A Facile Method to Control Pore Structure of PVDF/SiO2 Composite Membranes for Efficient Oil/Water Purification
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Characterization of PVDF/SiO2 Dope Solutions
2.2.1. Solution Preparation
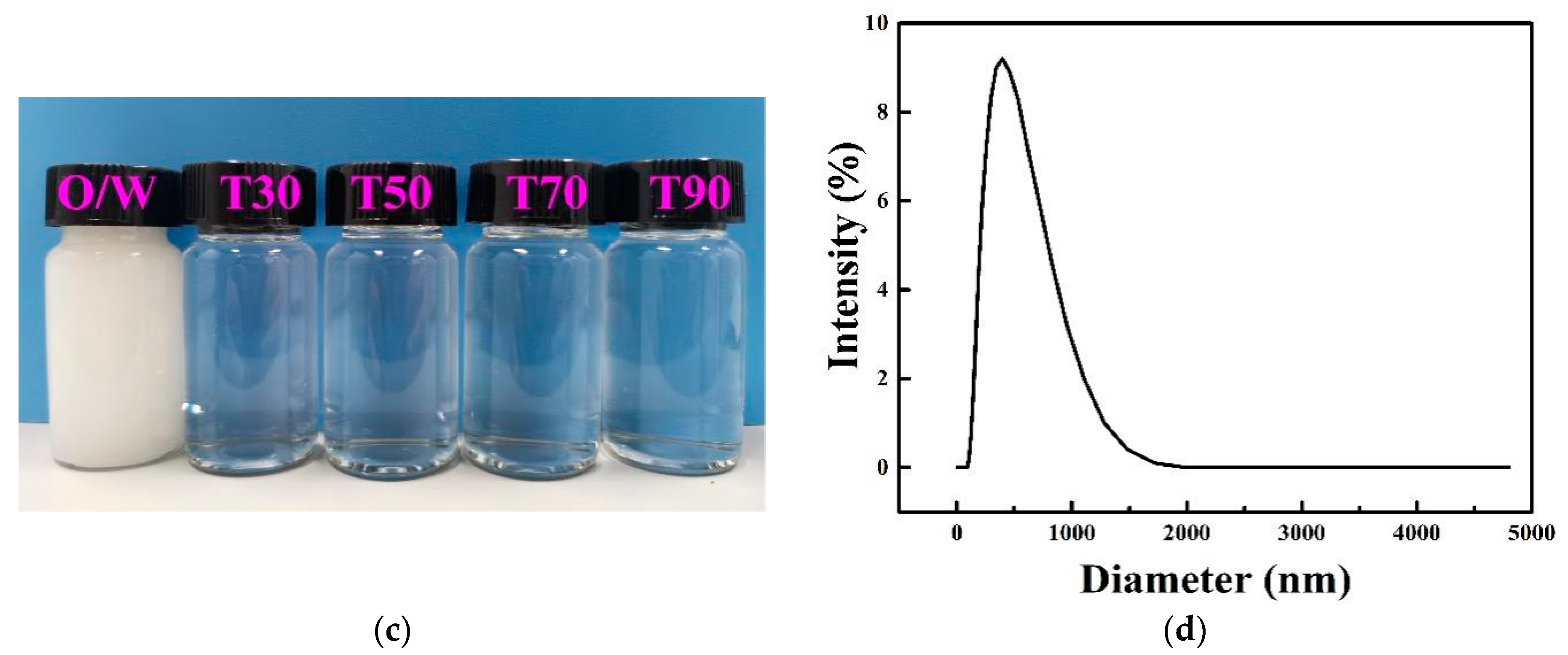
2.2.2. Cloud Points
2.3. Preparation of PVDF/SiO2 Composite Membranes
2.4. Membrane Characterization
2.5. Membrane Performance Testing
2.5.1. Preparation of Oil-in-Water Emulsion
2.5.2. Filtration Performance with Pure Water and Oil/Water Emulsion
2.5.3. Fouling Evaluation Experiments
3. Results and Discussion
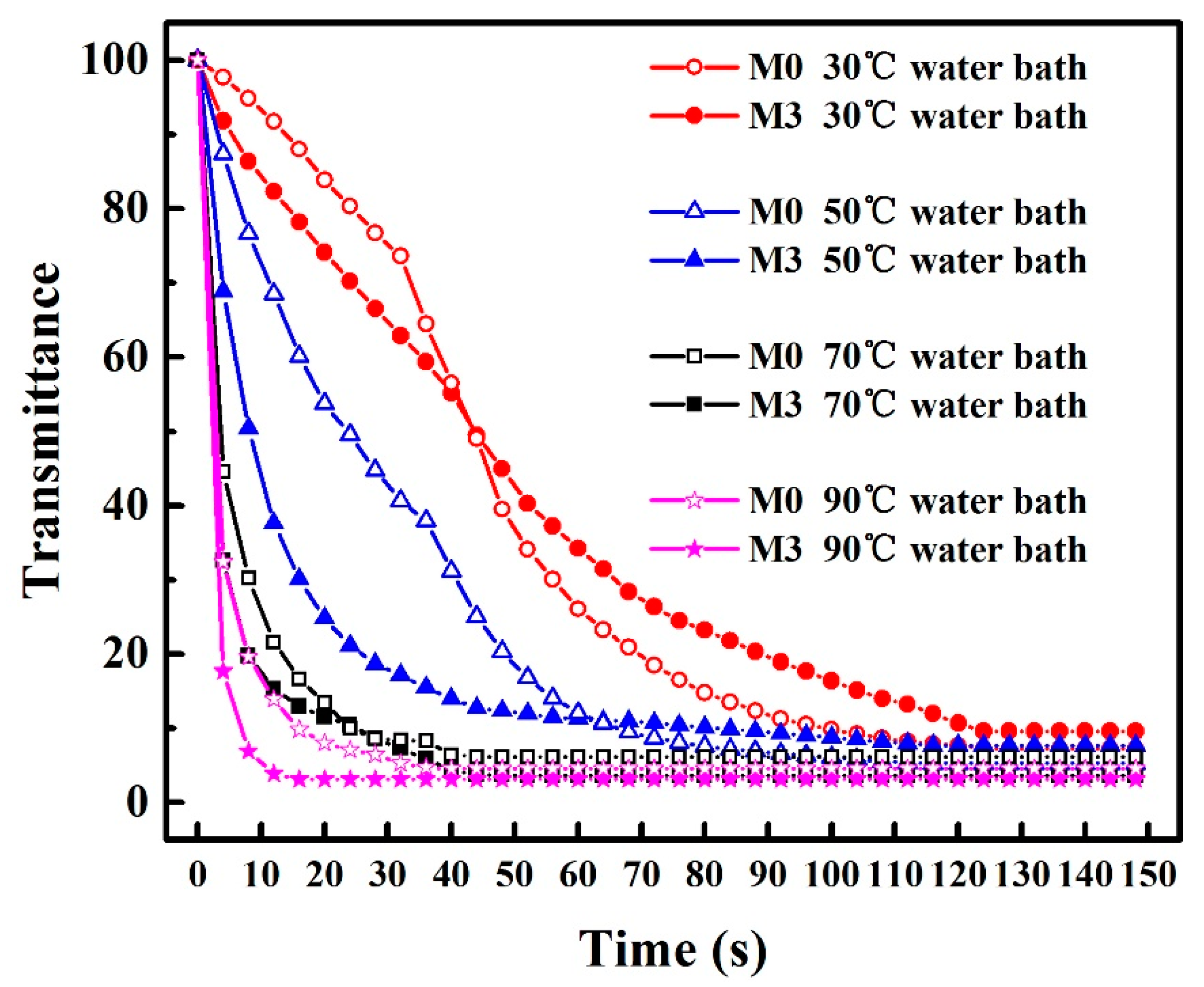
3.1. The Cloud Point of PVDF/SiO2 Dope Solutions
3.2. The Morphology of PVDF/SiO2 Composite Membranes
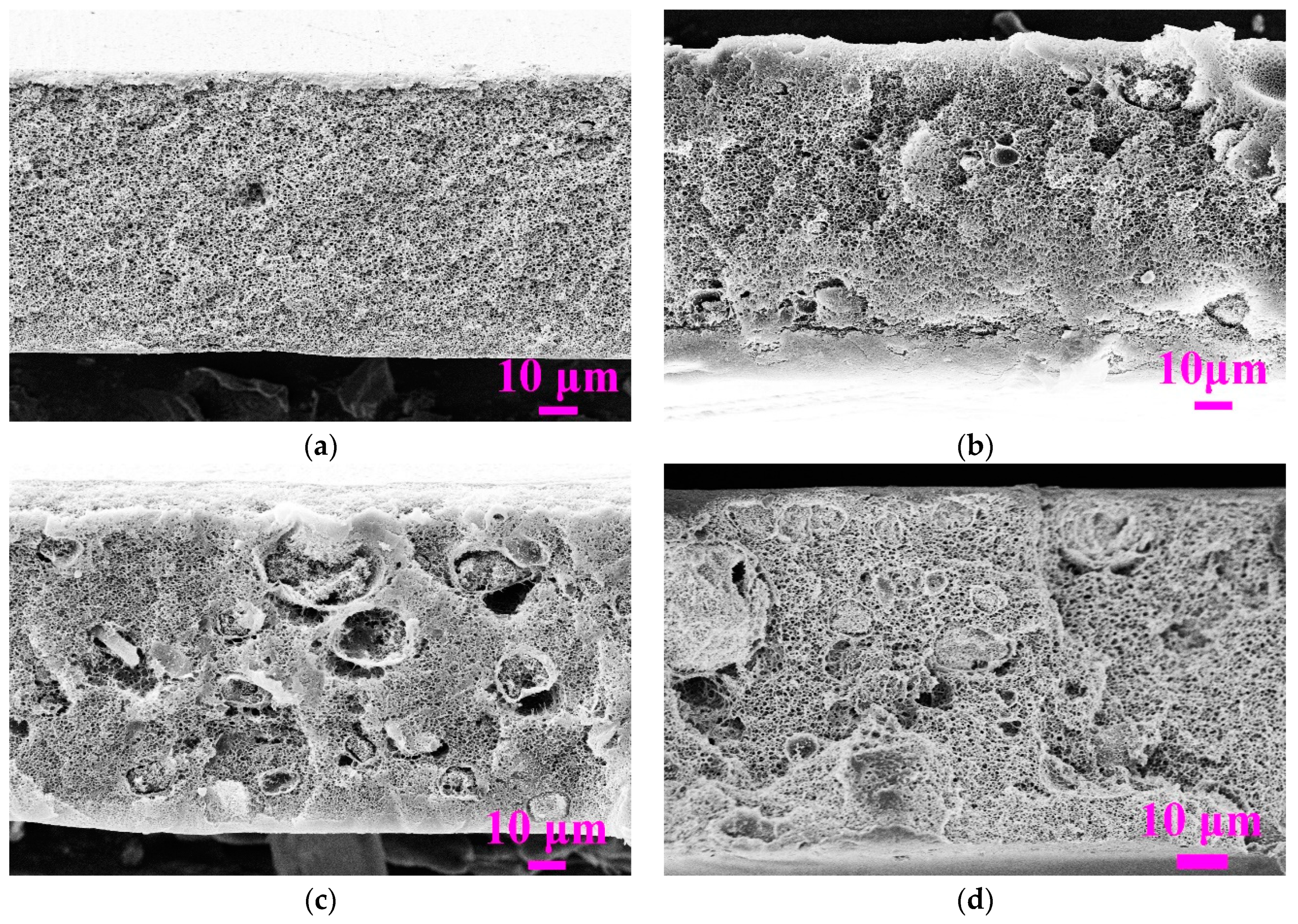
3.2.1. Effect of PVDF Concentration
3.2.2. Effect of Weight Ratio of SiO2/PVDF on Membrane Morphology
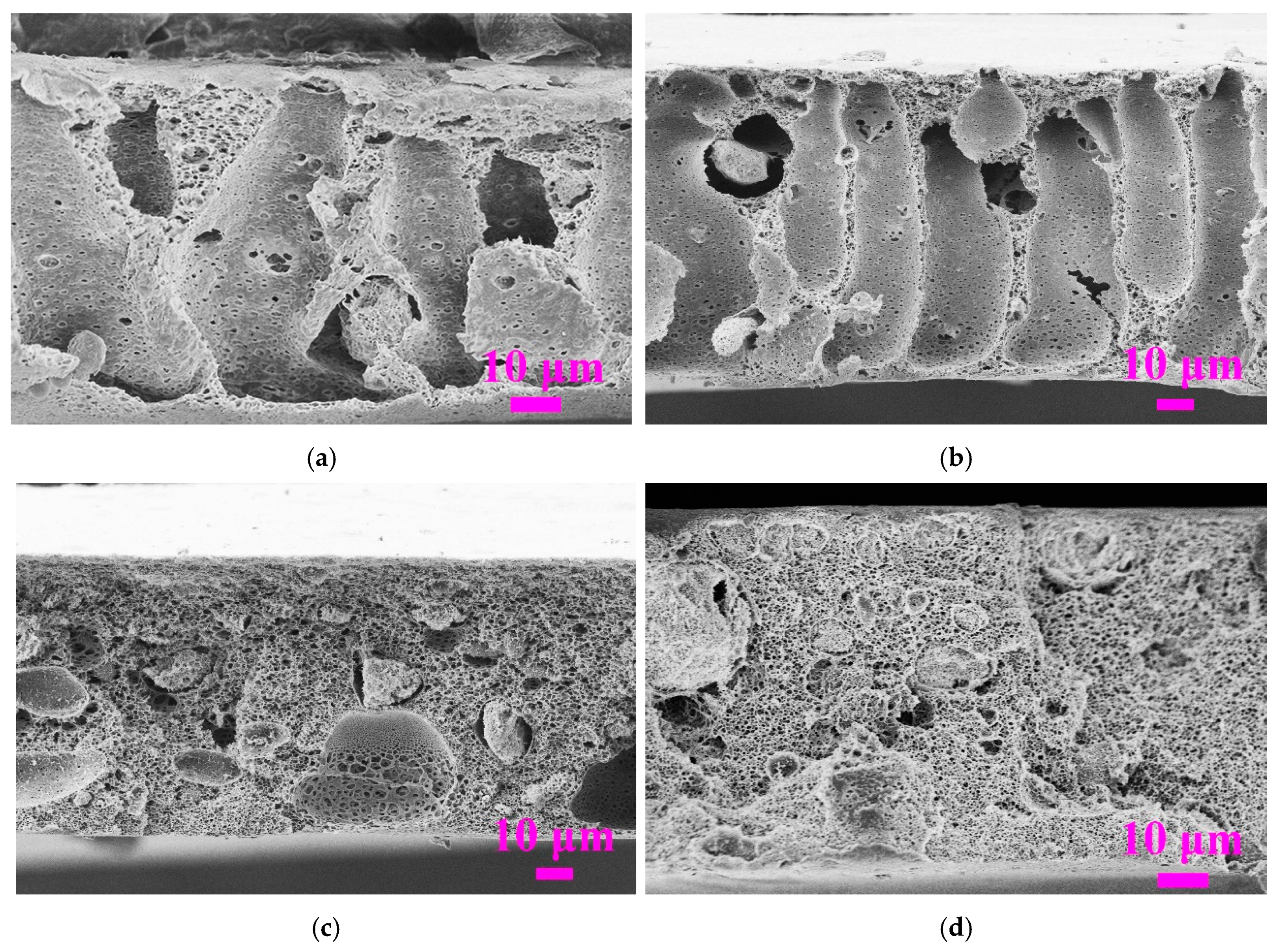
3.2.3. Effect of Coagulation Temperature
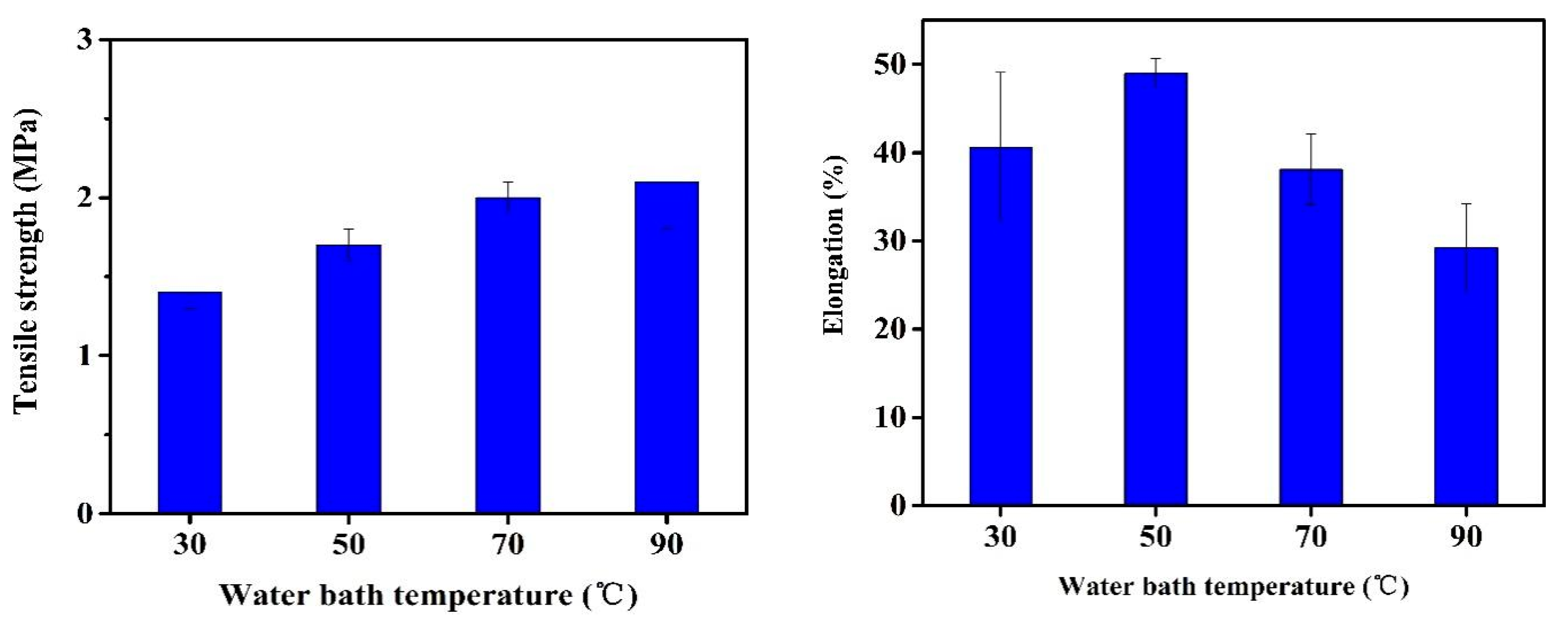
3.3. Membrane Pore Size, Contact Angle, and Mechanical Properties
3.4. Evaluation of Separation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Han, M.; He, F. A Review of Treating Oily Wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pan, K.; Li, L.; Cao, B. Surface Hydrophilicity and Structure of Hydrophilic Modified PVDF Membrane by Nonsolvent Induced Phase Separation and Their Effect on Oil/Water Separation Performance. Ind. Eng. Chem. Res. 2014, 53, 6401–6408. