Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas
Abstract
:1. Introduction
2. Materials and Methods
2.1. HCMV Neutralizing Monoclonal and Control Antibodies
2.2. Primary Cells and Human Placentas
2.3. Virus Neutralization Assays
2.4. Virus Spread Inhibition Assays
2.5. HCMV Titration
2.6. Antibodies and Reagents
2.7. Immunofluorescence and Imaging
3. Results
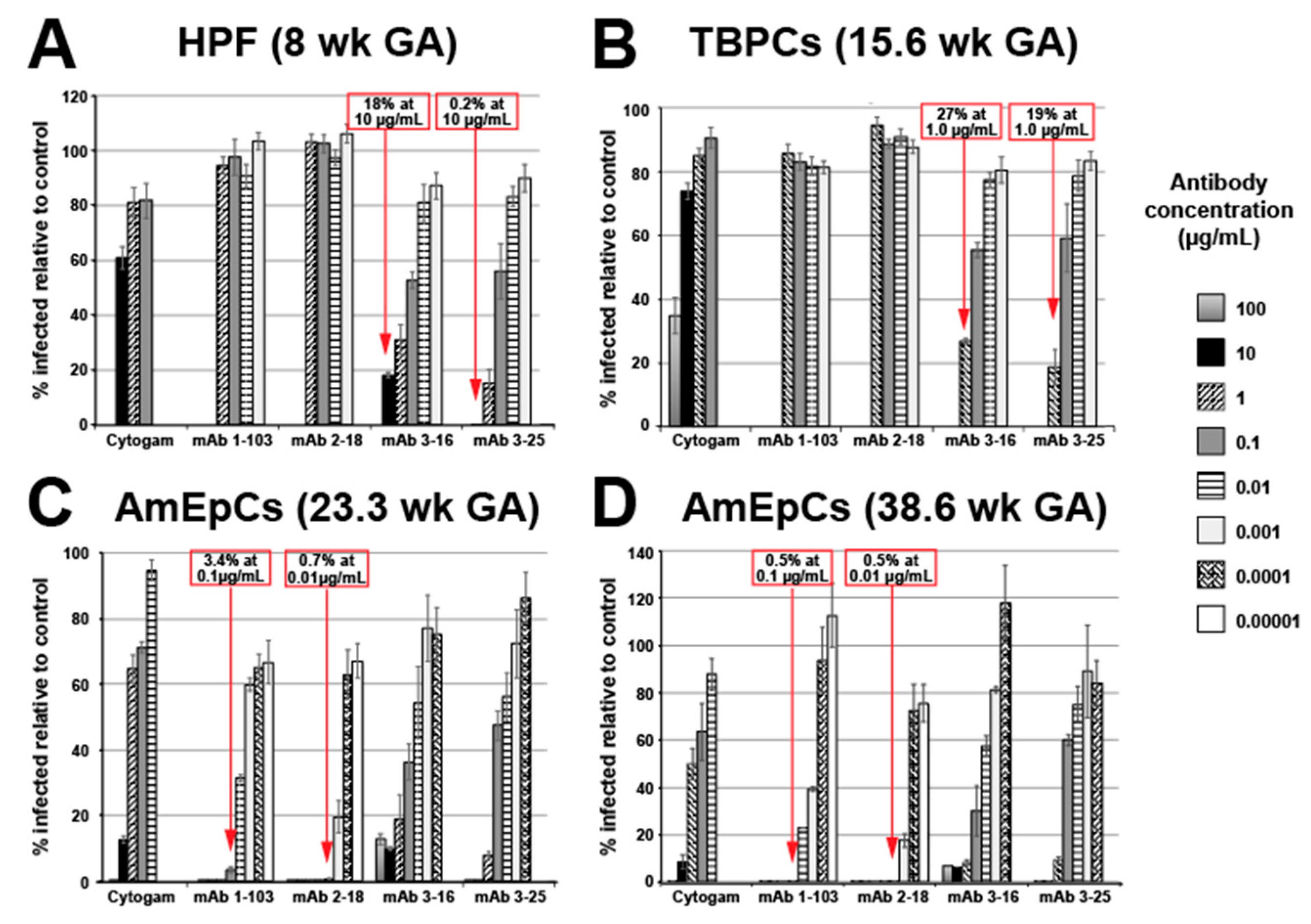
3.1. mAbs Have Potent HCMV Neutralizing Activities in Primary Placental Cells
3.1.1. Human Placental Fibroblast (HPF) Infection Is Blocked by mAbs to gB and gH/gL
3.1.2. Trophoblast Progenitor Cell (TBPC) Infection Is Blocked by mAbs to gB and gH/gL
3.1.3. Amniotic Epithelial Cell (AmEpC) Infection Is Strongly Inhibited by Anti-Pentamer mAbs
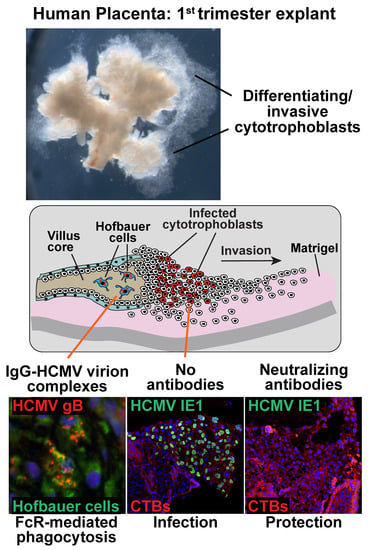
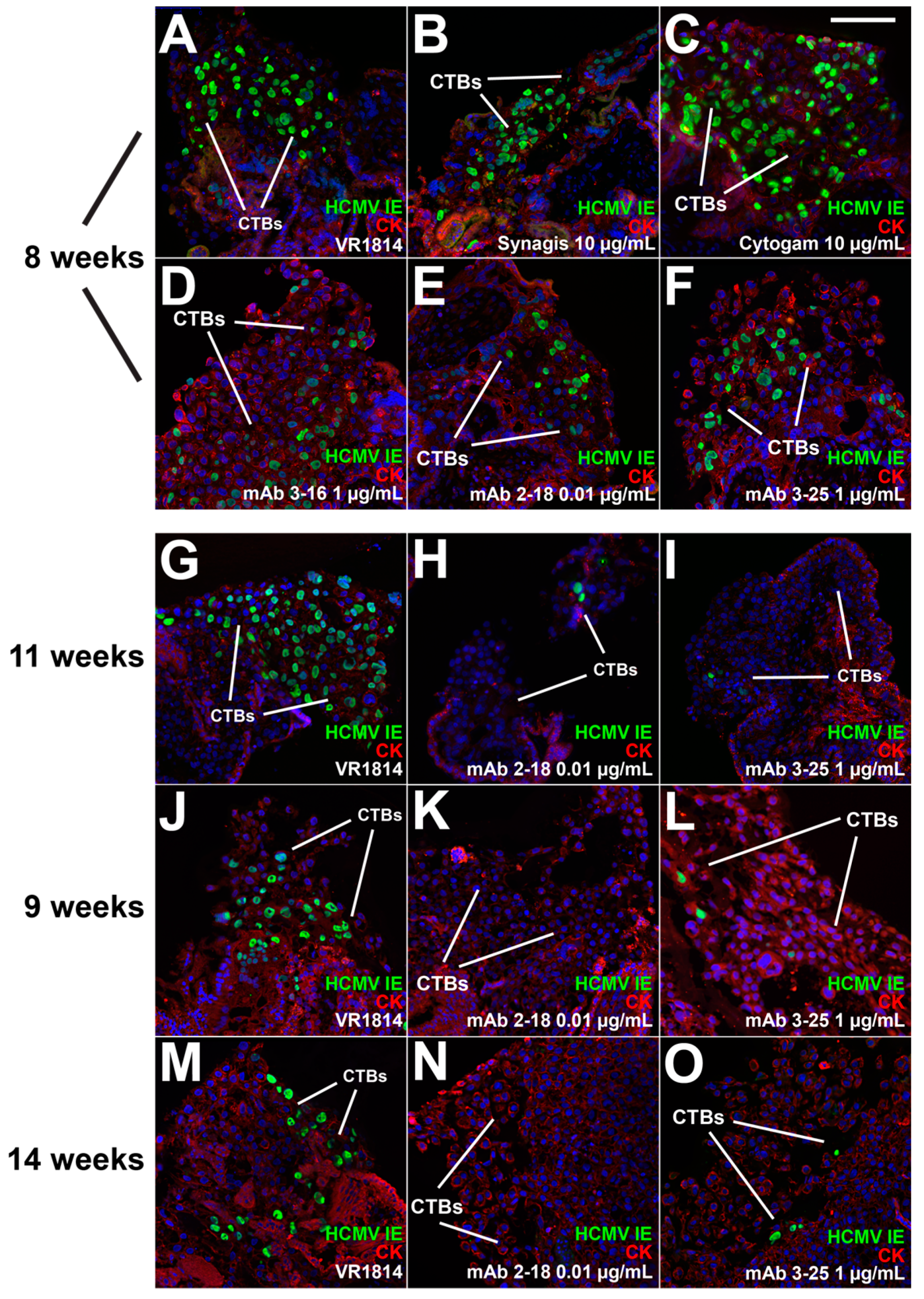
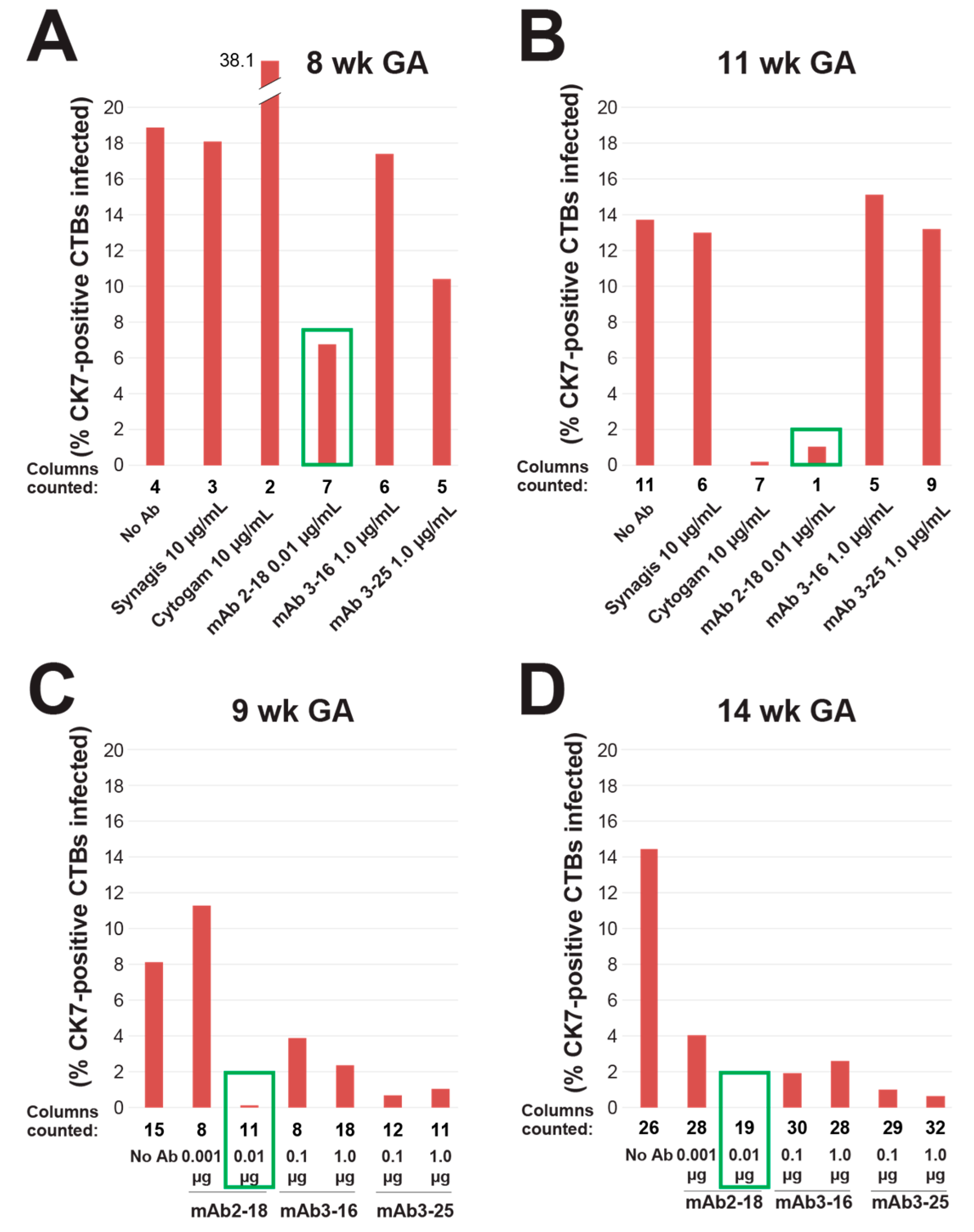
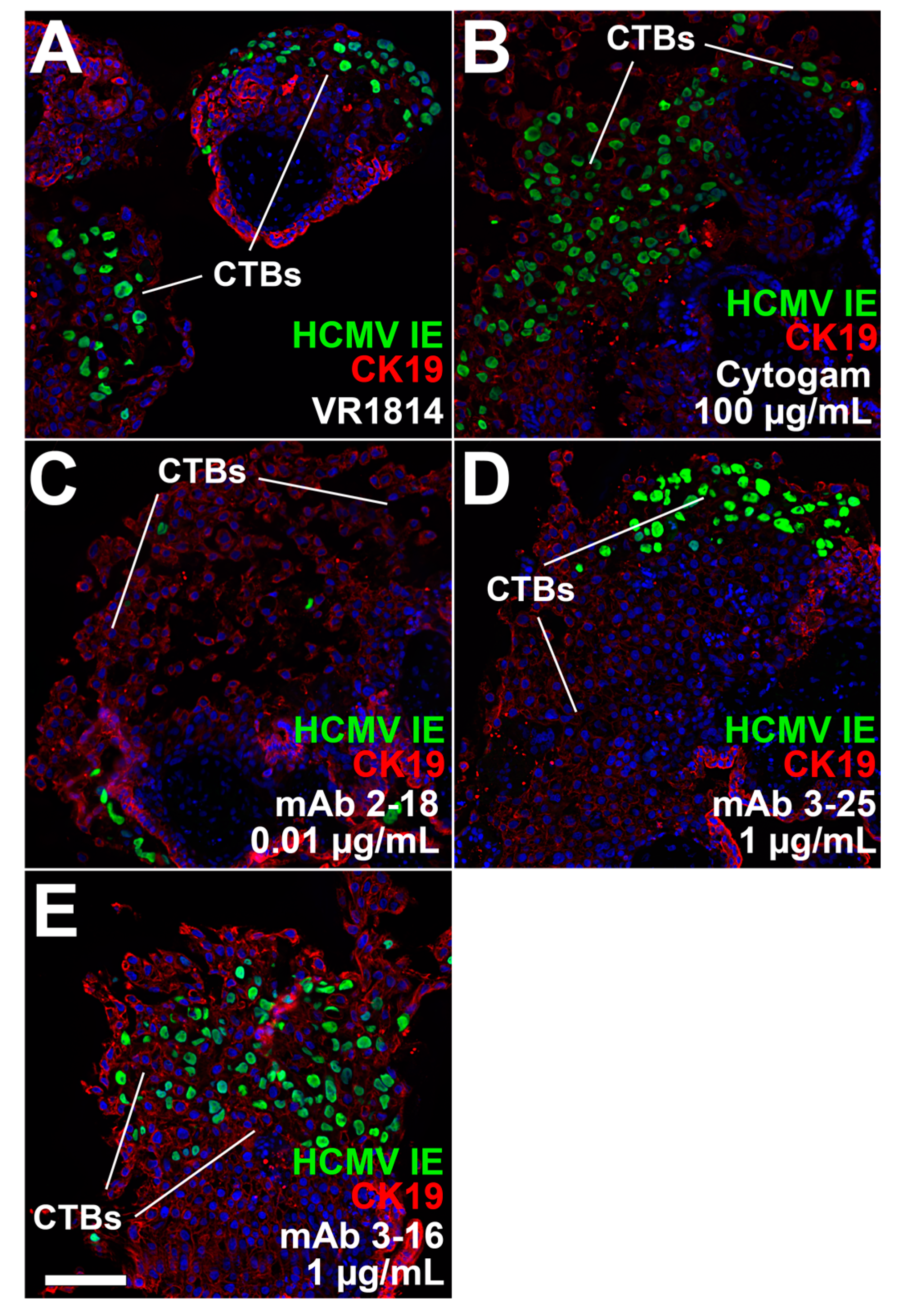
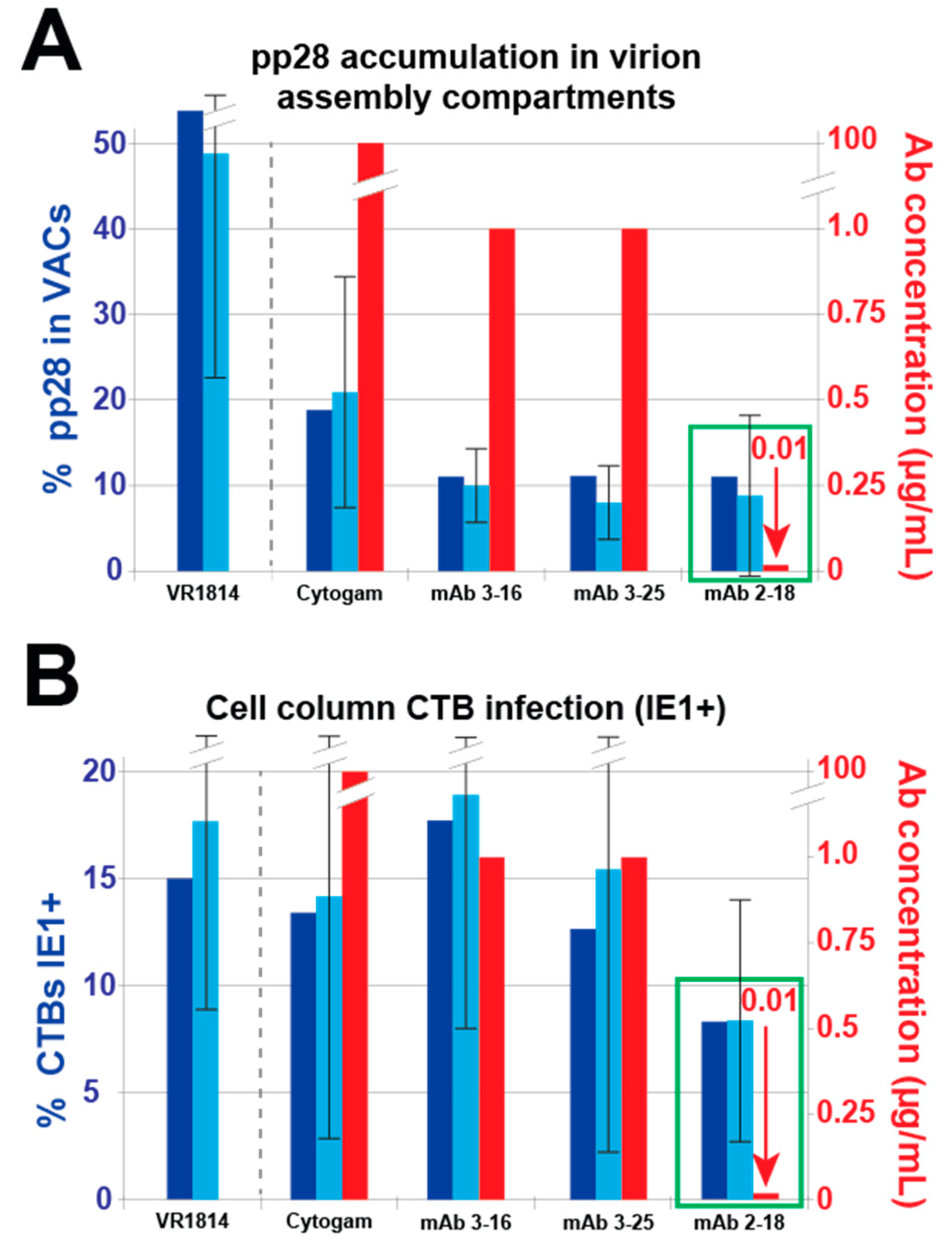
3.2. mAbs Specific to HCMV Proteins Neutralize Infection of Cell Column CTBs in Anchoring Villus Explants
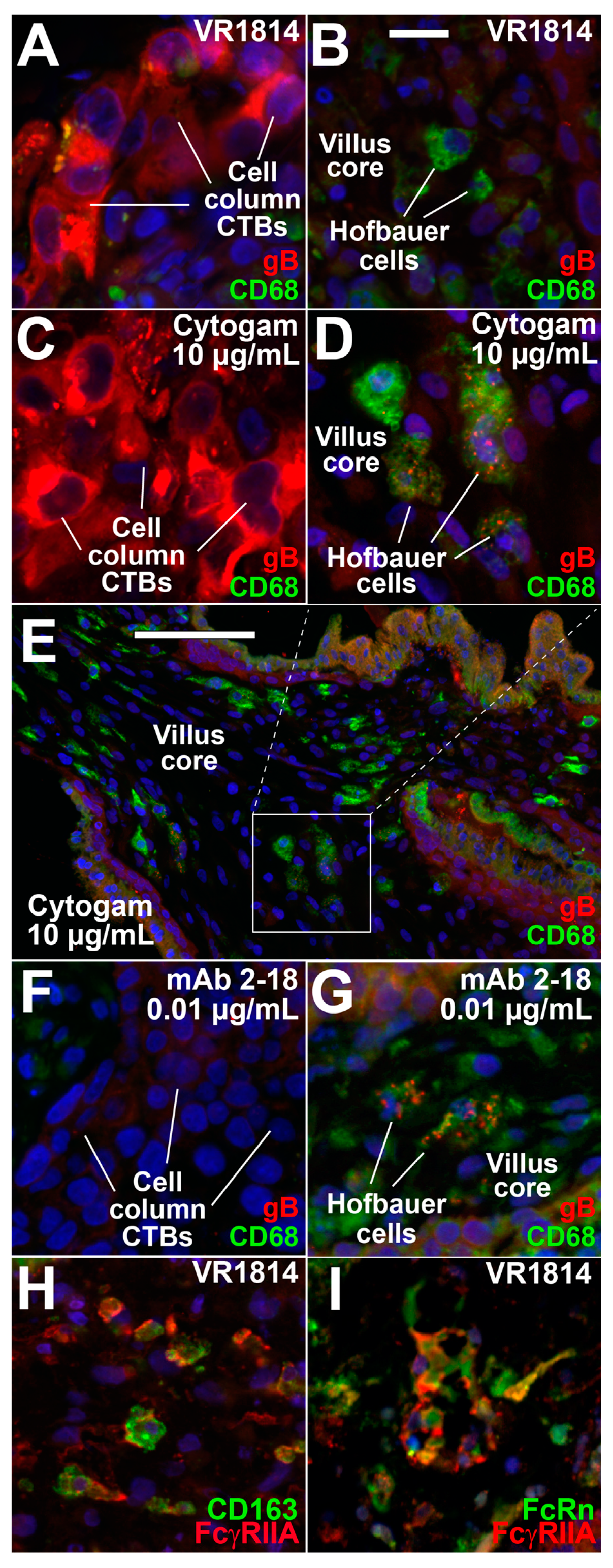
3.3. Hofbauer Cells in Villus Cores Phagocytose IgG-Virion Complexes
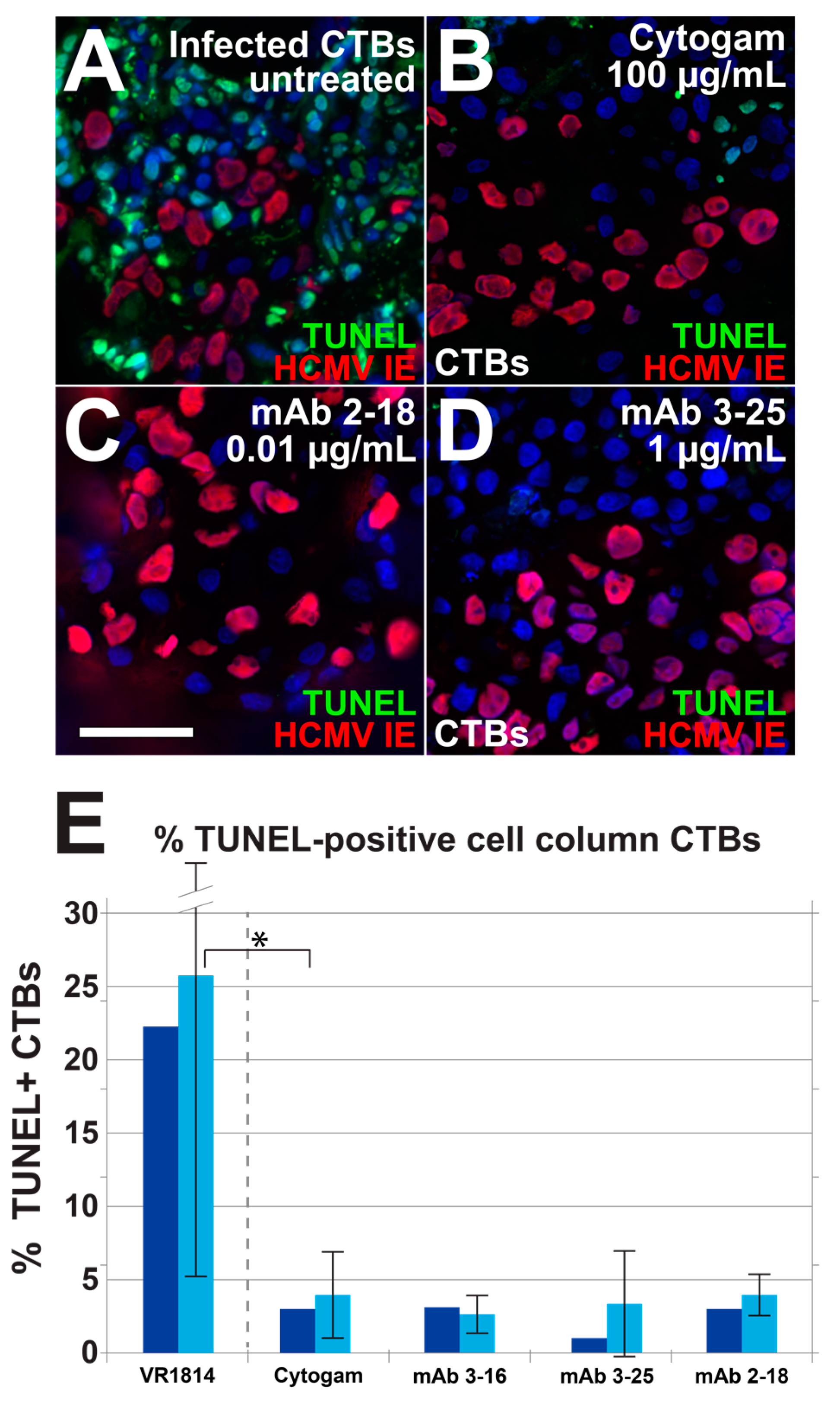
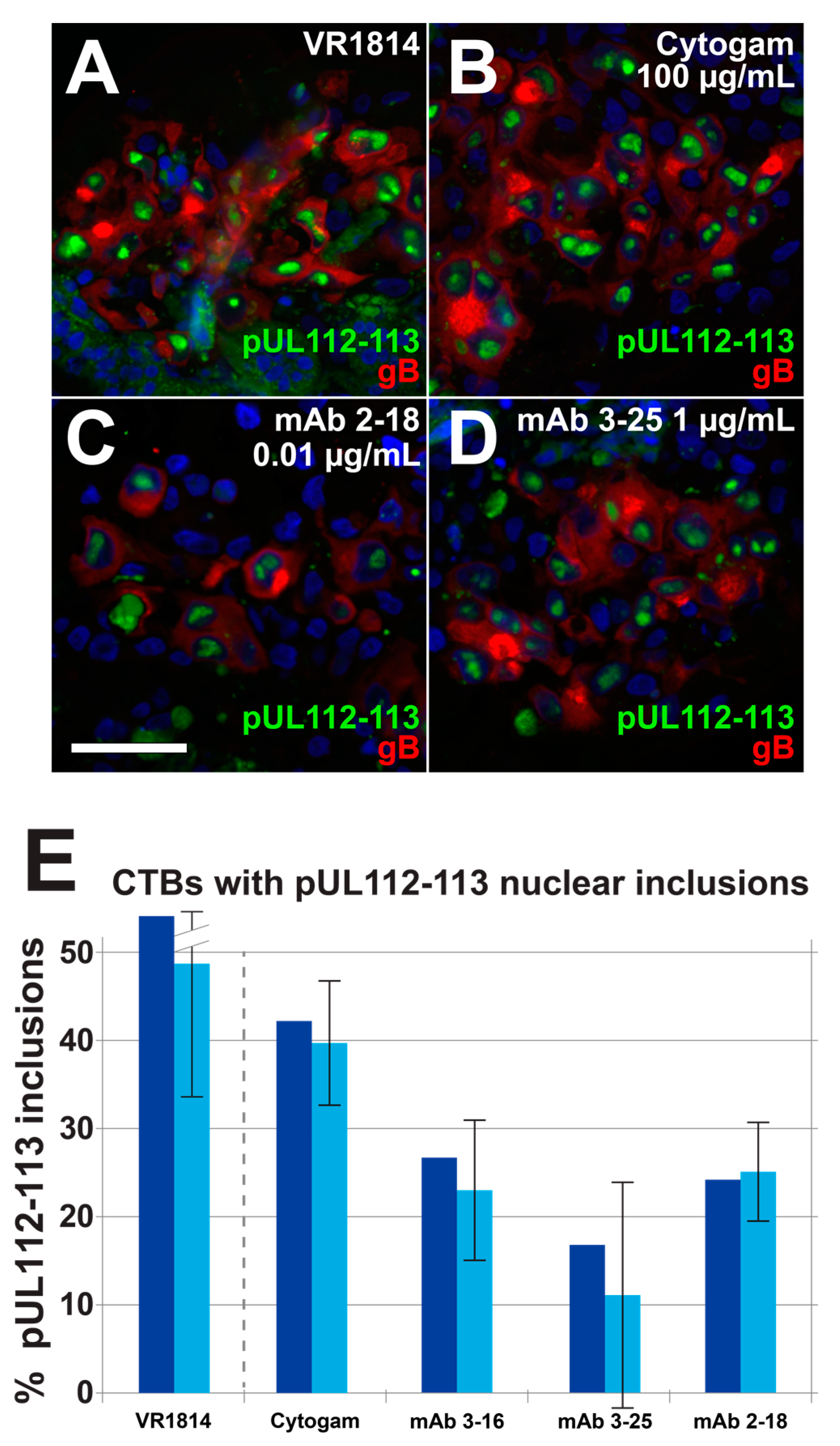
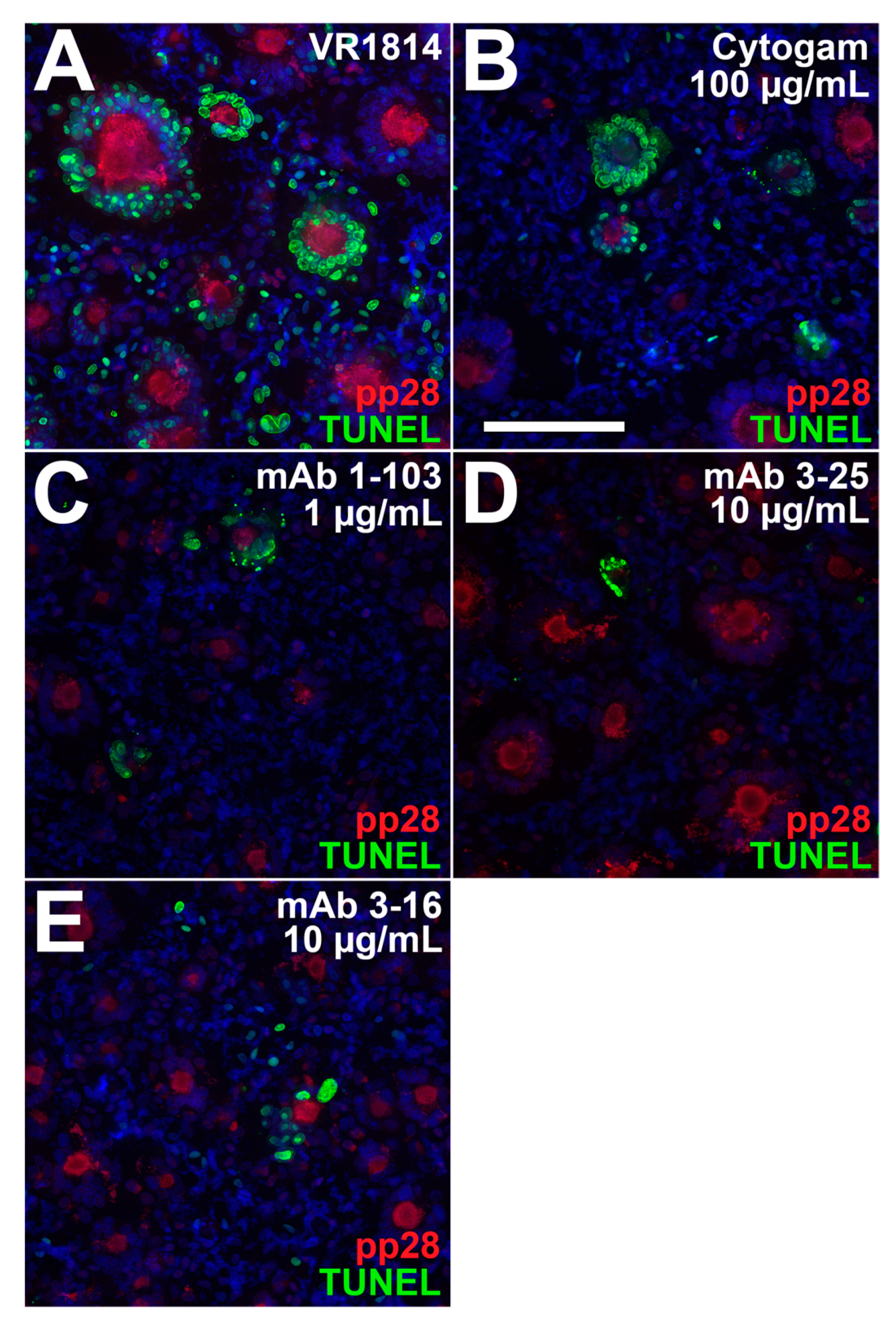
3.4. Antibody Inhibition of Cell–Cell Spread of HCMV and Apoptosis in CTB Cell Columns in Anchoring Villus Explants
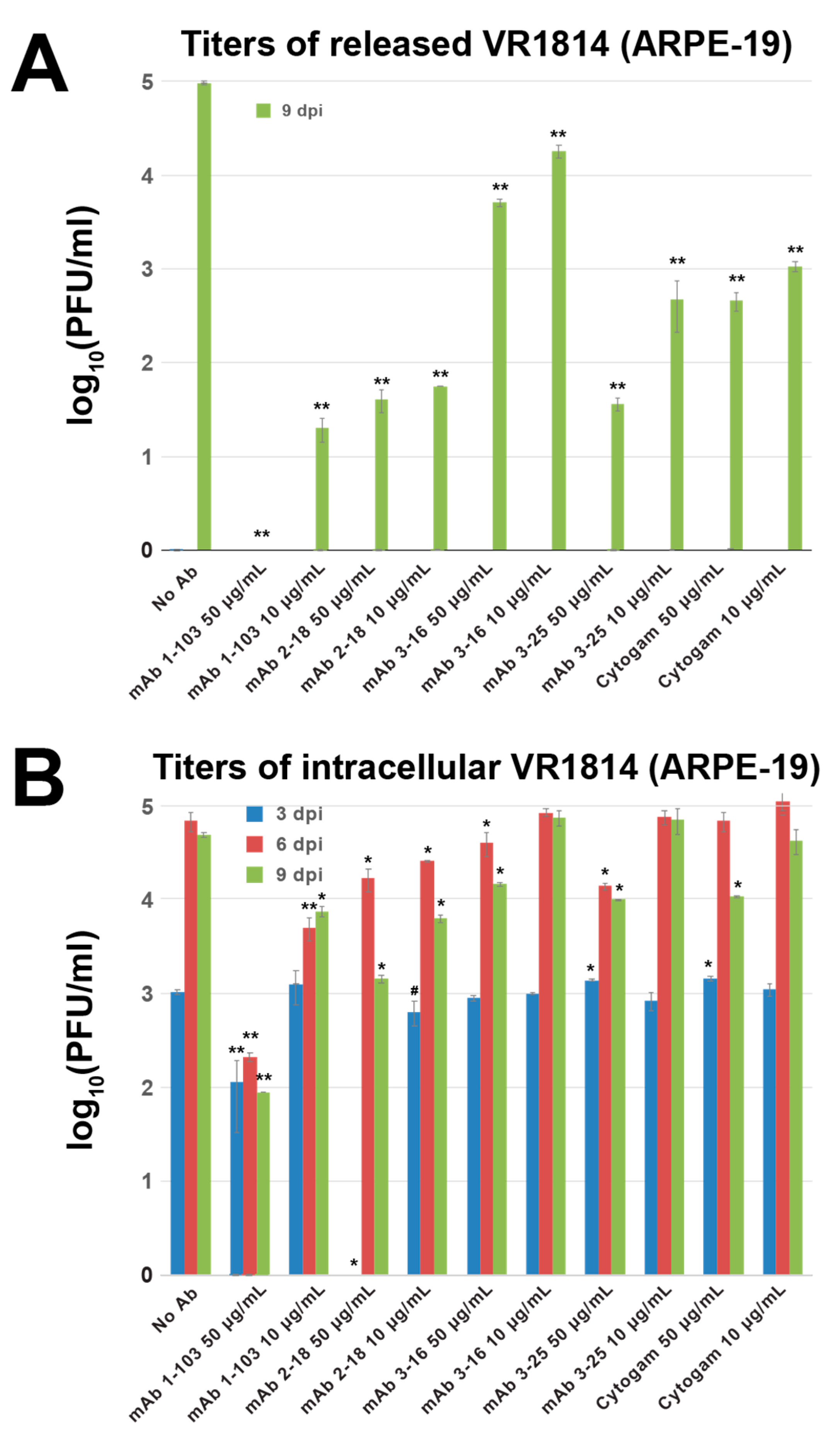
3.5. Neutralizing Antibodies Inhibit Cell–Cell Spread of HCMV in ARPE-19 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pass, R.F.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Stagno, S. Congenital cytomegalovirus infection following first trimester maternal infection: Symptoms at birth and outcome. J. Clin. Virol. 2006, 35, 216–220. [Google Scholar] [CrossRef]
- Pereira, L.; Petitt, M.; Fong, A.; Tsuge, M.; Tabata, T.; Fang-Hoover, J.; Maidji, E.; Zydek, M.; Zhou, Y.; Inoue, N.; et al. Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection. J. Infect. Dis. 2014, 209, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Iwasenko, J.M.; Howard, J.; Arbuckle, S.; Graf, N.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J. Infect. Dis. 2011, 203, 1526–1533. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Magny, J.F.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.M.; Benard, M.; Pinquier, D.; Astruc, D.; Patural, H.; et al. Sequelae of congenital cytomegalovirus (cCMV) following maternal primary infection are limited to those acquired in the first trimester of pregnancy. