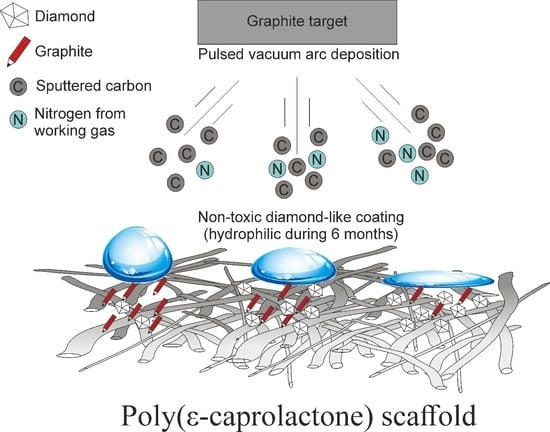
Pulsed Vacuum Arc Deposition of Nitrogen-Doped Diamond-like Coatings for Long-Term Hydrophilicity of Electrospun Poly(ε-caprolactone) Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun Polycaprolactone Scaffolds for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Biazar, E.; Kamalvand, M.; Avani, F. Recent Advances in Surface Modification of Biopolymeric Nanofibrous Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 493–512. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent Advances in Modification Strategies of Pre- and Post-Electrospinning of Nanofiber Scaffolds in Tissue Engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipovka, A.; Rodriguez, R.; Bolbasov, E.; Maryin, P.; Tverdokhlebov, S.; Sheremet, E. Time-Stable Wetting Effect of Plasma-Treated Biodegradable Scaffolds Functionalized with Graphene Oxide. Surf. Coat. Technol. 2020, 388, 125560. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An Overview on Diamond-like Carbon Coatings in Medical Applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Kolpakov, A.Y.; Poplavsky, A.I.; Galkina, M.E.; Manokhin, S.S.; Gerus, J.V. The Local Crystallization in Nanoscale Diamond-like Carbon Films during Annealing. Appl. Phys. Lett. 2014, 105, 233110. [Google Scholar] [CrossRef]
- Poplavsky, A.I.; Kolpakov, A.Y.; Kudriavtsev, Y.; Asomoza, R.; Goncharov, I.Y.; Galkina, M.E.; Manokhin, S.S.; Kharchenko, V.A. Effect of Nitrogen Ion Irradiation Parameters on Properties of Nitrogen-Containing Carbon Coatings Prepared by Pulsed Vacuum Arc Deposition Method. Vacuum 2018, 152, 193–199. [Google Scholar] [CrossRef]
- Yuriev, Y.; Goreninskii, S.; Runts, A.; Prosetskaya, E.; Plotnikov, E.; Shishkova, D.; Kudryavtseva, Y.; Bolbasov, E. DLC-Coated Ferroelectric Membranes as Vascular Patches: Physico-Chemical Properties and Biocompatibility. Membranes 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Kotula, A.P.; Snyder, C.R.; Migler, K.B. Determining Conformational Order and Crystallinity in Polycaprolactone via Raman Spectroscopy. Polymer 2017, 117, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Afandi, A.; Jackman, R.B. Graphene Diamond-like Carbon Films Heterostructure. Appl. Phys. Lett. 2015, 106, 102108. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, N.; Kahn, M.; Berghauser, R.; Waldhauser, W.; Mizaikoff, B. Nitrogen-Doped Diamond-like Carbon as Optically Transparent Electrode for Infrared Attenuated Total Reflection Spectroelectrochemistry. Analyst 2011, 136, 1831. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(Caprolactone) (PCL) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 27–31. [Google Scholar] [CrossRef]
- Murata, Y.; Choo, C.-K.; Ono, H.; Nagai, Y.; Tanaka, K. Characterization of N-Doped DLC Thin Films Prepared by Hydrocarbons Pyrolysis Method. Mater. Today Proc. 2016, 3, S197–S202. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Asadian, M.; Cools, P.; Thukkaram, M.; Van Der Voort, P.; Morent, R.; De Geyter, N.; Lukas, D. Plasma Treatment Effects on Bulk Properties of Polycaprolactone Nanofibrous Mats Fabricated by Uncommon AC Electrospinning: A Comparative Study. Surf. Coat. Technol. 2020, 399, 126203. [Google Scholar] [CrossRef]
