Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception
Abstract
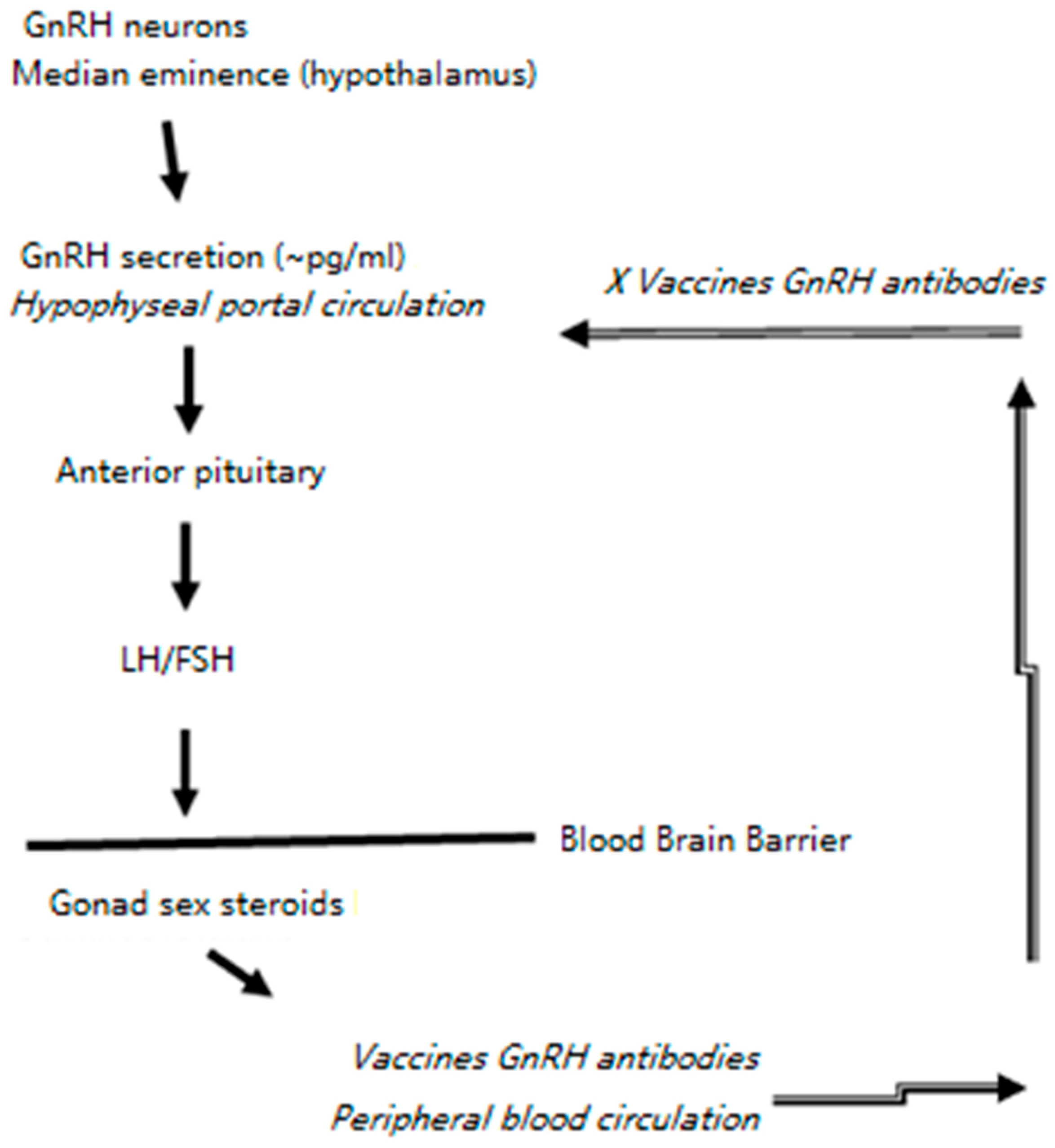
1. Introduction
2. Materials and Methods
2.1. Cells, ERA-2GnRH Virus Growth and Concentration
2.2. ERA-2GnRH Virus Inactivation, Lyophilization and Hydrogel Formulation
