Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Supply and Characteristics
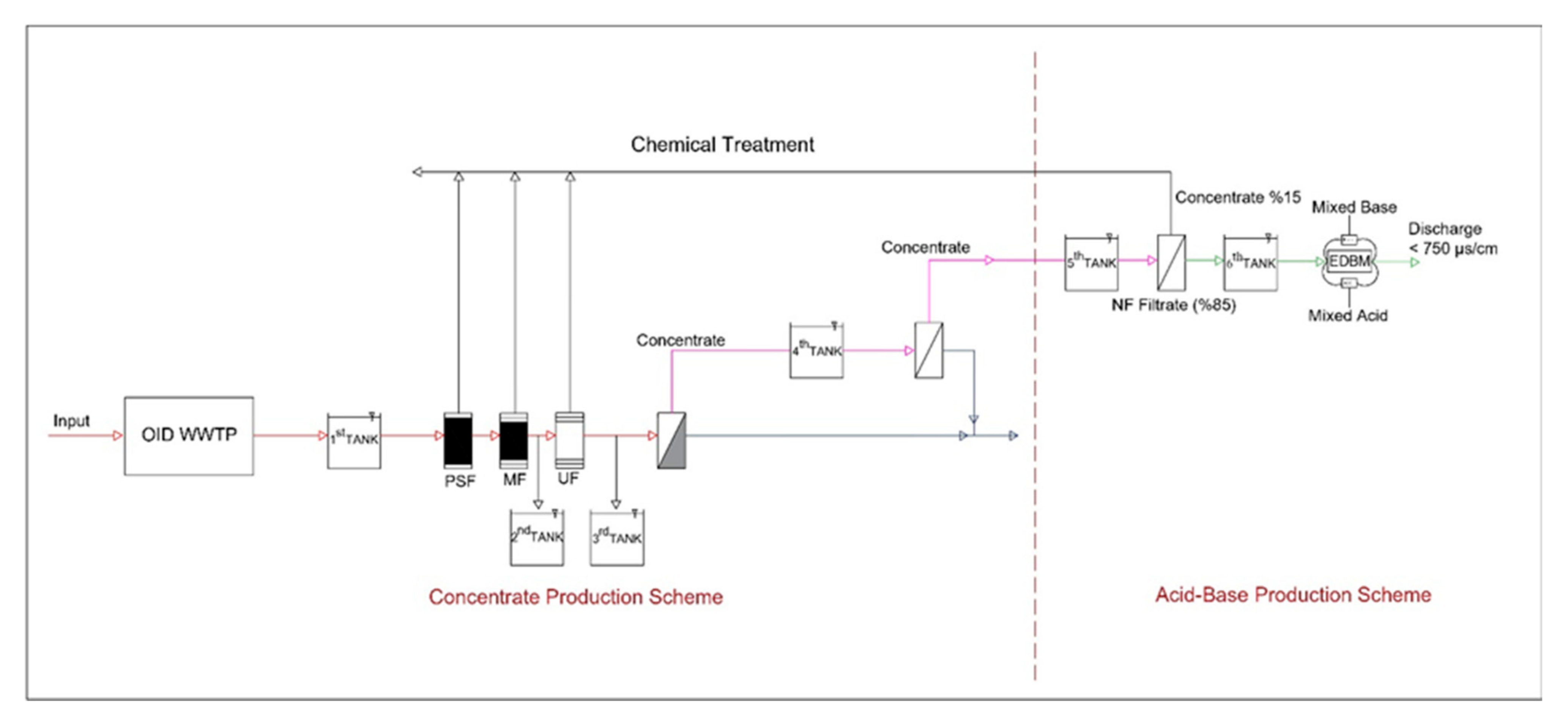
2.2. Pilot WWTP Concentrate Production and Disposal System Equipment
2.2.1. Pilot WWTP Concentrate Disposal System Equipment
2.2.2. Nanofiltration (NF)
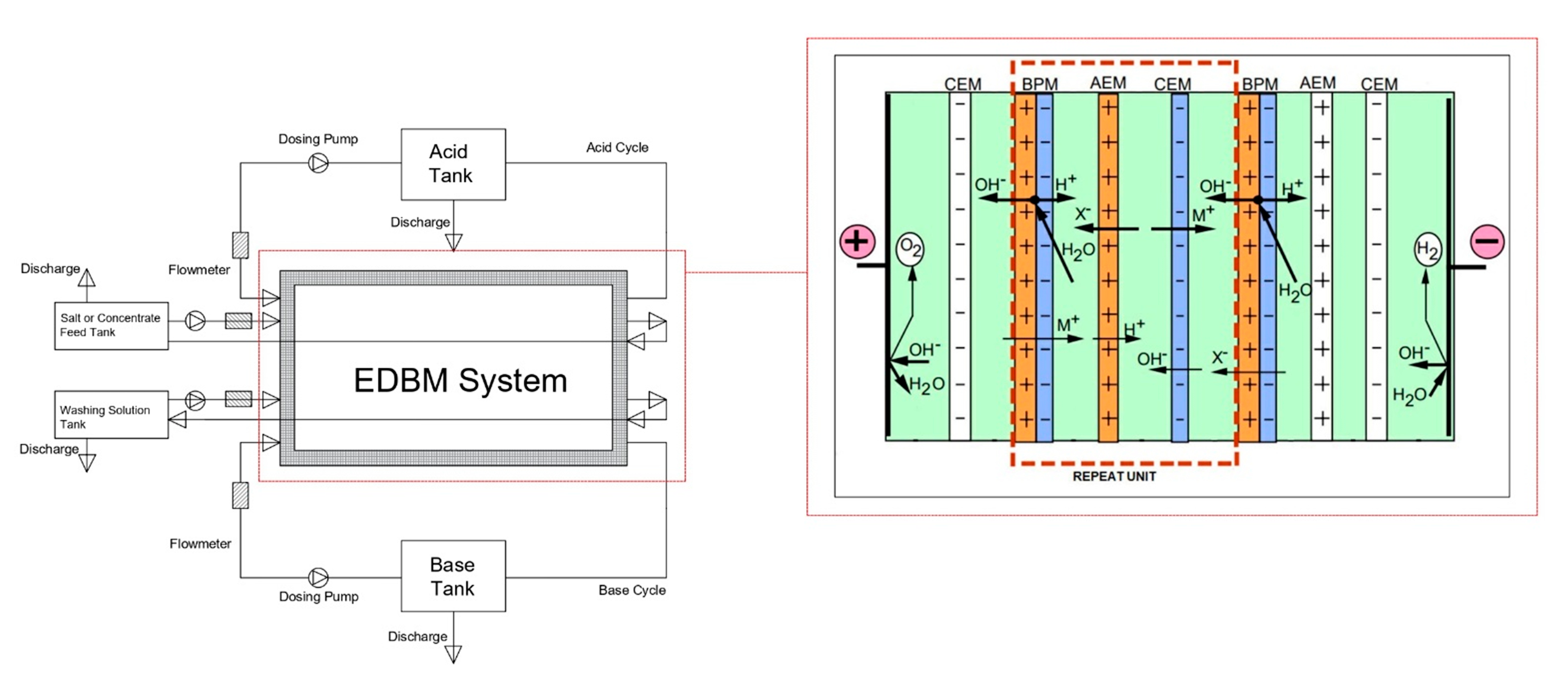
2.2.3. Electrodialysis Bipolar Membrane System (EDBM)
2.2.4. EDBM System Performance Calculation
- Ie: Available current efficiency)
- Vi: Inlet volume of acid or base (Liters)
- Ci: Concentration of input acid and base (equivalents/ L)
- Vf: Final acid and base volume (Liters)
- Cf: Final acid and base concentration (equivalents/ L)
- Id: Current density (A/m2)
- t: Times (s)
- S: Active membrane area (m2).