- Ostrovskaya, L.; Perevertailo, V.; Ralchenko, V.; Dementjev, A.; Loginova, O. Wettability and Surface Energy of Oxidized and Hydrogen Plasma-Treated Diamond Films. Diam. Relat. Mater. 2002, 11, 845–850. [Google Scholar] [CrossRef]
- Subramanian, B.; Thanka Rajan, S.; Martin, P.J.; Vaithilingam, V.; Bean, P.A.; Evans, M.D.M.; Bendavid, A. Biomineralization of Osteoblasts on DLC Coated Surfaces for Bone Implants. Biointerphases 2018, 13, 041002. [Google Scholar] [CrossRef]
- Kashyap, V.; Ramkumar, P. DLC Coating over Pre-Oxidized and Textured Ti6Al4V for Superior Adhesion and Tribo-Performance of Hip Implant. Surf. Coat. Technol. 2022, 440, 128492. [Google Scholar] [CrossRef]





| Sample | Carbon, at. % | Oxygen, at. % | Nitrogen, at. % |
|---|---|---|---|
| Control | 77.0 ± 2.0 | 23.0 ± 1.0 | - |
| NDLC-1 | 85.0 ± 1.0 | 14.0 ± 1.0 | 1.7 ± 0.8 |
| NDLC-2 | 86.3 ± 1.3 | 8.8 ± 1.0 | 4.9 ± 0.8 |
| NDLC-3 | 76.2 ± 0.9 | 9.9 ± 1.3 | 16.1 ± 0.9 |
| Sample | Water Contact Angle, Deg | |||
|---|---|---|---|---|
| Immediately after Deposition (n = 5) | 1 Month after Deposition (n = 5) | 3 Months after Deposition (n = 5) | 6 Months after Deposition (n = 5) | |
| Control | 126 ± 2 | 126 ± 3 | 130 ± 3 | 133 ± 2 |
| NDLC-1 | 107 ± 6 | 110 ± 3 | 115 ± 2 | 121 ± 6 |
| NDLC-2 | 89 ± 2 * | 91 ± 5 * | 95 ± 5 * | 107 ± 7 * |
| NDLC-3 | 22 ± 3 * | 23 ± 4 * | 21 ± 3 * | 21 ± 3 * |
| Sample | Cultivation Time | |||||
|---|---|---|---|---|---|---|
| 24 h | 72 h | 120 h | ||||
| Cell Viability, % | Cell Density, cells/mm2 | Cell Viability, % | Cell Density, cells/mm2 | Cell viability, % | Cell Density, cells/mm2 | |
| Control culture medium | 100 ± 3 | 210 ± 11 | 100 ± 3 | 1148 ± 88 | 100 ± 7 | 1450 ± 75 |
| Control | 99 ± 6 | 215 ± 14 | 100 ± 6 | 1130 ± 81 | 97 ± 4 | 1419 ± 57 |
| NDLC-1 | 96 ± 5 | 215 ± 15 | 98 ± 8 | 1128 ± 58 | 100 ± 7 | 1431 ± 105 |
| NDLC-2 | 102 ± 4 | 207 ± 12 | 101 ± 4 | 1110 ± 90 | 100 ± 6 | 1458 ± 79 |
| NDLC-3 | 101 ± 4 | 223 ± 36 | 99 ± 2 | 1132 ± 118 | 96 ± 7 | 1425 ± 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goreninskii, S.; Yuriev, Y.; Runts, A.; Prosetskaya, E.; Sviridova, E.; Plotnikov, E.; Stankevich, K.; Bolbasov, E. Pulsed Vacuum Arc Deposition of Nitrogen-Doped Diamond-like Coatings for Long-Term Hydrophilicity of Electrospun Poly(ε-caprolactone) Scaffolds. Membranes 2022, 12, 1080. https://doi.org/10.3390/membranes12111080
Goreninskii S, Yuriev Y, Runts A, Prosetskaya E, Sviridova E, Plotnikov E, Stankevich K, Bolbasov E. Pulsed Vacuum Arc Deposition of Nitrogen-Doped Diamond-like Coatings for Long-Term Hydrophilicity of Electrospun Poly(ε-caprolactone) Scaffolds. Membranes. 2022; 12(11):1080. https://doi.org/10.3390/membranes12111080
Chicago/Turabian StyleGoreninskii, Semen, Yuri Yuriev, Artem Runts, Elisaveta Prosetskaya, Elizaveta Sviridova, Evgenii Plotnikov, Ksenia Stankevich, and Evgeniy Bolbasov. 2022. "Pulsed Vacuum Arc Deposition of Nitrogen-Doped Diamond-like Coatings for Long-Term Hydrophilicity of Electrospun Poly(ε-caprolactone) Scaffolds" Membranes 12, no. 11: 1080. https://doi.org/10.3390/membranes12111080