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Bialek, S.; Cannon, M.J. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin. Infect. Dis. 2011, 52, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Fowler, K.B.; Pass, R.F.; Rivera, L.B.; Bradford, R.D.; Lakeman, F.D.; Britt, W.J. Congenital cytomegalovirus infection: Association between virus burden in infancy and hearing loss. J. Pediatr. 2005, 146, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Dreher, A.M.; Arora, N.; Fowler, K.B.; Novak, Z.; Britt, W.J.; Boppana, S.B.; Ross, S.A. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J. Pediatr. 2014, 164. [Google Scholar] [CrossRef]
- Arvin, A.M.; Fast, P.; Myers, M.; Plotkin, S.; Rabinovich, R. Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 2004, 39, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Boppana, S.B. Vaccination against the human cytomegalovirus. Vaccine 2018. [Google Scholar] [CrossRef]
- Permar, S.R.; Schleiss, M.R.; Plotkin, S.A. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Wang, D.; Fu, T.M. Progress on human cytomegalovirus vaccines for prevention of congenital infection and disease. Curr. Opin. Virol. 2014, 6, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Feire, A.L.; Roy, R.M.; Manley, K.; Compton, T. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J. Virol. 2010, 84, 10026–10037. [Google Scholar] [CrossRef] [PubMed]
- Lopper, M.; Compton, T. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 2004, 78, 8333–8341. [Google Scholar] [CrossRef]
- Compton, T. Receptors and immune sensors: The complex entry path of human cytomegalovirus. Trends Cell Biol. 2004, 14, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Macagno, A.; Bernasconi, N.L.; Vanzetta, F.; Dander, E.; Sarasini, A.; Revello, M.G.; Gerna, G.; Sallusto, F.; Lanzavecchia, A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J. Virol. 2010, 84, 1005–1013. [Google Scholar] [CrossRef]
- Fu, T.M.; Wang, D.; Freed, D.C.; Tang, A.; Li, F.; He, X.; Cole, S.; Dubey, S.; Finnefrock, A.C.; ter Meulen, J.; et al. Restoration of viral epithelial tropism improves immunogenicity in rabbits and rhesus macaques for a whole virion vaccine of human cytomegalovirus. Vaccine 2012, 30, 7469–7474. [Google Scholar] [CrossRef]