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based Separation for Oily Wastewater: A Practical Perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Long, N.B.; Xiao, T.H.; Yang, X. Tuning the Pore Structure of Poly(vinylidene fluoride) Membrane for Efficient Oil/Water Separation: A Novel Vapor Induced Phase Separation Method based on Lower Critical Solution Temperature system. Ind. Eng. Chem. Res. 2020, 59, 14947–14959. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane Technology Enhancement in Oil-water Separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Luo, L.; Han, G.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Oil/water Separation via Ultrafiltration by Novel Triangle-shape Tri-bore Hollow Fiber Membranes from Sulfonated Polyphenylenesulfone. J. Membr. Sci. 2015, 476, 162–170. [Google Scholar] [CrossRef]
- Szep, A.; Kohlheb, R. Water Treatment Technology for Produced Water. Water Sci. Technol. 2010, 62, 2372–2380. [Google Scholar] [CrossRef]
- Bilstad, T.; Espedal, E. Membrane Separation of Produced Water. Water Sci. Technol. 1996, 34, 239–246. [Google Scholar] [CrossRef]
- Hassankiadeh, N.T.; Cui, Z.; Kim, J.H.; Shin, D.W.; Lee, S.Y.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Microporous Poly(vinylidene fluoride) Hollow Fiber Membranes Fabricated with PolarClean as Water-soluble Green Diluent and Additives. J. Membr. Sci. 2015, 479, 204–212. [Google Scholar] [CrossRef]
