Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
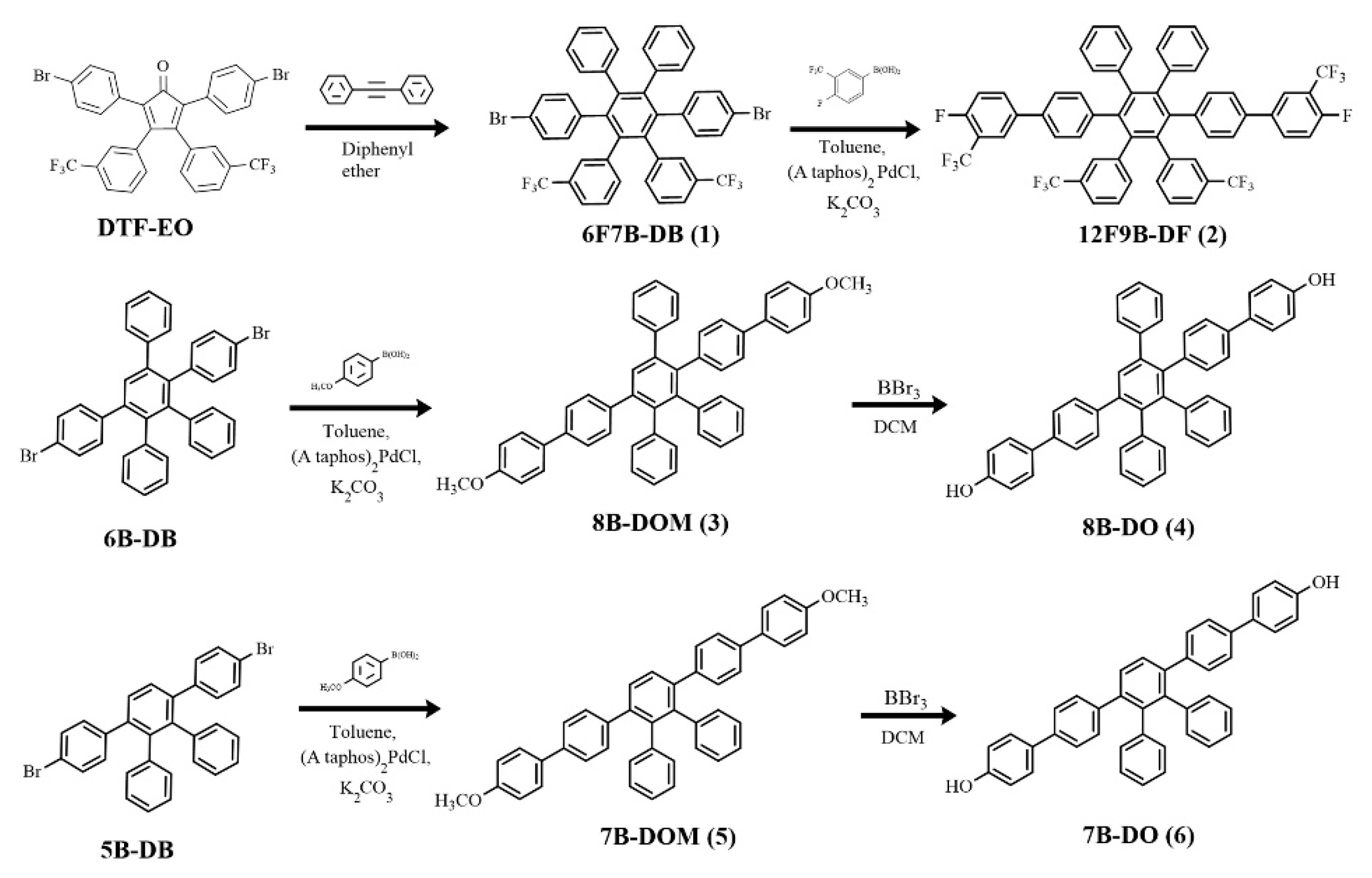
2.2. 3′,6′-Bis(4-bromophenyl)-4′,5′-diphenyl-3,3″-bis(trifluoromethyl)-1,1′:2′,1″-terphenyl (6F7B-DB) (1)
2.3. 4,4′′′′-Difluoro-3,3′′′′-bis(trifluoromethyl)-2″,3″,5″,6″,4,4′′′′-difluoro-3,3′′′′-bis(trifluoromethyl)-2″,3″,5″,6″-tetra (trifluoromethyl)phenyl-[1,1′:4′,1″:4″,1′′′:4′′′,1′′′′-quinquephenyl] (12F9B-DF) (2)
2.4. 4,4′′′′-Dimethoxy-2″,3″,5″-triphenyl-1,1′:4′,1″:4″,1′′′:4′′′,1′′′′-quinquephenyl (8B-DMO) (3)
2.5. 2″,3″,5″-Triphenyl-[1,1′:4′,1″:4″,1′′′:4′′′,1′′′′-quinquephenyl]-4,4′′′′-diol (8B-DO) (4)
2.6. 4,4′′′′-Dimethoxy-2″,3″-diphenyl-1,1′:4′,1″:4″,1′′′4′′′1′′′-quinquephenyl (7B-DMO) (5)
2.7. 2″,3″-Diphenyl-[1,1′:4′,1″:4″,1′′′:4′′′,1′′′-quinquephenyl]-4,4′′′′-diol (7B-DO) (6)
2.8. General Procedure for the Synthesis of Polymers
2.9. General Procedure for Sulfonation
2.10. Measurements
2.11. Stability
2.12. Water Uptake and Dimensional Stability
2.13. Ion Exchange Capacity
2.14. Hydrated Molar Volume per Charge
2.15. Percent Conducting Volume
2.16. Proton Conductivity
2.17. Microstructure Analysis
2.18. Single-Cell Performance
3. Results and Discussion
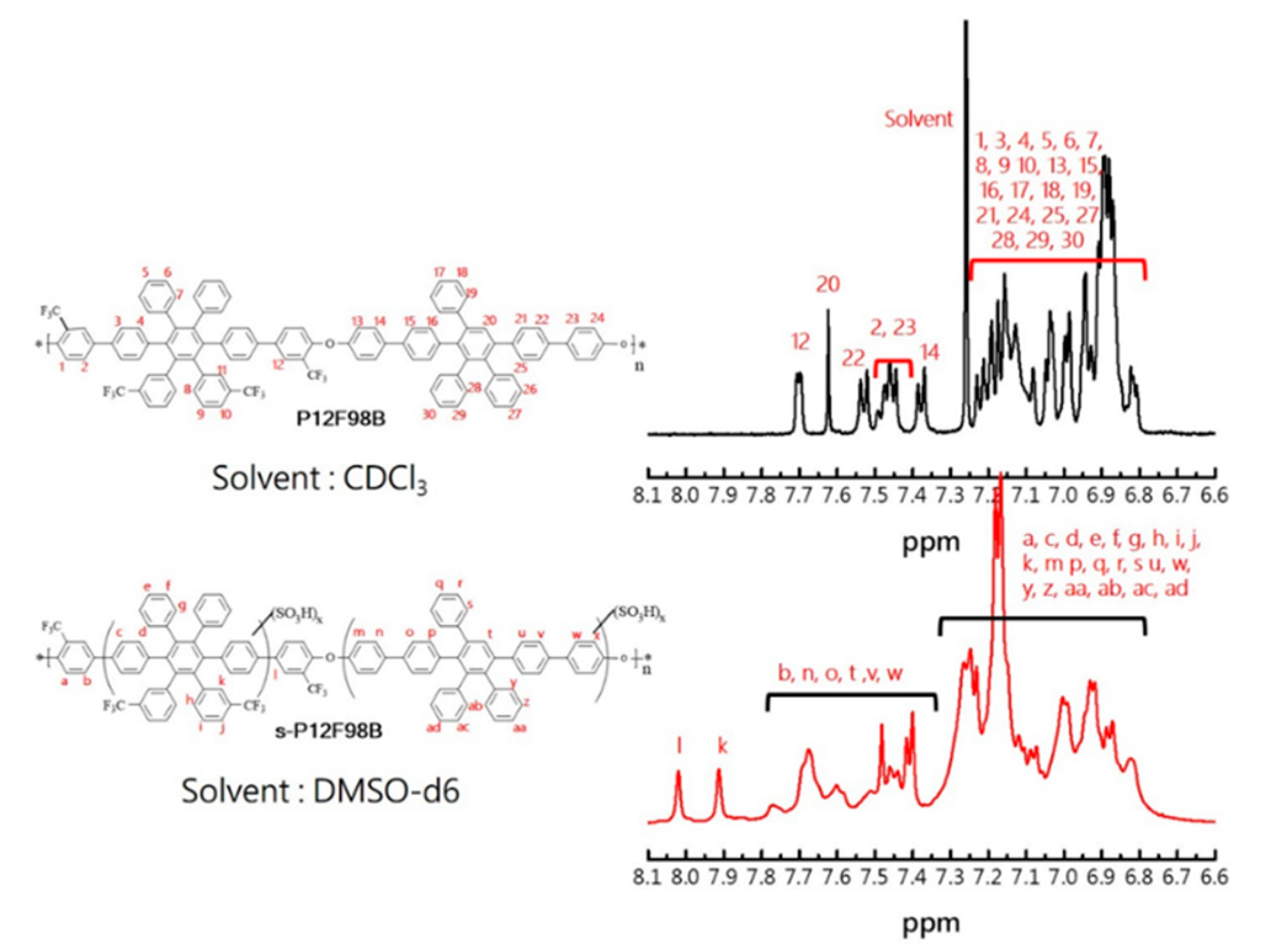
3.1. Synthesis and Characterization of the Monomers and Polymers
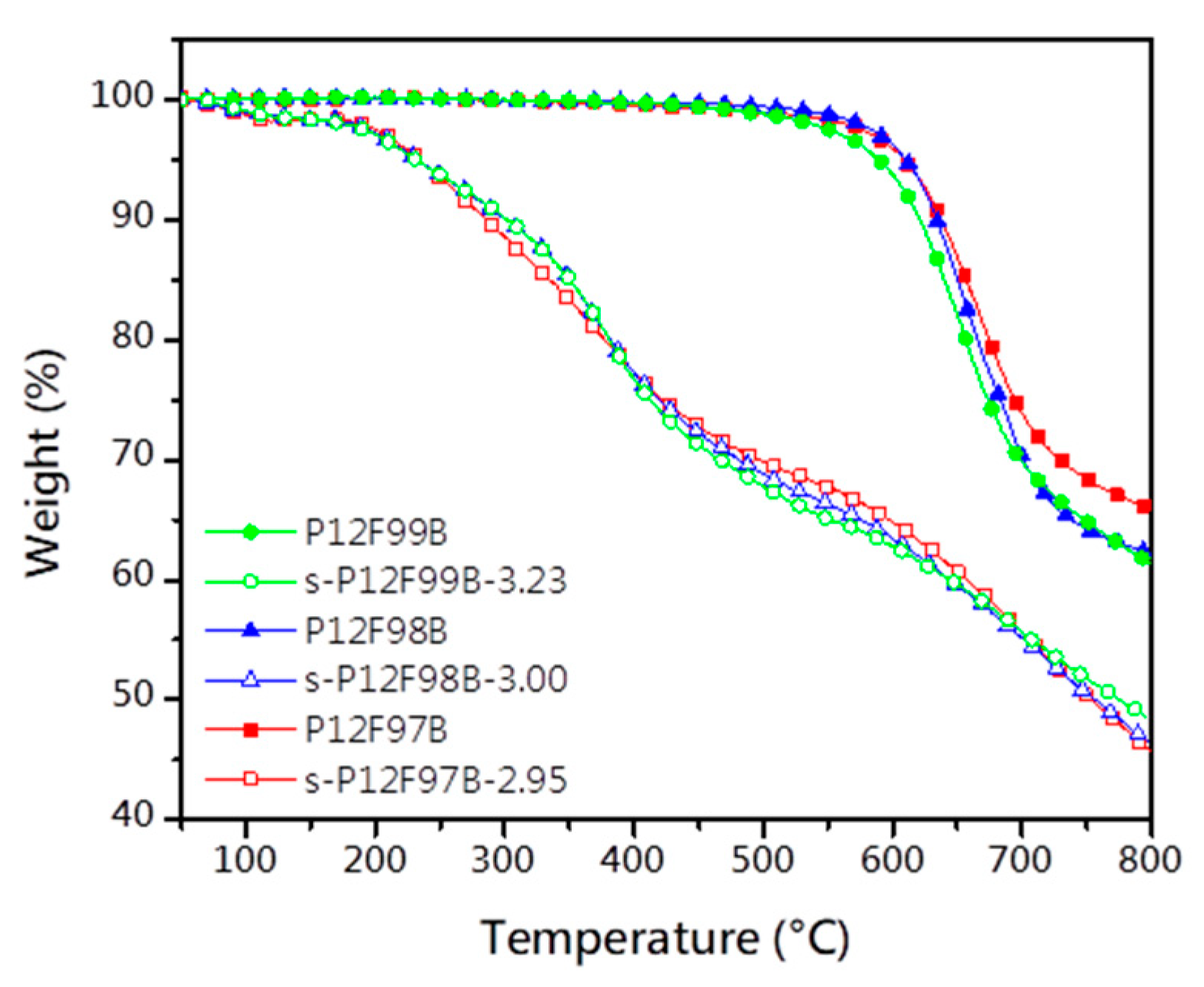
3.2. Thermal Stability
3.3. Mechanical Properties
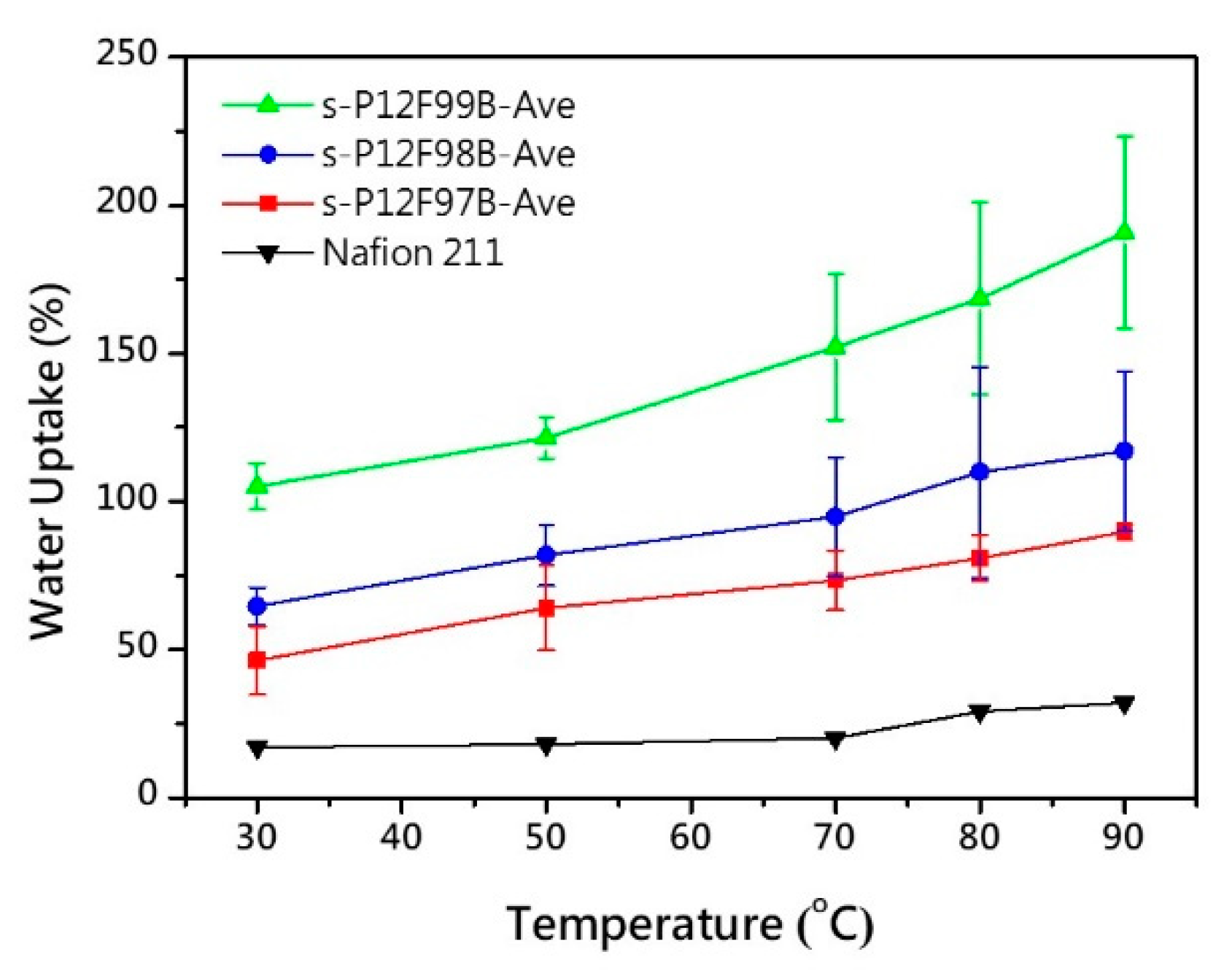
3.4. Hydration Behavior
3.5. Proton Conductivity
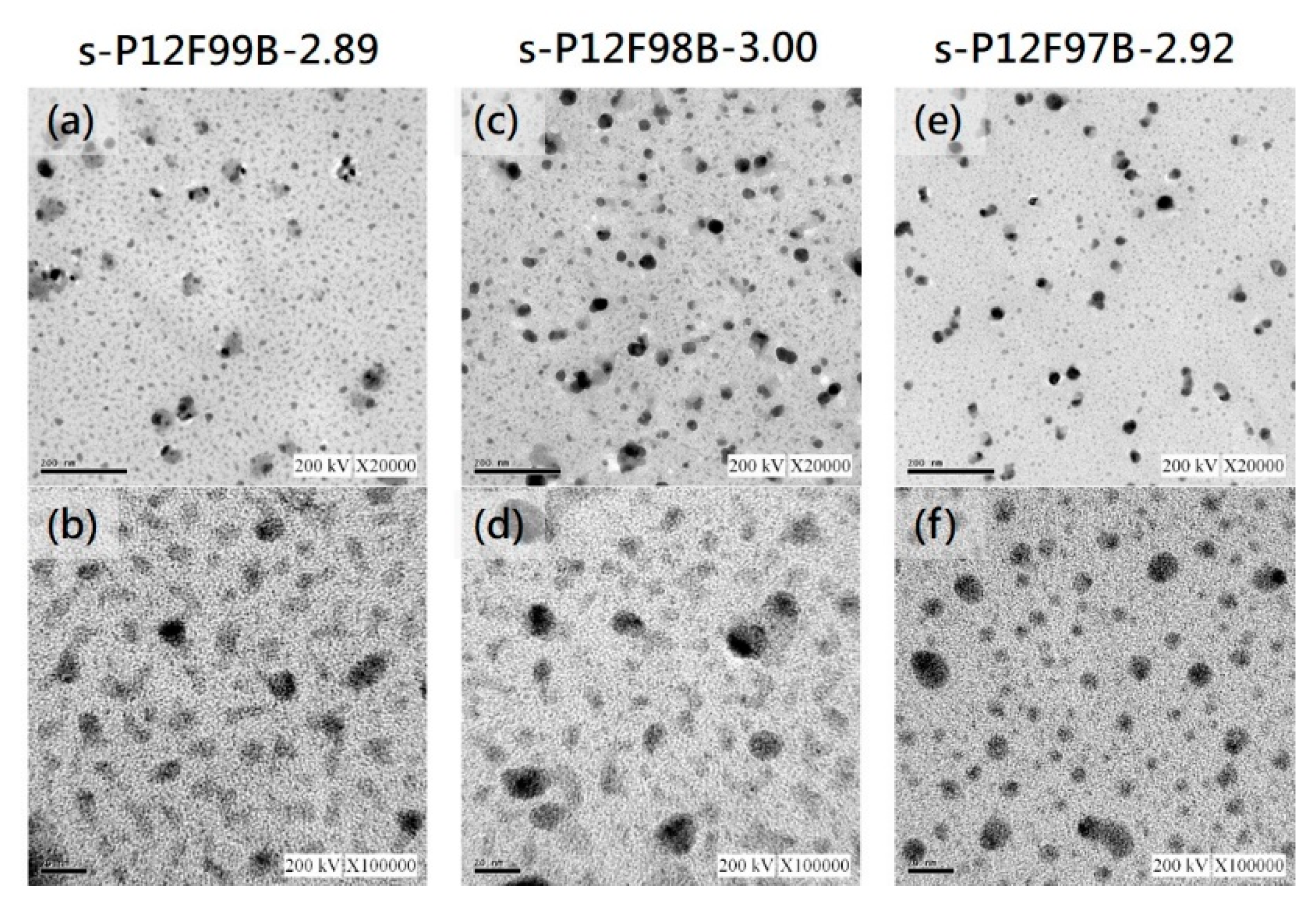
3.6. Microstructure Analysis