- Kabanova, A.; Perez, L.; Lilleri, D.; Marcandalli, J.; Agatic, G.; Becattini, S.; Preite, S.; Fuschillo, D.; Percivalle, E.; Sallusto, F.; et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2014, 111, 17965–17970. [Google Scholar] [CrossRef] [Green Version]
- Gerna, G.; Sarasini, A.; Patrone, M.; Percivalle, E.; Fiorina, L.; Campanini, G.; Gallina, A.; Baldanti, F.; Revello, M.G. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J. Gen. Virol. 2008, 89, 853–865. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Wussow, F.; Johnson, E.; Bian, C.; Zhuo, M.; Rajakumar, A.; Barry, P.A.; Britt, W.J.; Chakraborty, R.; Diamond, D.J. Vaccine-derived neutralizing antibodies to the human cytomegalovirus gH/gL pentamer potently block primary cytotrophoblast infection. J. Virol. 2015, 89, 11884–11898. [Google Scholar] [CrossRef] [PubMed]
- Lilleri, D.; Kabanova, A.; Revello, M.G.; Percivalle, E.; Sarasini, A.; Genini, E.; Sallusto, F.; Lanzavecchia, A.; Corti, D.; Gerna, G. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS ONE 2013, 8, e59863. [Google Scholar] [CrossRef] [PubMed]
- Schampera, M.S.; Arellano-Galindo, J.; Kagan, K.O.; Adler, S.P.; Jahn, G.; Hamprecht, K. Role of pentamer complex-specific and IgG subclass 3 antibodies in HCMV hyperimmunoglobulin and standard intravenous IgG preparations. Med. Microbiol. Immunol. 2018. [Google Scholar] [CrossRef]
- Nigro, G.; Adler, S.P.; La Torre, R.; Best, A.M. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Adler, S.P.; Parruti, G.; Anceschi, M.M.; Coclite, E.; Pezone, I.; Di Renzo, G.C. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy--a case-control study of the outcome in children. J. Infect. Dis. 2012, 205, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Buxmann, H.; Stackelberg, O.M.; Schlosser, R.L.; Enders, G.; Gonser, M.; Meyer-Wittkopf, M.; Hamprecht, K.; Enders, M. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: A retrospective analysis. J. Perinat. Med. 2012, 40, 439–446. [Google Scholar] [CrossRef]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Enders, M.; Schampera, M.S.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; De Catte, L.; Wallwiener, D.; et al. Prevention of maternal-fetal transmission of CMV by hyperimmunoglobulin (HIG) administered after a primary maternal CMV infection in early gestation. Ultrasound Obstet. Gynecol. 2018. [Google Scholar] [CrossRef]
- Pereira, L.; Maidji, E.; McDonagh, S.; Tabata, T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005, 13, 164–174. [Google Scholar] [CrossRef]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef]
