Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
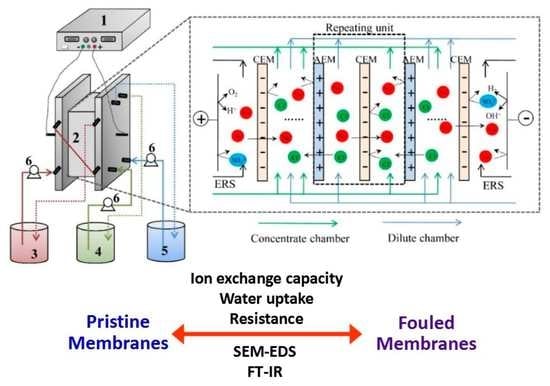
2.2. ED Set-Up
2.3. Characterization of Ion Exchange Membranes
2.4. Desalination Rate
3. Results
3.1. Determination of Limiting Current Density (LCD)
3.2. Continuous Batch ED Operation
3.3. The Main Property Comparison between Pristine and Fouled Membranes
3.4. Characterization of the Fouled Membranes
3.5. Membrane Fouling Speculation
3.6. Membrane Fouling Cleaning Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, J.G.; He, J.W.; Wu, F.G.; Tu, S.X.; Yan, T.J.; Si, H.; Xie, H. Comparative analysis on chemical components and sensory quality of aging flue-cured tobacco from four main tobacco areas of china. Agric. Sci. China 2011, 10, 1222–1231. [Google Scholar] [CrossRef]
- Wang, W.S.; Wang, Y.; Yang, L.J.; Liu, B.Z.; Lan, M.B.; Sun, W.L. Studies on thermal behavior of reconstituted tobacco sheet. Thermochim. Acta 2005, 437, 7–11. [Google Scholar] [CrossRef]
- Potts, R.J.; Bombick, B.R.; Meckley, D.R.; Ayres, P.H.; Pence, D.H. A summary of toxicological and chemical data relevant to the evaluation of cast sheet tobacco. Exp. Toxicol. Pathol. 2010, 62, 117–126. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, P.B.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, C.H.; Xu, Y.B.; Hu, Y. The pyrolysis of cigarette paper under the conditions that simulate cigarette smouldering and puffing. J. Therm. Anal. Calorim. 2011, 104, 1097–1106. [Google Scholar] [CrossRef]
- Zhou, S.; Ning, M.; Zhang, Y.P.; He, Q.; Wang, X.F.; Zhu, D.L.; Guo, S.; Hong, N.P.; Hu, Y. Significant removal of harmful compounds in mainstream cigarette smoke using carbon nanotubes mixture prepared by catalytic pyrolysis. Adsorpt. Sci. Technol. 2014, 32, 453–464. [Google Scholar] [CrossRef]
- Seyler, T.H.; Kim, J.G.; Hodgson, J.A.; Cowan, E.A.; Blount, B.C.; Wang, L.Q. Quantitation of urinary volatile nitrosamines from exposure to tobacco smoke. J. Anal. Toxicol. 2013, 37, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Narkowicz, S.; Polkowska, Z.; Kielbratowska, B.; Namiesnik, J. Environmental tobacco smoke: Exposure, health effects and analysis. Crit. Rev. Environ. Sci. Technol. 2013, 43, 121–161. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis. 2008, 83, 12–25. [Google Scholar] [CrossRef]
- Uhl, R.G.R.L. Denitration separete burley processing. 1977. Available online: http://legacy.library.ucsf.edutid/kzm02a00/pdf (accessed on 19 September 2020).
- Jeanneret, C.; Morris, P. Legislation 800124800224. 1981. Available online: http://industrydocuments.library.ucsf.edu/tobacco/docs/klxk0000 (accessed on 19 September 2020).