- Liu, F.; Awanis Hashim, N.; Liu, Y.T.; Moghareh Abed, M.R.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, T.H.; Yang, X. Facile Pore Structure Control of Poly(vinylidene fluoride) Membrane for Oil/Water Separation. Sep. Purif. Technol. 2020, 251, 117305. [Google Scholar] [CrossRef]
- Mueller, J.; Cen, Y.; Davis, R.H. Crossflow Microfiltration of Oily Water. J. Membr. Sci. 1997, 129, 221–235. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of Membrane Fouling at Constant Flux and Constant Transmembrane Pressure Conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, M.; Zhang, J.; Wang, X.; Tao, H.; Ge, L. Formation of Poly(vinylidene fluoride) (PVDF) Membranes via Thermally Induced Phase Separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.P.; Bouyer, D.; Drioli, E.; Tavajohi Hassan Kiadeh, N. Investigating the Potential of Membranes Formed by the Vapor Induced Phase Separation Process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Qin, J.J.; Oo, M.H.; Cao, Y.M.; Lee, L.S. Development of A LCST Membrane Forming System for Cellulose Acetate Ultrafiltration Hollow Fiber. Sep. Purif. Technol. 2005, 42, 291–295. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of High Flux Ultrafiltration Polyphenylsulfone Membranes Applying the Systems with Upper and Lower Critical Solution Temperatures: Effect of Polyethylene Glycol Molecular Weight and Coagulation Bath Temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Kim, J.F.; Jung, J.T.; Wang, H.H.; Lee, S.Y.; Moore, T.; Sanguineti, A.; Drioli, E.; Lee, Y.M. Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS) and Stretching Methods. J. Membr. Sci. 2016, 509, 94–104. [Google Scholar] [CrossRef]