- Cross, J.C.; Werb, Z.; Fisher, S.J. Implantation and the placenta: Key pieces of the development puzzle. Science 1994, 266, 1508–1518. [Google Scholar] [CrossRef]
- Damsky, C.H.; Librach, C.; Lim, K.H.; Fitzgerald, M.L.; McMaster, M.T.; Janatpour, M.; Zhou, Y.; Logan, S.K.; Fisher, S.J. Integrin switching regulates normal trophoblast invasion. Development 1994, 120, 3657–3666. [Google Scholar]
- Zhou, Y.; Fisher, S.J.; Janatpour, M.; Genbacev, O.; Dejana, E.; Wheelock, M.; Damsky, C.H. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Investig. 1997, 99, 2139–2151. [Google Scholar] [CrossRef]
- Maidji, E.; McDonagh, S.; Genbacev, O.; Tabata, T.; Pereira, L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 2006, 168, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tang, A.; Meng, W.; Freed, D.C.; He, L.; Wang, D.; Li, F.; Li, L.; Xiong, W.; Gui, X.; et al. Active evolution of memory B-cells specific to viral gH/gL/pUL128/130/131 pentameric complex in healthy subjects with silent human cytomegalovirus infection. Oncotarget 2017, 8, 73654–73669. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Fang-Hoover, J.; Zydek, M.; Pereira, L. Persistent cytomegalovirus infection in amniotic membranes of the human placenta. Am. J. Pathol. 2016, 186, 2970–2986. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.; Donne, M.; Kapidzic, M.; Gormley, M.; Lamb, J.; Gilmore, J.; Larocque, N.; Goldfien, G.; Zdravkovic, T.; McMaster, M.T.; et al. Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells 2011, 29, 1427–1436. [Google Scholar] [CrossRef]
- Damsky, C.H.; Fitzgerald, M.L.; Fisher, S.J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Investig. 1992, 89, 210–222. [Google Scholar] [CrossRef]
- Ilic, D.; Kapidzic, M.; Genbacev, O. Isolation of human placental fibroblasts. Curr. Protoc. Stem Cell Biol. 2008. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki, K.A.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Tugizov, S.; Maidji, E.; Pereira, L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J. Gen. Virol. 1996, 77, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Baldanti, F.; Percivalle, E.; Sarasini, A.; De-Giuli, L.; Genini, E.; Lilleri, D.; Labo, N.; Gerna, G. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J. Gen. Virol. 2001, 82, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozawa, N.; Fang-Hoover, J.; Tabata, T.; Maidji, E.; Pereira, L. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J. Clin. Virol. 2009, 46 (Suppl. 4), S58–S63. [Google Scholar] [CrossRef] [Green Version]
