, D.G.; Kornienko, L.A.; Aleksenko, V.O.; Dontsov, Y.V.; Ovechkin, B.B.; Shil’ko, S.V. Comparative Analysis of Tribological and Mechanical Properties of Extrudable Polymer–Polymer UHMWPE Composites Fabricated by 3D Printing and Hot-Pressing Methods. J. Frict. Wear 2020, 41, 228–235. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D Printing for Membrane Separation, Desalination and Water Treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Low, Z.X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D Printing of Separation Membranes and Comparison to Related Unconventional Fabrication Techniques. J. Memb. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Wang, F.; Zhou, X.; Liao, W. Finely Controlled Swelling: A Shortcut to Construct Ion-Selective Channels in Polymer Membranes. Polymer 2021, 225, 123793. [Google Scholar] [CrossRef]










| No. | MW of the UHMWPE Used, 106 g/mol | UHMWPE Concentration in Solution, % wt. | Solvent | Solution Preparation Temperature, °C | Equipment Used, Mixing Conditions | Presence of Antioxidant | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 10 | Liquid paraffin (LP) | – | rheometer | – | [189] |
| 2 | 2–4 | 25 | LP | 250 | Twin screw extruder (TSE) | – | [190] |
| 3 | 2 | 10 | LP | 165 | Batch mixer, 100 rpm | – | [191] |
| 4 | 3.2 | 1.5 | LP | 180 | – | + | [52] |
| 5 | 0.5–2 | 10–50 | LP | – | TSE | + | [192] |
| 6 | 4 | 5 | LP | 200 | Batch mixer, 60 rpm | Irganox 1076 | [193] |
| 7 | 4 | 5 | LP | 200 | Batch mixer, 60 rpm | – | [194] |
| 8 | 2–6 | 10–60 | LP | 200 | Rheometer, 40 rpm | – | [195] |
| 9 | 3.7 | – | LP | – | TSE | – | [196] |
| 10 | 4 | – | LP | – | – | – | [197] |
| 11 | 1 | 70 | LP | – | TSE | – | [35] |
| 12 | 2.5–3.5 | 23.6–55.6 | LP (MW ~500) | 160 | Rheometer, 60 rpm | 1010 | [198] |
| 13 | 1.5–2 | 10–50 | LP (MW ~150–250) | 160 | Batch mixer, 60 rpm | 1% Irganox 1010 | [33] |
| 14 | 2 | 10 | LP (MW ~150–250) | 165 | Batch mixer, 60 rpm | – | [199] |
| 15 | 2 | 10 | LP (MW ~150–250) | 165 | Batch mixer, 80 rpm | – | [200] |
| 16 | 2.5–4 | 3–8 | LP (MW ~250–450) | 200 | Batch mixer, 60 rpm | 0.7% Irganox 1076 | [201] |
| 17 | 1.2–1.5 | 10–30.8 | LP (MW ~150–300) | 190–210 | TSE | 0.5% Irganox 1010 | [202] |
| 18 | 0.6–5 | 2–10 | LP (MW ~150–300) | 190–210 | TSE | – | [203] |
| 19 | 1.2 | 10–30 | LP (MW ~150–300) | 185–205 | TSE | – | [50] |
| 20 | 0.5–10 | – | light mineral oil | – | – | – | [204] |
| 21 | >1 | 2–20 | mineral oil | 200 | TSE | – | [205] |
| 22 | 0.4–15 | 2–30 | mineral oil | 160 | TSE | – | [206] |
| 23 | 3.65 | 3.6 | Mineral oil | 175 | – | – | [207] |
| 24 | 3.65 | 7.5–15 | mineral oil | 190 | TSE | 0.3% Irganox 1076 | [208] |
| 25 | 4.5 | 10–25 | mineral oil | 160 | torque rheometer | Irganox 1010 | [209] |
| 26 | 3.65 | – | Mineral oil 7# | 175 | – | Irganox 1076 | [210] |
| 27 | 3.65 | 5–12 | Mineral oil 7# | – | TSE | 0.3% Irganox 1076 | [211] |