3.7. Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andujar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Perry, M.L.; Fuller, T.F. A historical perspective of fuel cell technology in the 20th century. J. Electrochem. Soc. 2002, 149, S59–S67. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and challenges of lithium ion batteries at temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Schiavone, M.-M.; Lamparelli, D.H.; Zhao, Y.; Zhu, F.; Revay, Z.; Radulescu, A. The Effects of Temperature and Humidity on the Microstructure of Sulfonated Syndiotactic-polystyrene Ionic Membranes. Membranes 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Ryu, S.K.; Kim, H.G.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Ameliorated Performance of Sulfonated Poly(Arylene Ether Sulfone) Block Copolymers with Increased Hydrophilic Oligomer Ratio in Proton-Exchange Membrane Fuel Cells Operating at 80% Relative Humidity. Polymers 2020, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid lonomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. Hydrog. Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 3, 1022–1077. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.P.; Kim, D.-S.; Guiver, M.; Hu, A.W.; Jiang, Z. Aromatic poly(ether ketone)s with pendant sulfonic acid phenyl groups prepared by a mild sulfonation method for proton exchange membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef] [Green Version]
- Kaliaguine, S.; Mikhailenko, S.; Wang, K.; Xing, P.; Robertson, G.; Guiver, M. Properties of SPEEK based PEMs for fuel cell application. Catal. Today 2003, 82, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; He, G.; Zhang, F. Sulfonated poly(phenylene sulfide) grafted polysulfone proton exchange membrane with improved stability. Int. J. Hydrog. Energy 2017, 42, 2360–2369. [Google Scholar] [CrossRef]