- Zydek, M.; Petitt, M.; Fang-Hoover, J.; Adler, B.; Kauvar, L.M.; Pereira, L.; Tabata, T. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 2014, 6, 1346–1364. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Fang-Hoover, J.; Rivera, J.; Nozawa, N.; Shiboski, S.; Inoue, N.; Pereira, L. Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model. Am. J. Pathol. 2012, 181, 1540–1559. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Freed, D.C.; Wang, D.; Qiu, P.; Li, F.; Fu, T.M.; Kauvar, L.M.; McVoy, M.A. Impact of antibodies and strain polymorphisms on cytomegalovirus entry and spread in fibroblasts and epithelial cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Hoffman, M.; Gallo, D.; Cremer, N. Monoclonal antibodies to human cytomegalovirus. I. Three cell surface proteins with unique immunologic and electrophoretic properties specify cross-reactive determinants. Infect. Immun. 1982, 36, 924–932. [Google Scholar] [PubMed]
- Iwayama, S.; Yamamoto, T.; Furuya, T.; Kobayashi, R.; Ikuta, K.; Hirai, K. Intracellular localization and DNA-binding activity of a class of viral early phosphoproteins in human fibroblasts infected with human cytomegalovirus (Towne strain). J. Gen. Virol. 1994, 75 Pt 12, 3309–3318. [Google Scholar] [CrossRef]
- Simister, N.E.; Story, C.M.; Chen, H.L.; Hunt, J.S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 1996, 26, 1527–1531. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Zydek, M.; Fang-Hoover, J.; Larocque, N.; Tsuge, M.; Gormley, M.; Kauvar, L.M.; Pereira, L. Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J. Virol. 2015, 89, 5134–5147. [Google Scholar] [CrossRef]
- Chan, G.; Guilbert, L.J. Enhanced monocyte binding to human cytomegalovirus-infected syncytiotrophoblast results in increased apoptosis via the release of tumour necrosis factor alpha. J. Pathol. 2005, 207, 462–470. [Google Scholar] [CrossRef]
- Isomura, H.; Stinski, M.F. Coordination of late gene transcription of human cytomegalovirus with viral DNA synthesis: Recombinant viruses as potential therapeutic vaccine candidates. Expert Opin. Ther. Targets 2013, 17, 157–166. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, S.; Radsak, K.; Hirai, K. The UL112/113 gene products of human cytomegalovirus which colocalize with viral DNA in infected cell nuclei are related to efficient viral DNA replication. Virus Res. 1998, 56, 107–114. [Google Scholar] [CrossRef]