2.3. Measurement and Analysis Methods
2.4. Determination of EDBM System Operating Conditions
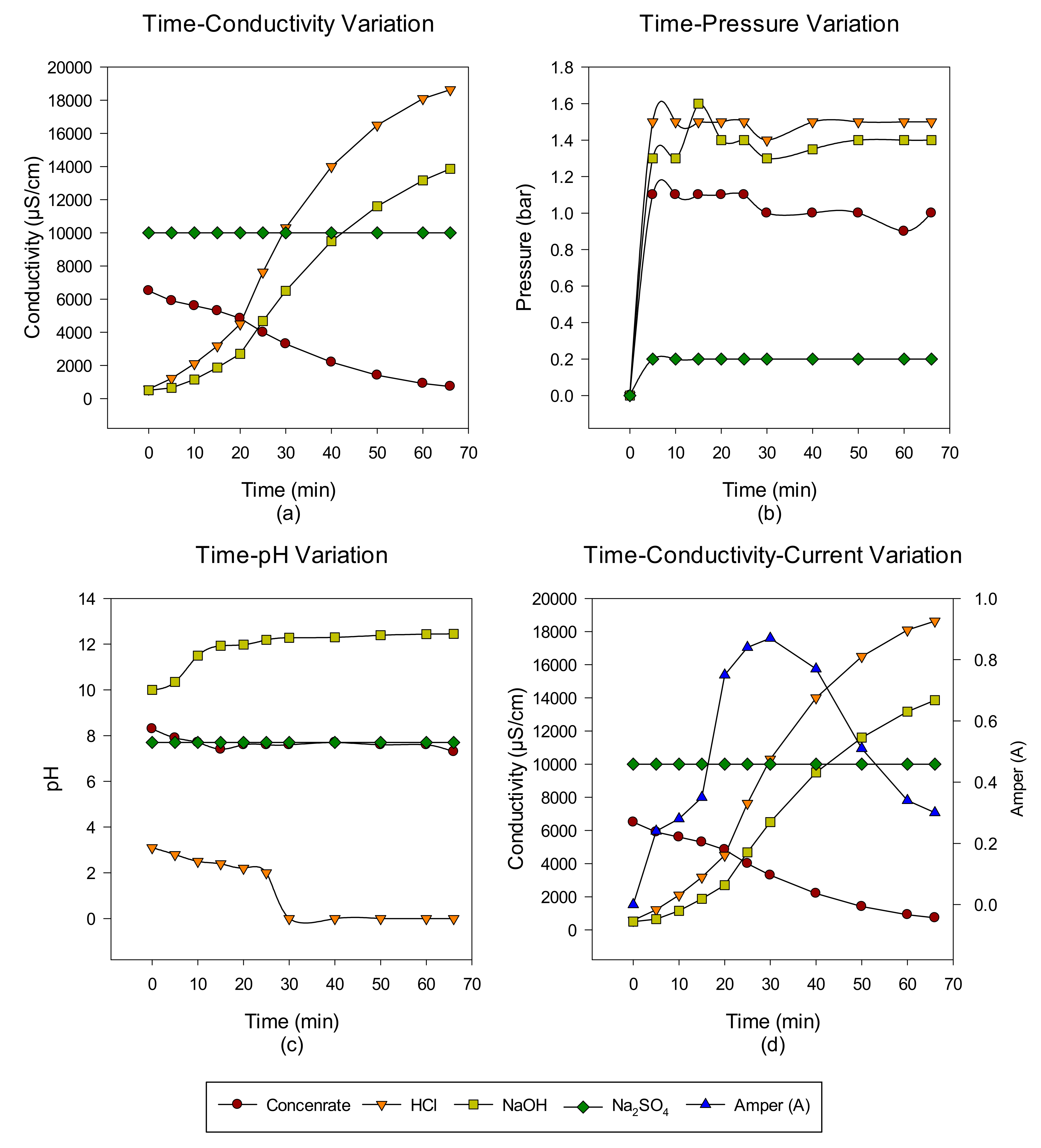
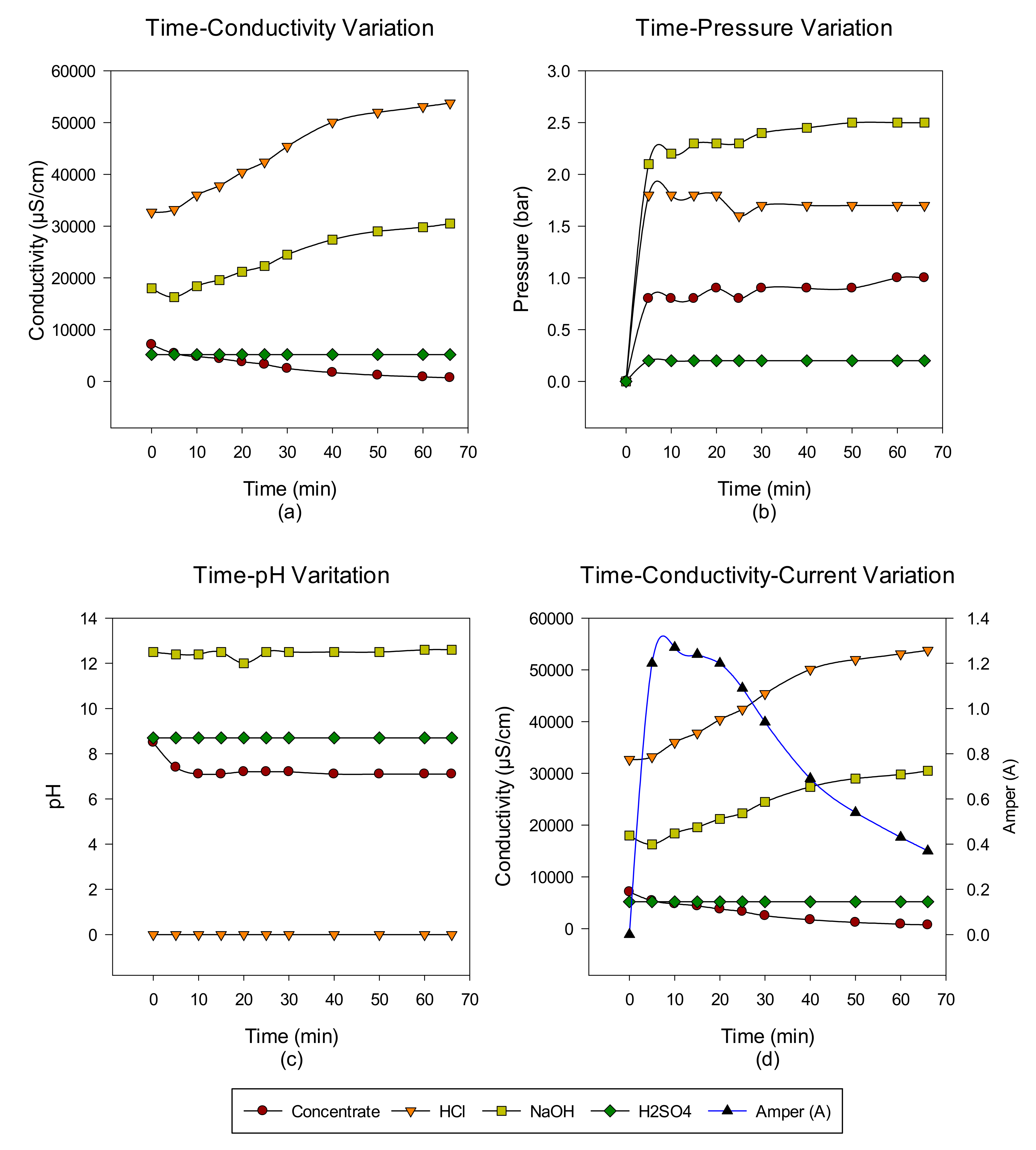
3. Results and Discussion
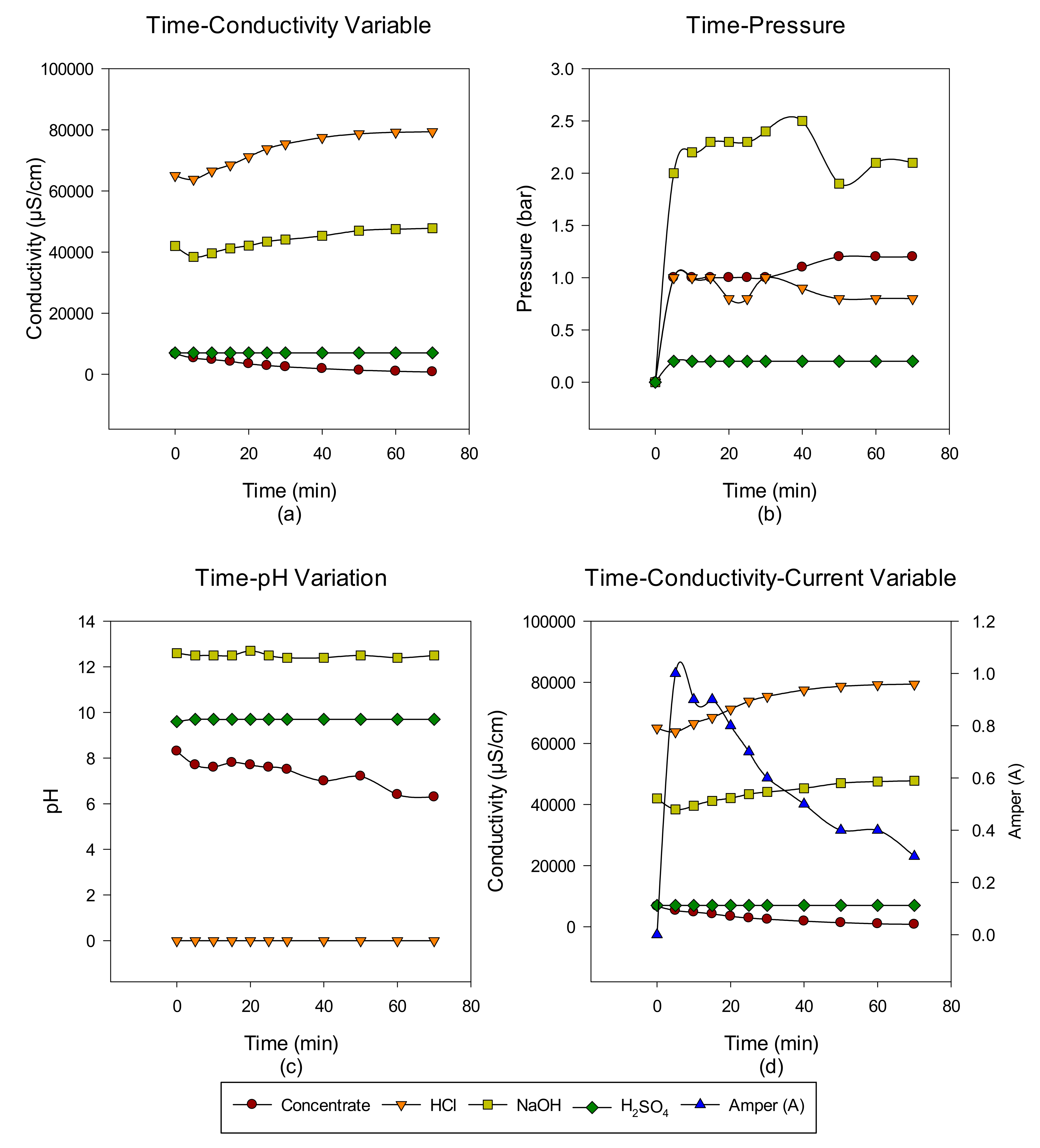
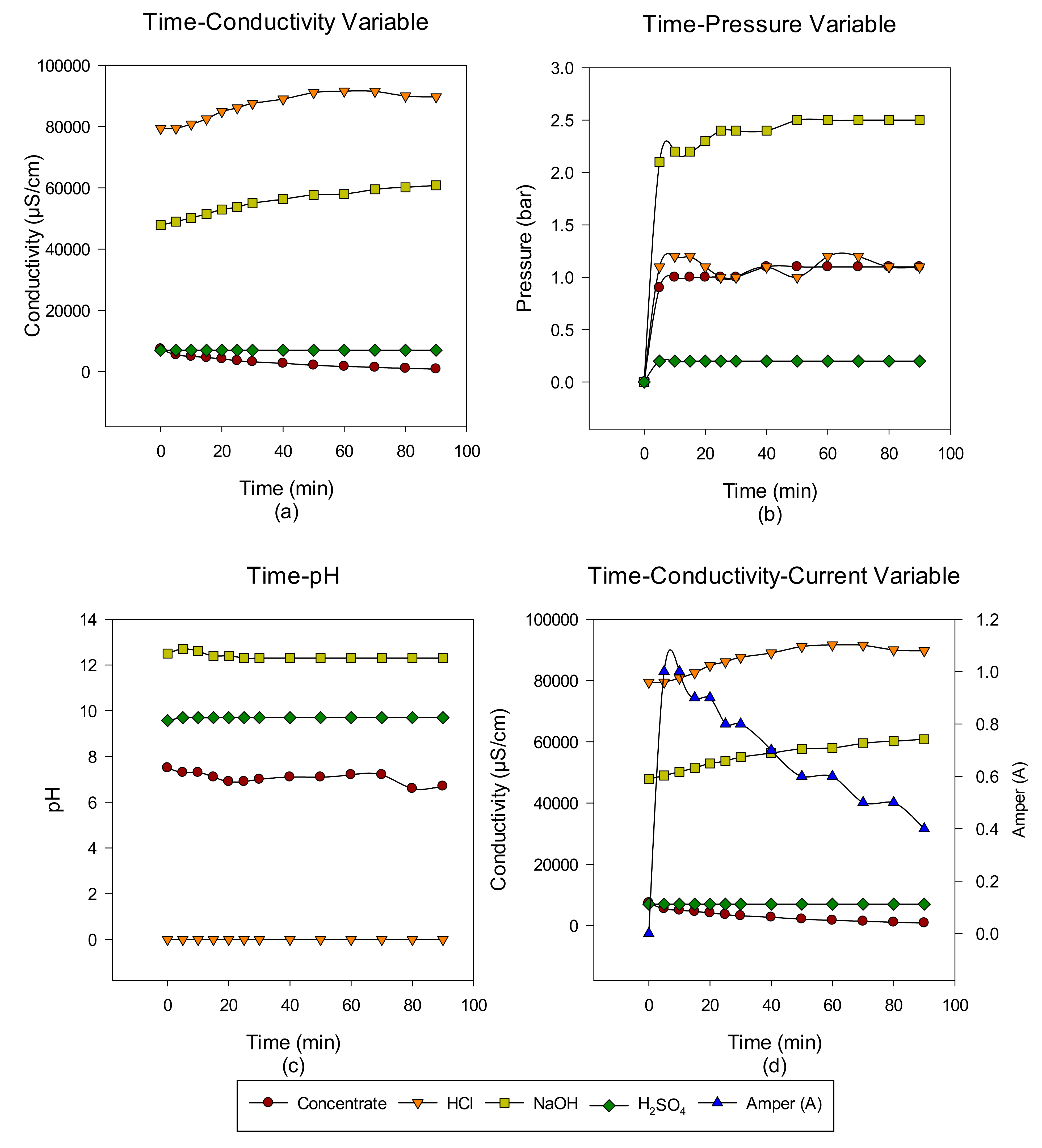
Concentrate Disposal Studies and Analysis Results with EDBM System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Gao, X.; Fan, A.; Fu, L.; Gao, C. An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J. Membr. Sci. 2014, 449, 119–126. [Google Scholar] [CrossRef]
- Mazhar, S.; Ditta, A.; Bulgariu, L.; Ahmad, I.; Ahmed, M.; Nadiri, A.A. Sequential treatment of paper and pulp industrial wastewater: Prediction of water quality parameters by Mamdani Fuzzy Logic model and phytotoxicity assessment. Chemosphere 2019, 227, 256–268. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Burn, S.; Hoang, M.; Zarzo, D.; Olewniak, F.; Campos, E.; Bolto, B.; Barron, O. Desalination techniques—A review of the opportunities for desalination in agriculture. Desalination 2015, 364, 2–16. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Birnhack, L.; Voutchkov, N.; Lahav, O. Fundamental chemistry and engineering aspects of post-treatment processes for desalinated water—A review. Desalination 2011, 273, 6–22. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Membr. Sci. 2009, 326, 392–399. [Google Scholar] [CrossRef]
- Rozoy, E.; Boudesocque, L.; Bazinet, L. Deacidification of Cranberry Juice by Electrodialysis with Bipolar Membranes. J. Agric. Food Chem. 2015, 63, 642–651. [Google Scholar] [CrossRef]