- Weiber, E.A.; Takamuku, S.; Jannasch, P. Highly Proton Conducting Electrolyte Membranes Based on Poly(arylene sulfone)s with Tetrasulfonated Segments. Macromolecules 2013, 46, 3476–3485. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Z.; Hu, M.; Hou, J.; Wu, L.; Xu, T. Perylene-based sulfonated aliphatic polyimides for fuel cell applications: Performance enhancement by stacking of polymer chains. J. Membr. Sci. 2018, 547, 43–50. [Google Scholar] [CrossRef]
- Chen, K.C.; Ji, M.D. High Tempreture Fuel Cell Performance and Anisotropy of Carbonyl and Sulfone Groups Co-crosslinked Sulfonated Polyimides Proton Exchange Membranes. Chem. J. Chin. Univ. 2016, 37, 989–995. [Google Scholar]
- Yin, Y.; Fang, J.; Watari, T.; Tanaka, K.; Kita, H.; Okamoto, K.-I. Synthesis and properties of highly sulfonated proton conducting polyimides from bis(3-sulfopropoxy)benzidine diamines. J. Mater. Chem. 2004, 14, 1062–1070. [Google Scholar] [CrossRef]
- Pan, H.; Chen, S.; Jin, M.; Chang, Z.; Pu, H. Preparation and properties of sulfonated polybenzimidazole-polyimide block copolymers as electrolyte membranes. Ionics 2018, 24, 1629–1638. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T.; Modestra, J.A.; Kumar, A.N.; Mohan, S.V. Highly efficient sulfonated polybenzimidazole as a proton exchange membrane for microbial fuel cells. J. Power Sources 2016, 317, 143–152. [Google Scholar] [CrossRef]
- Bai, H.; Ho, W.S.W. New sulfonated polybenzimidazole (SPBI) copolymer-based proton-exchange membranes for fuel cells. J. Taiwan Inst. Chem. Eng. 2009, 40, 260–267. [Google Scholar] [CrossRef]
- Shin, D.W.; Lee, S.Y.; Kang, N.R.; Lee, K.H.; Guiver, M.; Lee, Y.M. Durable Sulfonated Poly(arylene sulfide sulfone nitrile)s Containing Naphthalene Units for Direct Methanol Fuel Cells (DMFCs). Macromolecules 2013, 46, 3452–3460. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4611. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lee, H.-F.; Tseng, Y.-C.; Lee, C.-C.; Chang, M.-Y.; Huang, W.-Y. Synthesis of novel sulfonated poly(arylene ether)s containing a tetra-trifluoromethyl side chain and multi-phenyl for proton exchange membrane fuel cell application. RSC Adv. 2017, 7, 33068–33077. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-C.; Tai, R.-H.; Lee, H.-F.; Wang, P.-H.; Gopal, R.; Lee, C.-C.; Chang, M.-Y.; Huang, W.-Y. Synthesis of Highly Sulfonated Poly(arylene ether) Containing Multiphenyl for Proton Exchange Membrane Materials. Int. J. Polym. Sci. 2016, 2016, 6545362. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-F.; Wang, P.-H.; Huang, Y.-C.; Su, W.-H.; Gopal, R.; Lee, C.-C.; Holdcroft, S.; Huang, W.-Y. Synthesis and Proton Conductivity of Sulfonated, Multi-phenylated Poly(arylene ether)s. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2579–2587. [Google Scholar] [CrossRef]
- Adamski, M.; Skalski, T.J.; Schibli, E.M.; Killer, M.; Wu, Y.; Peressin, N.; Frisken, B.J.; Holdcroft, S. Molecular branching as a simple approach to improving polymer electrolyte membranes. J. Membr. Sci. 2020, 595, 117539. [Google Scholar] [CrossRef]
- Lee, K.-S.; Jeong, M.-H.; Lee, J.-P.; Kim, Y.-J.; Lee, J.-S. Synthesis and Characterization of Highly Fluorinated Cross-linked Aromatic Polyethers for Polymer Electrolytes. Chem. Mater. 2010, 22, 5500–5511. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Robertson, G.P.; Kim, Y.S.; Guiver, M.D. Copoly(arylene ether)s Containing Pendant Sulfonic Acid Groups as Proton Exchange Membranes. Macromolecules 2009, 42, 957–963. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Fuessl, A.; Yamamoto, M.; Schneller, A. Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mohanty, A.K.; Mistri, E.A.; Banerjee, S.; Komber, H.; Voit, B. Highly Fluorinated Sulfonated Poly(arylene ether sulfone) Copolymers: Synthesis and Evaluation of Proton Exchange Membrane Properties. Ind. Eng. Chem. Res. 2013, 52, 2772–2783. [Google Scholar] [CrossRef]