- Kinzer, K.E.; Tseng, H.S. Microporous Membrane Formation via Thermally Induced Phase Separetion. I. Solid-liquid Phase Separation. J. Membr. Sci. 1990, 52, 239–261. [Google Scholar]
- Kang, G.D.; Cao, Y.M. Application and Modification of Poly(vinylidene fluoride)(PVDF) Membranes—A Review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and Characterization of PVDF/TiO2 Organic-inorganic Composite Membranes for Fouling Resistance Improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Vatanpour, V. A Comprehensive Study on the Performance and Antifouling Enhancement of the PVDF Mixed Matrix Membranes by Embedding Different Nanoparticulates: Clay, Functionalized Carbon Nanotube, SiO2 and TiO2. Sep. Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Yu, L.Y.; Xu, Z.L.; Shen, H.M.; Yang, H. Preparation and Characterization of PVDF-SiO2 Composite Hollow Fiber UF Membrane by Sol-gel Method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Liao, C.; Zhao, J.; Yu, P.; Tong, H.; Luo, Y. Synthesis and Characterization of Low Content of Different SiO2 Materials Composite Poly(vinylidene fluoride) Ultrafiltration Membranes. Desalination 2012, 285, 117–122. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yub, L.E.; Laic, J.Y.; Chung, T.S. Developing Ultra-high Gas Permeance PVDF Hollow Fibers for Air Filtration Applications. Sep. Purif. Technol. 2018, 205, 184–195. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Yu, Y.; Deng, B.; Li, J.; Jin, J. Sol-gel Preparation of PAA-g-PVDF/TiO2 Nanocomposite Hollow Fiber Membranes with Extremely High Water Flux and Improved Antifouling Property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of Novel SiO2-GO Nanohybrid/polysulfone Membrane with Enhanced Performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and Characterization of Low Fouling Novel Hybrid Ultrafiltration Membranes Based on the Blends of GO−TiO2 Nanocomposite and Polysulfone for Humic Acid Removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Pan, Z.L.; Dong, Q.; Xu, N.; Wang, T.H. Inorganic Nanoparticles Incorporated in Polyacrylonitrile-based Mixed Matrix Membranes for Hydrophilic, Ultrafast, and Fouling-resistant Ultrafiltration. J. Appl. Polym. Sci. 2019, 136, 47902. [Google Scholar] [CrossRef]
- Xiao, T.H.; Wang, P.; Yang, X.; Cai, X.H.; Lu, J. Fabrication and Characterization of Novel Asymmetric Polyvinylidene Fluoride (PVDF) Membranes by the Nonsolvent Thermally Induced Phase Separation (NTIPS) Method for Membrane Distillation Applications. J. Membr. Sci. 2015, 489, 160–174. [Google Scholar] [CrossRef]
- Feng, C.; Shi, B.; Li, G.M.; Wu, Y. Preparation and Properties of Microporous Membrane From Poly(vinylidene fluoride-co-tetrafluoroethylene) (F2.4) For Membrane Distillation. J. Membr. Sci. 2004, 237, 15–24. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Xu, Z.; Guo, R.; Zhao, L. Preparation and Characterization of Polyethersulfone Microporous Membrane via Thermally Induced Phase Separation with Low Critical Solution Temperature System. J. Membr. Sci. 2013, 437, 169–178. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, J.; Qiu, M.; He, C. Antifouling Performance of Poly(lysine methacrylamide)-grafted PVDF Microfiltration Membrane for Solute Separation. Sep. Purif. Technol. 2016, 171, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in Phase-inversion Membranes. Part 1. Formation of Macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Xu, Z.L.; Yu, L.Y. Effects of Mixed Solvents and PVDF Types on Performances of PVDF Microporous Membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Sun, A.; Kosar, W.; Zhang, Y.; Feng, X. A Study of Thermodynamics and Kinetics Pertinent to Formation of PVDF Membranes by Phase Inversion. Desalination 2013, 309, 156–164. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Jiang, J.L.; Wang, X.D.; Huo, X.N.; Xu, Y.W.; Li, Q.Q.; Wang, L. Improving the Hydrophilic and Antifouling Properties of Polyvinylidene Fluoride Membrane by Incorporation of Novel Nanohybrid GO@SiO2 Particles. Chem. Eng. J. 2017, 314, 266–276. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical Cleaning/disinfection and Ageing of Organic UF Membranes: A Review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A Modified Mussel-inspired Method to Fabricate TiO2 Decorated Superhydrophilic PVDF Membrane for Oil/water Separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.; Huang, A.; Li, L. A