- Chen, Q.; Xue, C.; Zhang, W.-M.; Song, W.-G.; Wan, L.-J.; Ma, K.-S. Green Production of Ultrahigh-Basicity Polyaluminum Salts with Maximum Atomic Economy by Ultrafiltration and Electrodialysis with Bipolar Membranes. Ind. Eng. Chem. Res. 2014, 53, 13467–13474. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, Y.; Li, C.; Feng, H.; Xu, T. Production of Yellow Iron Oxide Pigments by Integration of the Air Oxidation Process with Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2014, 53, 1580–1587. [Google Scholar] [CrossRef]
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, H.; Zhang, Y.; Feng, H.; Shehzad, M.A.; Wang, Y.; Xu, T. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter. Chem. Eng. J. 2018, 348, 952–959. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Cai, Z.; Chen, R.; Sun, Q.; Sun, M. Removal of copper and nickel from municipal sludge using an improved electrokinetic process. Chem. Eng. J. 2017, 307, 1008–1016. [Google Scholar] [CrossRef]
- Herrero, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Photovoltaic solar electrodialysis with bipolar membranes. Desalination 2018, 433, 155–163. [Google Scholar] [CrossRef]
- Jarma, Y.A.; Çermikli, E.; Ipekçi, D.; Altıok, E.; Kabay, N. Comparison of two electrodialysis stacks having different ion exchange and bipolar membranes for simultaneous separation of boron and lithium from aqueous solution. Desalination 2021, 500, 114850. [Google Scholar] [CrossRef]
- Ilhan, F.; Kabuk, H.A.; Kurt, U.; Avşar, Y.; Gonullu, M.T. Recovery of mixed acid and base from wastewater with bipolar membrane electrodialysis—A case study. Desalination Water Treat. 2015, 57, 5165–5173. [Google Scholar] [CrossRef]
- Scelsi, L.; Hodzic, A.; Soutis, C.; Hayes, S.A.; Rajendran, S.; AlMa’Adeed, M.A.; Kahraman, R. A review on composite materials based on recycled thermoplastics and glass fibres. Plast. Rubber Compos. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Alvarez, F.; Alvarez, R.; Coca, J.; Sandeaux, J.; Sandeaux, R.; Gavach, C. Salicylic acid production by electrodialysis with bipolar membranes. J. Membr. Sci. 1997, 123, 61–69. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Peng, Z.; Liu, Y. Treatment of high salinity sulfanilic acid wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2021, 281, 119842. [Google Scholar] [CrossRef]