| 28 | 3.65 | 8 | Mineral oil 7# | – | TSE | 0.3% Irganox 1076 | [212] |
| 29 | 3.65 | 5 | Mineral oil 7# | 180 | TSE | 0.3% Irganox 1706 | [213] |
| 30 | 3.5 | 10 | mineral oil + petrolatum | 200 | TSE | 0.5% Irganox 1010 | [214] |
| 31 | 3.65 | 12–24 | mineral oil 7# + dibutyl phthalate | – | TSE | 0.2% Irganox 1076 | [215] |
| 32 | 9 | – | naphtenic oil | 165 | Batch mixer, 60 rpm | – | [216] |
| 33 | >1 | 10–55 | napthenic oil | 200 | TSE | – | [217] |
| 34 | >1 | 2–20 | hydrocarbons | 200 | TSE | – | [205] |
| 35 | 0.5–10 | – | hydrocarbons | – | – | – | [204] |
| 36 | 3 | 1 | paraffin | 145 | – | – | [218] |
| 37 | 1.6 | 5 | paraffin oil | 170 | 0.1% di-t-butyl-p-cresol | [219] | |
| 38 | 0.4–15 | 2–30 | paraffin oil | 160 | TSE | – | [206] |
| 39 | >1 | 10–55 | paraffin oil | 200 | TSE | – | [217] |
| 40 | 5 | 4–5 | paraffin oil | 150 | – | – | [220] |
| 41 | 4 | 2–3.2 | paraffin oil | 150 | Batch mixer | – | [221] |
| 42 | 1.6 | 5 | paraffin oil #70 | 220 | TSE, 60 rpm | 0.7% Irganox 1076 | [34] |
| 43 | 0.6 | 20–25 | paraffin oil | 220 | TSE | + | [47] |
| 44 | 2.7 | 4–12 | paraffin oil | 150–250 | TSE | – | [111] |
| 45 | – | – | paraffin oil | – | TSE | – | [51] |
| 46 | 1.6 | 5 | paraffin oil #70 | 220 | TSE | 0.7% Irganox 1076 | [222] |
| 47 | >1 | 2–20 | paraffin wax | 200 | TSE | – | [205] |
| 48 | 1.6 | 20 | white oil #70 | TSE | 0.7% Irganox 1076 | [223] | |
| 49 | 5 | 2–5 | hexadecane | 135 | – | – | [43] |
| 50 | 5 | 50 | hexadecane | 160–200 | Twin screw microcompounder, 100 rpm | 0.2% Irganox 1010 + 0.2% Irgafox 168 | [224] |
| 51 | >1 | 10–55 | aromatic oil | 200 | TSE | – | [217] |
| 52 | 4 | 2–3.2 | paraffin oil | 150 | Batch mixer | – | [221] |
| 53 | 2–6 | 10–60 | polybutene-1 (MW ~400) | 200 | Rheometer, 40 rpm | – | [195] |
| 54 | 1.6–9 | 0.5 | mixture of xylenes | 140 | – | – | [225] |
| 55 | 2–3 | 0.058 | xylene | 140 | – | – | [226] |
| 56 | 1 | 38 | p-xylene | 140 | TSE | – | [227] |
| 57 | 2 | 0.25–0.75 | p-xylene | 130 | – | – | [44] |
| 58 | 5 | 2–5 | o-xylene | 135 | – | – | [43] |
| 59 | 1.6–9 | 0.5 | o-xylene | 140 | – | – | [225] |
| 60 | 3.5 | 0.6–10 | decalin | 160 | – | 0.5% di-t-butyl-p-cresol | [228] |
| 61 | 0.4–15 | 2–30 | decalin | 160 | TSE | – | [206] |
| 62 | 1.6 | 5 | decalin | 150 | 0.1% di-t-butyl-p-cresol | [219] | |
| 63 | 6 | 0.4 | decalin | 135 | – | – | [229] |
| 64 | 3 | 10–40 | decalin | 160 | – | – | [230] |
| 65 | 3 | 1 | decalin | 145 | – | – | [218] |
| 66 | 4 | 2–3.2 | decalin | 150 | Batch mixer | – | [221] |
| 67 | 1.6 | 5–15 | decalin | 165 | TSE, 60 rpm | 0.7% Irganox 1076 | [39] |
| 68 | 5 | 50 | decalin | 160–200 | Twin screw microcompounder, 100 rpm | 0.2% Irganox 1010 + 0.2% Irgafox 168 | [224] |
| 69 | 5 | 50 | decalin + dodecanol | 160–200 | Twin screw microcompounder, 100 rpm | 0.2% Irganox 1010 + 0.2% Irgafox 168 | [224] |