- Schommartz, T.; Tang, J.; Brost, R.; Brune, W. Differential requirement of human cytomegalovirus UL112-113 protein isoforms for viral replication. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Stagno, S.; Pass, R.F.; Dworsky, M.E.; Henderson, R.E.; Moore, E.G.; Walton, P.D.; Alford, C.A. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 1982, 306, 945–949. [Google Scholar] [CrossRef]
- Fowler, K.B.; Stagno, S.; Pass, R.F.; Britt, W.J.; Boll, T.J.; Alford, C.A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 1992, 326, 663–667. [Google Scholar] [CrossRef]
- Fowler, K.B.; Stagno, S.; Pass, R.F. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003, 289, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, I.; Kropff, B.; McLean, G.R.; Pichon, S.; Piras-Douce, F.; Milne, R.S.B.; Smith, C.; Mach, M.; Griffiths, P.D.; Reeves, M.B. Epitope-specific humoral responses to human cytomegalovirus glycoprotein-B vaccine with MF59: Anti-AD2 levels correlate with protection from viremia. J. Infect. Dis. 2018, 217, 1907–1917. [Google Scholar] [CrossRef]
- Baraniak, I.; Kropff, B.; Ambrose, L.; McIntosh, M.; McLean, G.R.; Pichon, S.; Atkinson, C.; Milne, R.S.B.; Mach, M.; Griffiths, P.D.; et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, 6273–6278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.S.; Huffman, T.; Jenks, J.A.; Cisneros de la Rosa, E.; Xie, G.; Vandergrift, N.; Pass, R.F.; Pollara, J.; Permar, S.R. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 2018, 115, 6267–6272. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: Implications for transmission and pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef]
- Tabata, T.; McDonagh, S.; Kawakatsu, H.; Pereira, L. Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell-matrix and cell-cell adhesion molecules: A quantitative analysis. Placenta 2007, 28, 527–537. [Google Scholar] [CrossRef]
- Maidji, E.; Genbacev, O.; Chang, H.T.; Pereira, L. Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J. Virol. 2007, 81, 4701–4712. [Google Scholar] [CrossRef]
- Ha, S.; Li, F.; Troutman, M.C.; Freed, D.C.; Tang, A.; Loughney, J.W.; Wang, D.; Wang, I.M.; Vlasak, J.; Nickle, D.C.; et al. Neutralization of diverse human cytomegalovirus strains conferred by antibodies targeting viral gH/gL/pUL128-131 pentameric complex. J. Virol. 2017, 91, e02033. [Google Scholar] [CrossRef]