- Xu, L.; Dong, F.; Yang, J.; Liu, W.; Zhu, L.; He, Q.; Wang, X.; Li, H.; Wang, X. Electricity generation and acid and alkaline recovery from pickled waters/wastewaters through anaerobic digestion, bipolar membrane electrodialysis and solid oxide fuel cell hybrid system. Energy Convers. Manag. 2022, 251, 114973. [Google Scholar] [CrossRef]
- Yuzer, B.; Selcuk, H. Recovery of Biologically Treated Textile Wastewater by Ozonation and Subsequent Bipolar Membrane Electrodialysis Process. Membranes 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, H.; Xu, M.; Ruan, H. Sustainable reverse osmosis, electrodialysis and bipolar membrane electrodialysis application for cold-rolling wastewater treatment in the steel industry. J. Water Process. Eng. 2021, 40, 101968. [Google Scholar] [CrossRef]
- Rózsenberszki, T.; Komáromy, P.; Hülber-Beyer, É.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Demonstration of bipolar membrane electrodialysis technique for itaconic acid recovery from real fermentation effluent of Aspergillus terreus. Chem. Eng. Res. Des. 2021, 175, 348–357. [Google Scholar] [CrossRef]
- Gössi, A.; Burgener, F.; Kohler, D.; Urso, A.; Kolvenbach, B.A.; Riedl, W.; Schuur, B. In-situ recovery of carboxylic acids from fermentation broths through membrane supported reactive extraction using membrane modules with improved stability. Sep. Purif. Technol. 2020, 241, 116694. [Google Scholar] [CrossRef]
- Gao, W.; Fang, Q.; Yan, H.; Wei, X.; Wu, K. Recovery of Acid and Base from Sodium Sulfate Containing Lithium Carbonate Using Bipolar Membrane Electrodialysis. Membranes 2021, 11, 152. [Google Scholar] [CrossRef]