- Banerjee, S.; Maier, G.; Salunke, A.; Madhra, M. Novel high-T-g, high-strength poly(aryl ether) containing quadrephenyl unit. J. Appl. Polym. Sci. 2001, 82, 3149–3156. [Google Scholar] [CrossRef]
- Lee, H.-F.; Huang, Y.-C.; Wang, P.-H.; Lee, C.-C.; Hung, Y.-S.; Gopal, R.; Holdcroft, S.; Huang, W.-Y. Synthesis of highly sulfonated polyarylene ethers containing alternating aromatic units. Mater. Today Commun. 2015, 3, 114–121. [Google Scholar] [CrossRef]
- Parreno, R.P., Jr.; Liu, Y.-L.; Beltran, A.-B.; Carandang, M.-B. Effect of a direct sulfonation reaction on the functional properties of thermally-crosslinked electrospun polybenzoxazine (PBz) nanofibers. RSC Adv. 2020, 10, 14198–14207. [Google Scholar] [CrossRef]
- Pirali-Hamedani, M.; Mehdipour-Ataei, S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017, 20, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochimica Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Iulianelli, A.; Clarizia, G.; Gugliuzza, A.; Ebrasu, D.; Bevilacqua, A.; Trotta, F.; Basile, A. Sulfonation of PEEK-WC polymer via chloro-sulfonic acid for potential PEM fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 12688–12695. [Google Scholar] [CrossRef]
- Siengchum, T.; Isenberg, M.; Chuang, S.S.C. Fast pyrolysis of coconut biomass—An FTIR study. Fuel 2013, 105, 559–565. [Google Scholar] [CrossRef]
- Takenaka, M.; Kimura, Y.; Ohara, H. Influence of decomposition temperature of aromatic sulfonic acid catalysts on the molecular weight and thermal stability of poly(L-lactic acid) prepared by melt/solid state polycondenstaion. Polymer 2018, 155, 218–224. [Google Scholar] [CrossRef]