- Frenzel, K.; Ganepola, S.; Michel, D.; Thiel, E.; Kruger, D.H.; Uharek, L.; Hofmann, J. Antiviral function and efficacy of polyvalent immunoglobulin products against CMV isolates in different human cell lines. Med. Microbiol. Immunol. 2012, 201, 277–286. [Google Scholar] [CrossRef]
- Schampera, M.S.; Schweinzer, K.; Abele, H.; Kagan, K.O.; Klein, R.; Rettig, I.; Jahn, G.; Hamprecht, K. Comparison of cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: Impact of CMV IgG normalization. J. Clin. Virol. 2017, 90, 40–45. [Google Scholar] [CrossRef]
- Revello, M.G.; Furione, M.; Rognoni, V.; Arossa, A.; Gerna, G. Cytomegalovirus DNAemia in pregnant women. J. Clin. Virol. 2014, 61, 590–592. [Google Scholar] [CrossRef]
- Revello, M.G.; Fabbri, E.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Cena, C.; Arossa, A.; Montanari, L.; et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: A 20-year experience. J. Clin. Virol. 2011, 50, 303–307. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Sellier, Y.; Salomon, L.J.; Stirnemann, J.J.; Jacquemard, F.; Ville, Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: A retrospective cohort. Clin. Infect. Dis. 2013, 56, 1428–1435. [Google Scholar] [CrossRef]
- Delforge, M.L.; Costa, E.; Brancart, F.; Goldman, D.; Montesinos, I.; Zaytouni, S.; Marchant, A.; Donner, C. Presence of cytomegalovirus in urine and blood of pregnant women with primary infection might be associated with fetal infection. J. Clin. Virol. 2017, 90, 14–17. [Google Scholar] [CrossRef]
- Genini, E.; Percivalle, E.; Sarasini, A.; Revello, M.G.; Baldanti, F.; Gerna, G. Serum antibody response to the gH/gL/pUL128-131 five-protein complex of human cytomegalovirus (HCMV) in primary and reactivated HCMV infections. J. Clin. Virol. 2011, 52, 113–118. [Google Scholar] [CrossRef]
- Kauvar, L.M.; Liu, K.; Park, M.; DeChene, N.; Stephenson, R.; Tenorio, E.; Ellsworth, S.L.; Tabata, T.; Petitt, M.; Tsuge, M.; et al. A high-affinity native human antibody neutralizes human cytomegalovirus infection of diverse cell types. Antimicrob. Agents Chemother. 2015, 59, 1558–1568. [Google Scholar] [CrossRef]
- Rath, T.; Kuo, T.T.; Baker, K.; Qiao, S.W.; Kobayashi, K.; Yoshida, M.; Roopenian, D.; Fiebiger, E.; Lencer, W.I.; Blumberg, R.S. The immunologic functions of the neonatal Fc receptor for IgG. J. Clin. Immunol. 2013, 33 (Suppl. 1), S9–S17. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, G.; Dickinson, B.L.; Li, X.; Mizoguchi, E.; Miao, L.; Wang, Y.; Robert, C.; Wu, B.; Smith, P.D.; et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 2001, 166, 3266–3276. [Google Scholar] [CrossRef]