- Saabas, D.; Lee, J. Recovery of ammonia from simulated membrane contactor effluent using bipolar membrane electrodialysis. J. Membr. Sci. 2021, 644, 120081. [Google Scholar] [CrossRef]
- Kuldeep; Badenhorst, W.D.; Kauranen, P.; Pajari, H.; Ruismäki, R.; Mannela, P.; Murtomäki, L. Bipolar Membrane Electrodialysis for Sulfate Recycling in the Metallurgical Industries. Membranes 2021, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- José, C.; Briand, L.; Michlig, N.; Repetti, M.R.; Benedetich, C.; Cornaglia, L.M.; Bosko, M.L. Isolation of ibuprofen enantiomers and racemic esters through electrodialysis. J. Membr. Sci. 2021, 618, 118714. [Google Scholar] [CrossRef]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.A.W.; Budd, P.M.; Szekely, G. Graphene oxide–polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. J. Mater. Chem. A 2018, 6, 24728–24739. [Google Scholar] [CrossRef] [Green Version]
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite recovery from municipal-wastewater sludge centrifuge supernatant using seawater NF concentrate as a cheap Mg(II) source. Sep. Purif. Technol. 2013, 108, 103–110. [Google Scholar] [CrossRef]
- Yonar, T. A New Approach for Membrane Process Concentrate Management: Electrodialysis Bipolar Membrane Systems-A Short Communication. In Electrodialysis; IntechOpen: London, UK, 2020; pp. 1–13. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Valderrama, C.; Gibert, O.; Cortina, J.L. Integration of monopolar and bipolar electrodialysis for valorization of seawater reverse osmosis desalination brines: Production of strong acid and base. Desalination 2016, 398, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Xu, T. Electrodialysis with Bipolar Membranes for Sustainable Development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.; Wiśniewska, G.; Winnicki, T. Application of bipolar electrodialysis to the recovery of acids and bases from water solutions. Desalination 2004, 169, 11–20. [Google Scholar] [CrossRef]