| 70 | 2.2–4.4 | 0.5–4 | Decalin + cyclohexanone | 130 | – | – | [231] |
| 71 | 3 | 0.07 | decalin | 140 | – | 1076 | [232] |
| 72 | 1.6–9 | 0.5 | decalin | 140 | – | – | [225] |
| 73 | 0.4–15 | 2–30 | tetralin | 160 | TSE | – | [206] |
| 74 | 3 | 10–40 | diphenyl ether | 160 | – | – | [230] |
| 75 | >1 | 2–20 | dioctyl phthalate | 200 | TSE | – | [205] |
| 76 | >1 | 10–55 | dioctyl phthalate | 200 | TSE | – | [217] |
| 77 | 34 | 0.9–3 | dioctyl phthalate | 220 | – | – | [233] |
| 78 | >1 | 10–55 | butylbenzyl phthalate | 200 | TSE | – | [217] |
| 79 | >1 | 2–20 | dibutyl sebacate | 200 | TSE | – | [205] |
| 80 | >1 | 10–55 | dibutyl phthalate | 200 | TSE | – | [217] |
| 81 | 1 | 5 | 1,2-dichloroethane + cyclopentane | 180 | Autoclave with CO2, 5 MPa | Irganox 1010 | [234] |
| 82 | 1.2–6 | 4 | 1,2,4-trichlorobenzene | – | – | – | [45] |
| 83 | 5 | 50 | stearic acid | 160–200 | Twin screw microcompounder, 100 rpm | 0.2% Irganox 1010 + 0.2% Irgafox 168 | [224] |
| Method of UHMWPE Membrane Preparation | Advantages | Disadvantages | Proportion of Papers Devoted to the Formation of Membranes by the Respective Method |
|---|---|---|---|
| Powder sintering | No shrinkage, simplicity of hardware design, no need to prepare a homogeneos solution, high tensile strength | Low porosity, wide pore size distribution, low selectivity, difficulty in control of the porous structure and properties | ~18 |
| Stretching | No need to prepare a homogeneous solution, high tensile strength | High thermal shrinkage, difficulty in control of the porous structure and properties | ~5 |
| TIPS | High porosity, good possibilities of control of the porous structure and properties, possibility for modification by inclusion of fillers in the dope solution | Problem of homogeneous solution preparation, lower tensile strength, high shrinkage, low surface porosity | ~46 |
| Combination of TIPS and stretching | High porosity, excellent possibilities of control of the porous structure and properties, possibility for modification by inclusion of fillers in the dope solution, high tensile strength | Problem of homogeneous solution preparation, high shrinkage | ~23 |
| Other | – | – | ~8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basko, A.; Pochivalov, K. Current State-of-the-Art in Membrane Formation from Ultra-High Molecular Weight Polyethylene. Membranes 2022, 12, 1137. https://doi.org/10.3390/membranes12111137
Basko A, Pochivalov K. Current State-of-the-Art in Membrane Formation from Ultra-High Molecular Weight Polyethylene. Membranes. 2022; 12(11):1137. https://doi.org/10.3390/membranes12111137
Chicago/Turabian StyleBasko, Andrey, and Konstantin Pochivalov. 2022. "Current State-of-the-Art in Membrane Formation from Ultra-High Molecular Weight Polyethylene" Membranes 12, no. 11: 1137. https://doi.org/10.3390/membranes12111137