- Gaisch, H.; Krasna, B.; Schulthess, D. Continuous Method of Denitrating Tobacco Extracts. U.S. Patent 4,622,982, 18 November 1986. [Google Scholar]
- Mattina, C.F.; Selke, A.S. Method of Making Reconstituted Tobacco Having Reduced Nitrates. U.S. Patent 3,847,164, 12 November 1974. [Google Scholar]
- Baker, R.R.; da Silva, J.R.P.; Smith, G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food Chem. Toxicol. 2004, 42, S3–S37. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.B.; Yang, Z.J.; Wang, Y.M.; Jiang, C.X.; Ge, L.; Bakangura, E.; Xu, T.W. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhou, M.M.; Yan, B.H.; Sun, X.H.; Liu, Y.; Wang, Y.M.; Xu, T.W.; Zhang, Y. Waste conversion and resource recovery from wastewater by ion exchange membranes: State-of-the-art and perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Bazinet, L.; Degrandpre, Y.; Porter, A. Electromigration of tobacco polyphenols. Sep. Purif. Technol. 2005, 41, 101–107. [Google Scholar] [CrossRef]
- Bazinet, L.; DeGrandpre, Y.; Porter, A. Enhanced tobacco polyphenol electromigration and impact on membrane integrity. J. Membr. Sci. 2005, 254, 111–118. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ge, S.L.; Jiang, C.X.; Zhao, Y.; Wang, Y.M. Improving the smoking quality of papermaking tobacco sheet extract by using electrodialysis. Membr. Water Treat. 2014, 5, 31–40. [Google Scholar] [CrossRef]
- Ge, S.L.; Li, W.; Zhang, Z.; Li, C.R.; Wang, Y.M. Desalting tobacco extract using electrodialysis. Membr. Water Treat. 2016, 7, 341–353. [Google Scholar] [CrossRef]
- Li, C.R.; Ge, S.L.; Li, W.; Zhang, Z.; She, S.K.; Huang, L.; Wang, Y.M. Desalting of papermaking tobacco sheet extract using selective electrodialysis. Membr. Water. Treat. 2017, 8, 381–393. [Google Scholar]
- Lindstrand, V.; Sundstrom, G.; Jonsson, A.S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Lindstrand, V.; Jonsson, A.S.; Sundstrom, G. Organic fouling of electrodialysis membranes with and without applied voltage. Desalination 2000, 130, 73–84. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, M.K.; Han, S.D.; Cho, S.H.; Moon, S.H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Roberts, W.A. Foraging and spatial memory in pigeons(columba-livia). J. Comp. Psychol. 1988, 102, 108–117. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Klaysom, C.; Marschall, R.; Wang, L.Z.; Ladewig, B.P.; Lu, G.Q.M. Synthesis of composite ion-exchange membranes and their electrochemical properties for desalination applications. J. Mater. Chem. 2010, 20, 4669–4674. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Jiang, C.X.; Li, Y.Y.; Shehzad, M.A.; Wang, X.; Wang, Y.M.; Xu, T.W. Electro-driven in situ construction of functional layer using amphoteric molecule: The role of tryptophan in ion sieving. ACS Appl. Mater. Interfaces 2019, 11, 36626–36637. [Google Scholar] [CrossRef]
- Lee, H.J.; Strathmann, H.; Moon, S.H. Determination of the limiting current density in electrodialysis desalination as an empirical function of linear velocity. Desalination 2006, 190, 43–50. [Google Scholar] [CrossRef]
- Koter, S.; Piotrowski, P.; Kerres, J. Comparative investigations of ion-exchange membranes. J. Membr. Sci. 1999, 153, 83–90. [Google Scholar] [CrossRef]
- Clayton, P.; Vas, C.; McAdam, K. Use of Chiroptical Spectroscopy to Determine the Ionisation Status of (S)-Nicotine in Electronic Cigarette Formulations. In Proceedings of the ST 49, Coresta Congress, Québec, BC, Canada, 8–12 October 2017. [Google Scholar]
- Shahi, V.K.; Murugesh, A.P.; Makwana, B.S.; Thampy, S.K.; Rangarajan, R. Comparative investigations on electrical conductance of ion-exchange membranes. Ind. J. Chem. Sect. Inorgan. Bio-Inorgan. Phys. Theor. Anal. Chem. 2000, 39, 1264–1269. [Google Scholar]
- Guo, H.C.; You, F.C.; Yu, S.L.; Li, L.; Zhao, D.S. Mechanisms of chemical cleaning of ion exchange membranes: A case study of plant-scale electrodialysis for oily wastewater treatment. J. Membr. Sci. 2015, 496, 310–317. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Pismenskaya, N.; Grande, D. Evolution of anion-exchange membrane properties in a full scale electrodialysis stack. J. Membr. Sci. 2013, 446, 255–265. [Google Scholar] [CrossRef]
- Kang, M.S.; Choi, Y.J.; Moon, S.H. Characterization of anion-exchange membranes containing pyridinium groups. AIChE J. 2003, 49, 3213–3220. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Chermit, R.K.; Sharafan, M.V. Mass transfer mechanism and chemical stability of strongly basic anion-exchange membranes under overlimiting current conditions. Rus. J. Electrochem. 2014, 50, 38–45. [Google Scholar] [CrossRef]