- Woźniak, M.J.; Prochaska, K. Fumaric acid separation from fermentation broth using nanofiltration (NF) and bipolar electrodialysis (EDBM). Sep. Purif. Technol. 2014, 125, 179–186. [Google Scholar] [CrossRef]
- Szczygiełda, M.; Antczak, J.; Prochaska, K. Separation and concentration of succinic acid from post-fermentation broth by bipolar membrane electrodialysis (EDBM). Sep. Purif. Technol. 2017, 181, 53–59. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Bruinsma, O.; Branken, D.; Lemmer, T.; van der Westhuizen, L.; Rossouw, S. Sodium sulfate splitting as zero brine process in a base metal refinery: Screening and optimization in batch mode. Desalination 2021, 511, 115096. [Google Scholar] [CrossRef]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef]


| Parameter | OIE WWTP Outlet | PSF Outlet | MF Outlet | UF Outlet | RO Outlet |
|---|---|---|---|---|---|
| pH | 6.3 ± 0.15 | 8.06 ± 0.23 | 8.18 ± 0.21 | 8.24 ± 0.26 | 8.5 ± 0.12 |
| Temperature (°C) | 22.5 ± 0.53 | 21.4 ± 0.56 | 24.7 ± 0.85 | 21.6 ± 0.53 | 22.4 ± 0.18 |
| Conductivity (µS/cm) | 3906.0 ± 148 | 2750.0 ± 55.0 | 2760.0 ± 62.0 | 2760.0 ± 41.0 | 4510.0 ± 212.0 |
| SS (mg/L) | 48.7 ± 2.93 | 16.0 ± 0.92 | 6.0 ± 0.18 | 4.0 ± 0.13 | 4.0 ± 0.11 |
| COD (mg/L) | 105.1 ± 2.32 | 85.0 ± 1.54 | 100.0 ± 2.05 | 70.0 ± 1.85 | 106.0 ± 2.20 |
| Equipment Type | Unit | Number | Specifications |
|---|---|---|---|
| System Capacity | L/h/cell | 10 | 180 L/h |
| (10 cells, 18 L/h) | |||
| System Pressure | bar | - | 0.8 bar |
| Membranes | |||
| Membrane Size | mm | 10 | 160 × 160 |
| Effective Membrane Area | cm2 | 1 | 100 |