- Mukherjee, R.; Banerjee, S.; Komber, H.; Voit, B. Carboxylic acid functionalized fluorinated sulfonated poly(arylene ether sulfone) copolymers with enhanced oxidative stability. J. Membr. Sci. 2016, 510, 497–509. [Google Scholar] [CrossRef]
- Jo, S.G.; Kim, T.-H.; Yoon, S.J.; Oh, S.-G.; Cha, M.S.; Shin, H.Y.; Ahn, J.M.; Lee, J.Y.; Hong, Y.T. Synthesis and investigation of random-structured ionomers with highly sulfonated multi-phenyl pendants for electrochemical applications. J. Membr. Sci. 2016, 510, 326–337. [Google Scholar] [CrossRef]
- Ijaodola, O.S.; Hassan, Z.E.-; Ogungbemi, E.; Khatib, F.; Wilberforce, T.; Thompson, J.; Olabi, A. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Lee, S.; Ann, J.; Lee, H.; Kim, J.-H.; Yang, T.-H.; Bae, B. Synthesis and characterization of crosslink-free highly sulfonated multi-block poly(arylene ether sulfone) multi-block membranes for fuel cells. J. Mater. Chem. A 2015, 3, 1833–1836. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Kim, D.-S.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Comparison of PEM properties of copoly(aryl ether ether nitrile)s containing sulfonic acid bonded to naphthalene in structurally different ways. Macromolecules 2007, 40, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures-Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Kim, S.; Melnyk, A.; Andrienko, D.; Suzuki, Y. Solid-State Electron Affinity Analysis of Amorphous Fluorinated Polymer Electret. J. Phys. Chem. B 2020, 124, 10507–10513. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Mistri, E.A.; Ghosh, A.; Banerjee, S. Synthesis and characterization of novel fluorinated poly(arylene ether sulfone)s containing pendant sulfonic acid groups for proton exchange membrane materials. J. Membr. Sci. 2012, 409, 145–155. [Google Scholar] [CrossRef]