- Gerna, G.; Percivalle, E.; Perez, L.; Lanzavecchia, A.; Lilleri, D. Monoclonal antibodies to different components of the human cytomegalovirus (HCMV) pentamer gH/gL/pUL128L and trimer gH/gL/gO as well as antibodies elicited during primary HCMV infection prevent epithelial cell syncytium formation. J. Virol. 2016, 90, 6216–6223. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polanski, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Co, E.C.; Gormley, M.; Kapidzic, M.; Rosen, D.B.; Scott, M.A.; Stolp, H.A.; McMaster, M.; Lanier, L.L.; Barcena, A.; Fisher, S.J. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 2013, 88, 155. [Google Scholar] [CrossRef]
- Weisblum, Y.; Panet, A.; Zakay-Rones, Z.; Haimov-Kochman, R.; Goldman-Wohl, D.; Ariel, I.; Falk, H.; Natanson-Yaron, S.; Goldberg, M.D.; Gilad, R.; et al. Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture. J. Virol. 2011, 85, 13204–13213. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Fang-Hoover, J.; Pereira, L. Survey of cellular immune responses to human cytomegalovirus infection in the microenvironment of the uterine-placental interface. Med. Microbiol. Immunol. 2019. [Google Scholar] [CrossRef]
- Lilleri, D.; Fornara, C.; Furione, M.; Zavattoni, M.; Revello, M.G.; Gerna, G. Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J. Infect. Dis. 2007, 195, 1062–1070. [Google Scholar] [CrossRef]
- Wussow, F.; Chiuppesi, F.; Contreras, H.; Diamond, D.J. Neutralization of human cytomegalovirus entry into fibroblasts and epithelial cells. Vaccines 2017, 5, 39. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabata, T.; Petitt, M.; Fang-Hoover, J.; Freed, D.C.; Li, F.; An, Z.; Wang, D.; Fu, T.-M.; Pereira, L. Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas. Vaccines 2019, 7, 135. https://doi.org/10.3390/vaccines7040135
Tabata T, Petitt M, Fang-Hoover J, Freed DC, Li F, An Z, Wang D, Fu T-M, Pereira L. Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas. Vaccines. 2019; 7(4):135. https://doi.org/10.3390/vaccines7040135
Chicago/Turabian StyleTabata, Takako, Matthew Petitt, June Fang-Hoover, Daniel C. Freed, Fengsheng Li, Zhiqiang An, Dai Wang, Tong-Ming Fu, and Lenore Pereira. 2019. "Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas" Vaccines 7, no. 4: 135. https://doi.org/10.3390/vaccines7040135