| Membrane Cover | - | 20 | PVC/PET |
| 10 | PE/PVDF | ||
| Electrode | - | 1 | Anode |
| - | 1 | Cathode | |
| Parameter | Unit | Method | RO Outlet | Reinforced with NaCl | NF System Output | NF System Output |
|---|---|---|---|---|---|---|
| (Concentrate) | (8000 μS/cm) | (Filtrate) | (Concentrate) | |||
| pH | SM 4500 H+B | 8.85 | - | 8.22 | 8.34 | |
| Conductivity | μS/cm | SM 2510.B | 4100 | 8000 | 7300 | 20,940 |
| Na+2 | mg/L | * ISO 11885 ICP | 686 | 1463 | 1211 | 3271.5 |
| Ca+2 | mg/L | * ISO 11885 ICP | 60 | 49.95 | 20,4 | 189 |
| Mg+2 | mg/L | * ISO 11885 ICP | 38.7 | 44.8 | 20.6 | 212 |
| NO3− | mg/L | * TS 6231 | 38.5 | 36.5 | 37.4 | 43.7 |
| SO4−2 | mg/L | * TS 5095 | 593 | 703.25 | 31.7 | 3699 |
| Si+2 | mg/L | S.M 4500 SiO2.C | 22.7 | 16.125 | 12 | 48.05 |
| Cl− | mg/L | * SM 4500 Cl− B | 689 | 2972.4 | 2927.5 | 3231.5 |
| Fe+2 | mg/L | * ISO 11885 ICP | - | 0.84 | 0.04 | 4.5 |
| Ba+2 | mg/L | * ISO 11885 ICP | - | ˂0.1 | ˂0.1 | ˂0.1 |
| Colour | Pt-Co | SM 2120C | - | - | 16.9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazigil, L.; Er, E.; Kestioğlu, O.E.; Yonar, T. Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery. Membranes 2022, 12, 83. https://doi.org/10.3390/membranes12010083
Gazigil L, Er E, Kestioğlu OE, Yonar T. Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery. Membranes. 2022; 12(1):83. https://doi.org/10.3390/membranes12010083
Chicago/Turabian StyleGazigil, Leyla, Eren Er, O. Erdem Kestioğlu, and Taner Yonar. 2022. "Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery" Membranes 12, no. 1: 83. https://doi.org/10.3390/membranes12010083
APA StyleGazigil, L., Er, E., Kestioğlu, O. E., & Yonar, T. (2022). Pilot-Scale Test Results of Electrodialysis Bipolar Membrane for Reverse-Osmosis Concentrate Recovery. Membranes, 12(1), 83. https://doi.org/10.3390/membranes12010083