- Nandjou, E.; Poirot-Crouvezier, J.-P.; Chandesris, M.; Blachot, J.-F.; Bonnaud, C.; Bultel, Y. Impact of heat and water management on proton exchange membrane fuel cells degradation in automotive application. J Power Sources 2016, 326, 182–192. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer Science & Business Media: Vancouver, BC, Canada, 2008. [Google Scholar]


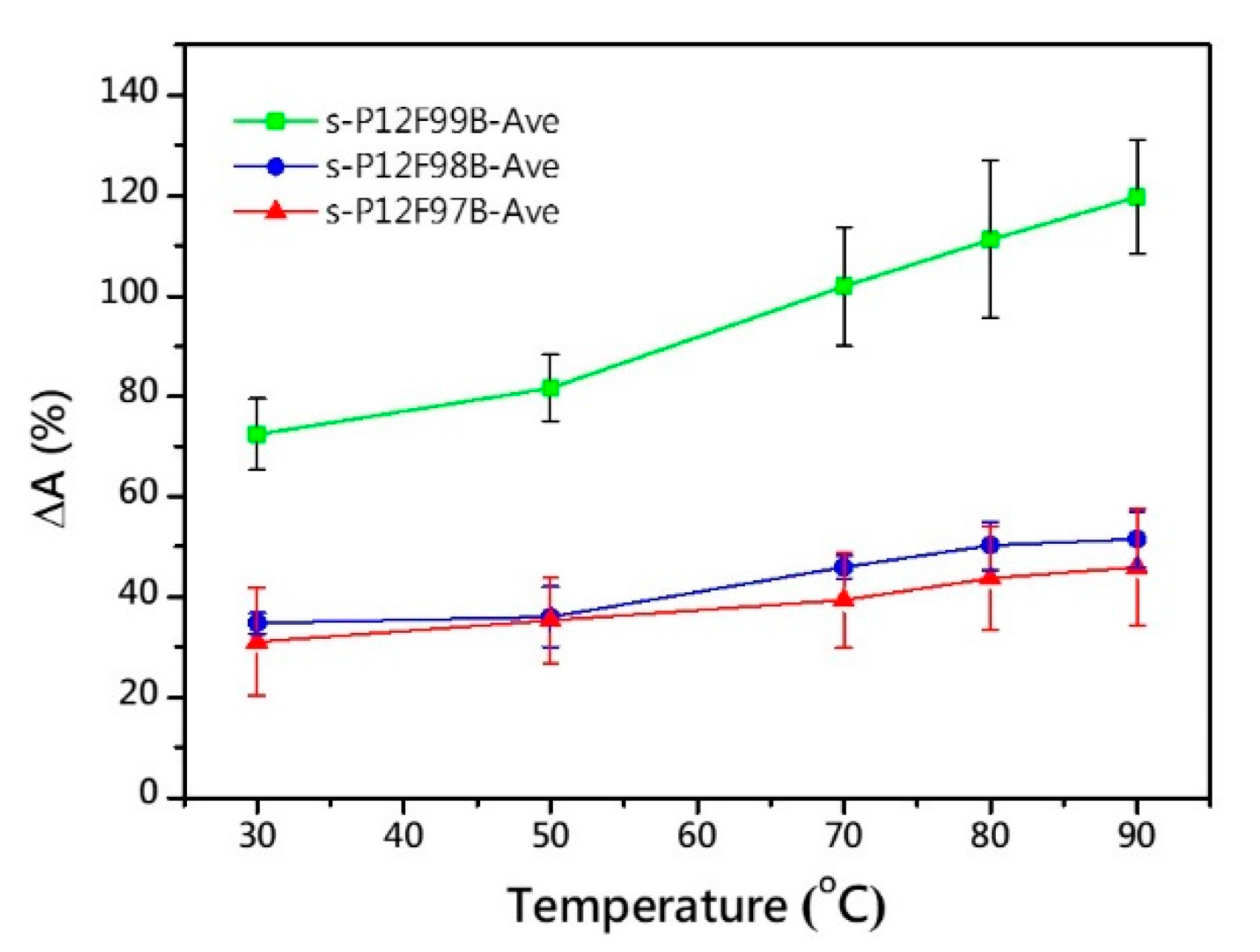


| Membrane | Td5% (°C) | Young’s Modulus a (GPa) | Tensile Strength a (MPa) | Elongation at Break a (%) |
|---|---|---|---|---|
| P12F99B | 589.0 | 1.69 | 86.2 | 25.6 |
| s-P12F99B-2.89 | 265.4 | 0.51 | 68.3 | 74.9 |
| s-P12F99B-3.23 | 230.8 | 0.45 | 48.7 | 57.7 |
| P12F98B | 610.1 | 1.83 | 80.3 | 34.9 |
| s-P12F98B-2.61 | 262.6 | 0.59 | 70.7 | 75.6 |
| s-P12F98B-3.00 | 232.2 | 0.53 | 56.2 | 66.9 |
| P12F97B | 608.9 | 1.60 | 97.1 | 40.3 |
| s-P12F97B-2.84 | 251.0 | 1.03 | 76.6 | 95.8 |
| s-P12F97B-2.92 | 234.7 | 0.73 | 59.6 | 82.7 |
| Nafion 211 | - | 0.28 | 34.2 | 101.5 |
| Membrane | IEC a | WU (%) | Λ b | ΔA (%) | ΔT c (%) | OS (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mmol g−1) | 30 °C | 80 °C | 30 °C | 80 °C | 30 °C | 80 °C | 30 °C | 80 °C | 80 °C | |
| s-P12F99B-2.89 | 2.89 | 96.6 | 114.3 | 18.6 | 28.0 | 64.3 | 100.1 | 35.9 | 61.5 | - |
| s-P12F99B-3.23 | 3.23 | 106.7 | 142.9 | 18.3 | 26.0 | 76.5 | 122.3 | 35.5 | - | - |
| s-P12F98B-2.61 | 2.61 | 65.4 | 88.5 | 13.9 | 18.8 | 36.9 | 52.0 | 29.7 | 54.1 | 55.9 |
| s-P12F98B-3.00 | 3.00 | 66.7 | 103.3 | 12.3 | 19.1 | 33.7 | 49.4 | 31.9 | 56.0 | 61.2 |