- Persico, M.; Mikhaylin, S.; Doyen, A.; Firdaous, L.; Hammami, R.; Bazinet, L. How peptide physicochemical and structural characteristics affect anion-exchange membranes fouling by a tryptic whey protein hydrolysate. J. Membr. Sci. 2016, 520, 914–923. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinee, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Ping, Q.Y.; Cohen, B.; Dosoretz, C.; He, Z. Long-term investigation of fouling of cation and anion exchange membranes in microbial desalination cells. Desalination 2013, 325, 48–55. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. Water splitting proton-barriers for mineral membrane fouling control and their optimization by accurate pulsed modes of electrodialysis. J. Membr. Sci. 2013, 447, 433–441. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Astudillo-Castro, C.; Bazinet, L. Mechanisms of mineral membrane fouling growth modulated by pulsed modes of current during electrodialysis: Evidences of water splitting implications in the appearance of the amorphous phases of magnesium hydroxide and calcium carbonate. J. Colloid Interfaces Sci. 2014, 426, 221–234. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. How electrodialysis configuration influences the acid whey deacidification and membrane scaling. J. Dairy Sci. 2018, 101, 7833–7850. [Google Scholar] [CrossRef]
- Yan, H.Y.; Wu, C.M.; Wu, Y.H. Separation of alumina alkaline solution by electrodialysis: Membrane stack configuration optimization and repeated batch experiments. Sep. Purif. Technol. 2015, 139, 78–87. [Google Scholar] [CrossRef]
- Lemay, N.; Mikhaylin, S.; Mareev, S.; Pismenskay, N.; Nikonenko, V.; Bazinet, L. How demineralization duration by electrodialysis under high frequency pulsed electric field can be the same as in continuous current condition and that for better performances? J. Membr. Sci. 2020, 603, 117878. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Systematic study of the impact of pulsed electric field parameters (pulse/pause duration and frequency) on ED performances during acid whey treatment. Membranes 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa-Fernandez, P.A.; Post, J.W.; Ramdlan, M.S.; Leermakers, F.A.M.; Bruning, H.; Rijnaarts, H.H.M. Improving the performance of polymer-flooding produced water electrodialysis through the application of pulsed electric field. Desalination 2020, 484, 114424. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, S.H.; Tsai, S.P. Effects of pulsed electric fields on membrane fouling in electrodialysis of NaCl solution containing humate. Sep. Purif. Technol. 2002, 27, 89–95. [Google Scholar] [CrossRef]
- Zhu, A.M.; Teng, H.K.; Zheng, S.Z.; Chen, J.; Zhang, D.M.; Zhang, L. A Cleaning Agent and its Manufacturing Methods for Ion Exchange Membrane Used for Electrodialysis. U.S. Patent 1,034,639,91A, 26 May 2013. [Google Scholar]






| Characteristics | Data |
|---|---|
| Total dissolved solids (wt.%) | 7 |
| Sugar (g/L) | 17.31 |
| Nicotine (g/L) | 1.91 |
| Nitrate (g/L) | 672 |
| Potassium (mg/L) | 4255 |
| Magnesium (mg/L) | 654 |
| Calcium (mg/L) | 1217 |
| Chloride (mg/L) | 893 |
| Sulfate (mg/L) | 668 |
| Malate (mg/L) | 3537 |
| Oxalate (mg/L) | 35 |
| Phosphate (mg/L) | 501 |
| Citrate (mg/L) | 32 |
| Tobacco extract solution pH | 5.4 |
| Tobacco extract conductivity (mS/cm) | 22.4 |
| Membrane | IEC (meq/g) | Area Resistance (Ω cm2) | Water Uptake (%) | Transport Number (%) |
|---|---|---|---|---|
| CEM-pristine | 0.8−1.0 | 2.5−3.5 | 40–45 | >93 |
| CEM-fouled | 0.74 | 7.25 | 36.2 | 93 |
| AEM-pristine | 0.5−0.6 | 3.5−4.5 | 15–20 | >93 |
| AEM-fouled | 1.11 | 16.04 | 16.7 | 90 |
| Compositions | Content |
|---|---|
| NaOH | 30% |
| Tripolyphosphate | 20% |
| Sodium dodecyl benzene sulfonate (SDS) | 15% |
| Na2CO3 | 15% |
| NaCl | 15% |
| EDTA | 5% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Zhang, Z.; Yan, H.; Irfan, M.; Xu, Y.; Li, W.; Wang, H.; Wang, Y. Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies. Membranes 2020, 10, 245. https://doi.org/10.3390/membranes10090245
Ge S, Zhang Z, Yan H, Irfan M, Xu Y, Li W, Wang H, Wang Y. Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies. Membranes. 2020; 10(9):245. https://doi.org/10.3390/membranes10090245
Chicago/Turabian StyleGe, Shaolin, Zhao Zhang, Haiyang Yan, Muhammad Irfan, Yingbo Xu, Wei Li, Huangying Wang, and Yaoming Wang. 2020. "Electrodialytic Desalination of Tobacco Sheet Extract: Membrane Fouling Mechanism and Mitigation Strategies" Membranes 10, no. 9: 245. https://doi.org/10.3390/membranes10090245