Low-cost Mullite-titania Composite Ceramic Hollow Fiber Microfiltration Membrane for Highly Efficient Separation of Oil-in-water Emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, S.; Mao, H.; Li, M.; Xue, A.; Zhao, Y.; Xing, W. A Novel Ceramic Microfiltration Membrane Fabricated by Anthurium Andraeanum-like Attapulgite Nanofibers for High-efficiency Oil-in-water Emulsions Separation. J. Membr. Sci. 2021, 630, 119291. [Google Scholar] [CrossRef]
- Nayak, K.; Tripathi, B.P. Molecularly Grafted PVDF Membranes with In-air Superamphiphilicity and Underwater Superoleophobicity for Oil/water Separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Song, C.; Rutledge, G.C. Electrospun Polyimide Fiber Membranes for Separation of Oil-in-water Emulsions. Sep. Purif. Technol. 2021, 270, 118825. [Google Scholar] [CrossRef]





| Dope Solution Code | Composition of Dope Solution (wt%) | ||||
|---|---|---|---|---|---|
| PVDF | SiO2 | PVP | DMAc | MgCl2 | |
| M0 | 16 | 0 | 8 | 68.0 | 8 |
| M1 | 16 | 0.53 | 8 | 67.5 | 8 |
| M2 | 16 | 1.07 | 8 | 66.9 | 8 |
| M3 | 16 | 1.6 | 8 | 66.4 | 8 |
| M4 | 14 | 1.4 | 8 | 68.6 | 8 |
| M5 | 12 | 1.2 | 8 | 70.8 | 8 |
| M6 | 10 | 1.0 | 8 | 73.0 | 8 |
| Membrane ID | Coagulation Temperature (°C) | Porosity (ε, %) | rmax (μm) | rm (μm) | rmax/rm | Contact Angle (°) |
|---|---|---|---|---|---|---|
| T30 | 30 | 84.8 ± 0.5 | 0.161 ± 0.013 | 0.072 ± 0.014 | 2.2 | 81 ± 2 |
| T50 | 50 | 80.6 ± 0.4 | 0.187 ± 0.014 | 0.101 ± 0.016 | 1.9 | 73 ± 1 |