| s-P12F97B-2.84 | 2.84 | 40.0 | 74.6 | 7.8 | 14.6 | 28.6 | 45.1 | 26.3 | 42.1 | 50.0 |
| s-P12F97B-2.92 | 2.92 | 39.3 | 78.6 | 7.5 | 14.9 | 32.4 | 43.1 | 29.1 | 42.5 | 55.0 |
| Nafion 211 | 0.91 | 17.0 | 29.0 | 7.5 | 22.0 | - | - | - | - | 88.6 |
| Membrane | Proton Conductivity at 80 °C (mS/cm) | λ | MVC | MCV (Wet) at 80 °C | PCV at 80 °C | |||
|---|---|---|---|---|---|---|---|---|
| 40%RH | 60%RH | 80%RH | 95%RH | (cm3 eq.−1 mol−1) | (cm3 eq.−1 mol−1) | |||
| s-P12F99B-2.89 | 15.8 | 55.8 | 134.2 | 294.1 | 28.0 | 151.8 | 713.5 | 0.71 |
| s-P12F99B-3.23 | 19.5 | 60.2 | 151.0 | 301.8 | 26.0 | 139.5 | 658.4 | 0.71 |
| s-P12F98B-2.61 | 11.3 | 42.7 | 103.3 | 212.6 | 18.8 | 161.6 | 562.8 | 0.60 |
| s-P12F98B-3.00 | 17.2 | 55.8 | 128.0 | 255.1 | 19.1 | 145.1 | 543.8 | 0.63 |
| s-P12F97B-2.84 | 14.2 | 43.8 | 100.9 | 173.9 | 14.6 | 142.6 | 458.1 | 0.57 |
| s-P12F97B-2.92 | 11.2 | 40.7 | 101.6 | 187.3 | 14.9 | 139.7 | 460.3 | 0.58 |
| Nafion 211 | 14.6 | 38.8 | 64.5 | 123.8 | 14.8 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-S.; Hsieh, T.-L.; Lai, C.-C.; Wen, H.-Y.; Huang, W.-Y.; Chang, M.-Y. Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications. Membranes 2021, 11, 626. https://doi.org/10.3390/membranes11080626
Huang T-S, Hsieh T-L, Lai C-C, Wen H-Y, Huang W-Y, Chang M-Y. Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications. Membranes. 2021; 11(8):626. https://doi.org/10.3390/membranes11080626
Chicago/Turabian StyleHuang, Tzu-Sheng, Tung-Li Hsieh, Chih-Ching Lai, Hsin-Yi Wen, Wen-Yao Huang, and Mei-Ying Chang. 2021. "Highly Proton-Conducting Membranes Based on Poly(arylene ether)s with Densely Sulfonated and Partially Fluorinated Multiphenyl for Fuel Cell Applications" Membranes 11, no. 8: 626. https://doi.org/10.3390/membranes11080626