| T70 | 70 | 79.7 ± 1.1 | 0.186 ± 0.024 | 0.084 ± 0.021 | 2.2 | 74 ± 1 |
| T90 | 90 | 81.4 ± 0.7 | 0.203 ± 0.007 | 0.068 ± 0.014 | 3.0 | 68 ± 2 |
| Membrane Material | Pore Size (μm) | Type of Oil Emulsion | Oil Droplet Diameter (μm) | Permeate Flux (L·m−2·h−1·bar−1 ) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|
| PVDF/TiO2 | / | Diesel oil (10 g/L) | 1–20 | 382 | 99 | [40] |
| Ceramic (modified) | 0.11 | Soybean oil (0.2 g/L) | 1.09 | 150 | 97.0 | [41] |
| Ceramic (FATP) | 0.6 | Engine oil (2 g/L) | 5 | 209 | 98.7 | [42] |
| PVDF (grafted) | 0.45 | Soybean oil (100 g/L) | 0.7–1.4 | 10 | 99 | [43] |
| Polyimide | 0.16 | Dodecane (5 g/L) | 0.63 | 121 | 86.6 | [44] |
| PVDF/SiO2 (T70) | 0.17 | Soybean oil (5 g/L) | 0.1–1.7 | 413 | 99.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Chen, Y.; Xiao, T.; Yang, X. A Facile Method to Control Pore Structure of PVDF/SiO2 Composite Membranes for Efficient Oil/Water Purification. Membranes 2021, 11, 803. https://doi.org/10.3390/membranes11110803
Xu Q, Chen Y, Xiao T, Yang X. A Facile Method to Control Pore Structure of PVDF/SiO2 Composite Membranes for Efficient Oil/Water Purification. Membranes. 2021; 11(11):803. https://doi.org/10.3390/membranes11110803
Chicago/Turabian StyleXu, Qianqian, Yuchao Chen, Tonghu Xiao, and Xing Yang. 2021. "A Facile Method to Control Pore Structure of PVDF/SiO2 Composite Membranes for Efficient Oil/Water Purification" Membranes 11, no. 11: 803. https://doi.org/10.3390/membranes11110803