2.3. Vaccine Gelling Studies In Vitro
2.4. Mouse Vaccination Using the ERA-2GnRH Virus
2.5. Rabies Virus Neutralizing Antibodies and GnRH Antibodies in Mice
2.6. Statistics
3. Results
3.1. The ERA-2gnrh Vaccine Was Formulated in a Thermo-Responsive Chitosan Hydrogel
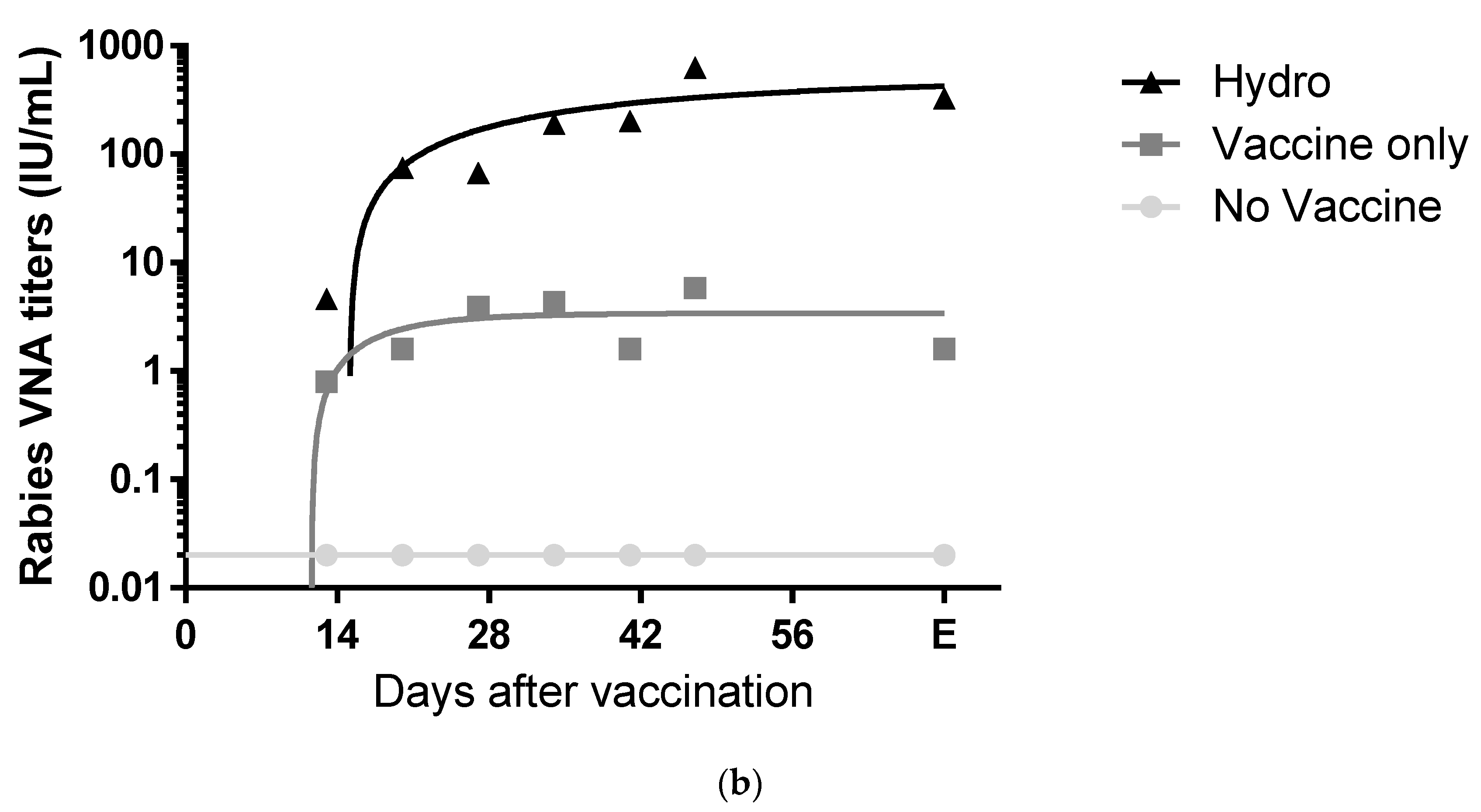
3.2. The Chitosan Hydrogel ERA-2GnRH Vaccine Induced High and Persistent Rabies-Neutralizing Antibodies in Mice
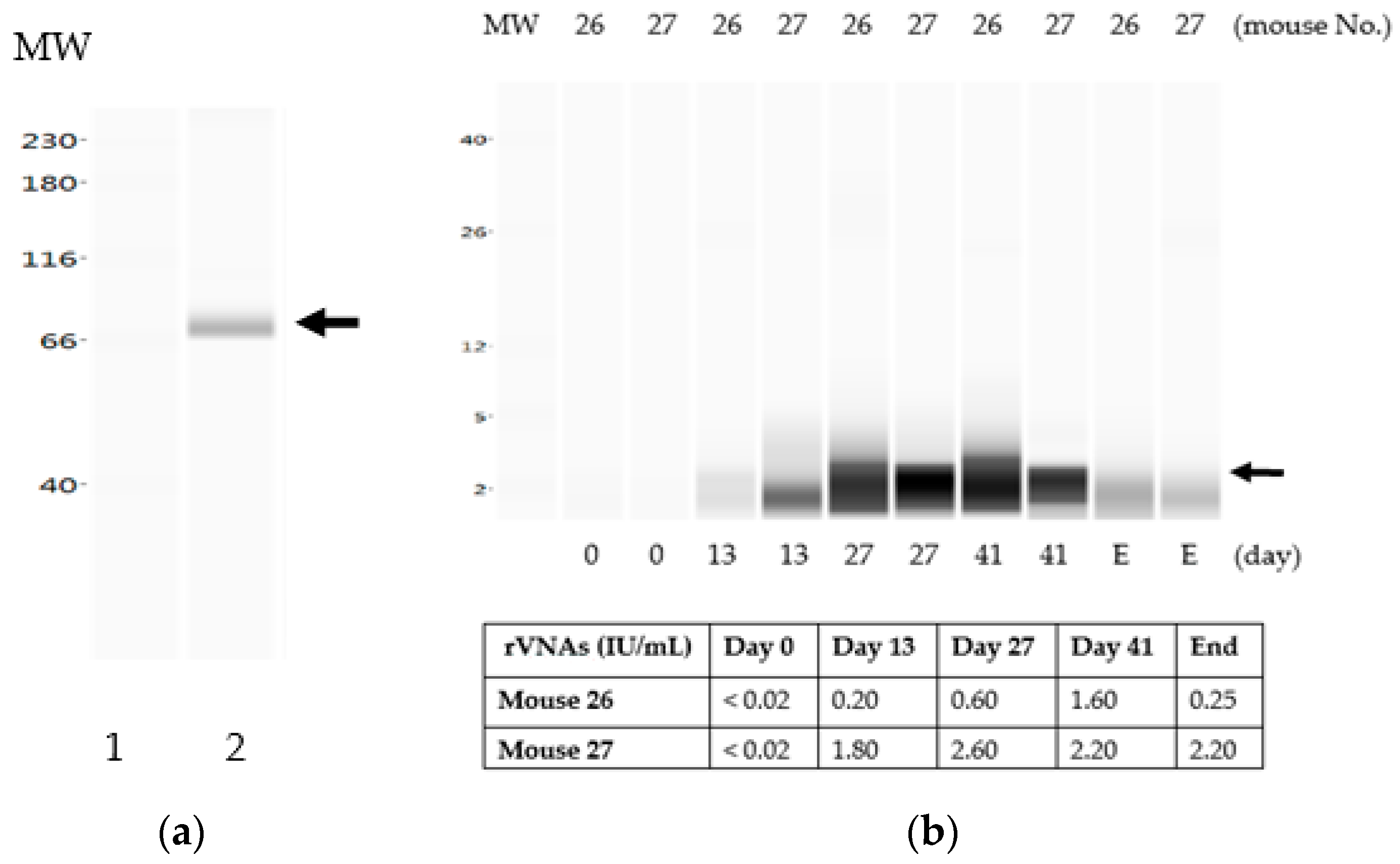
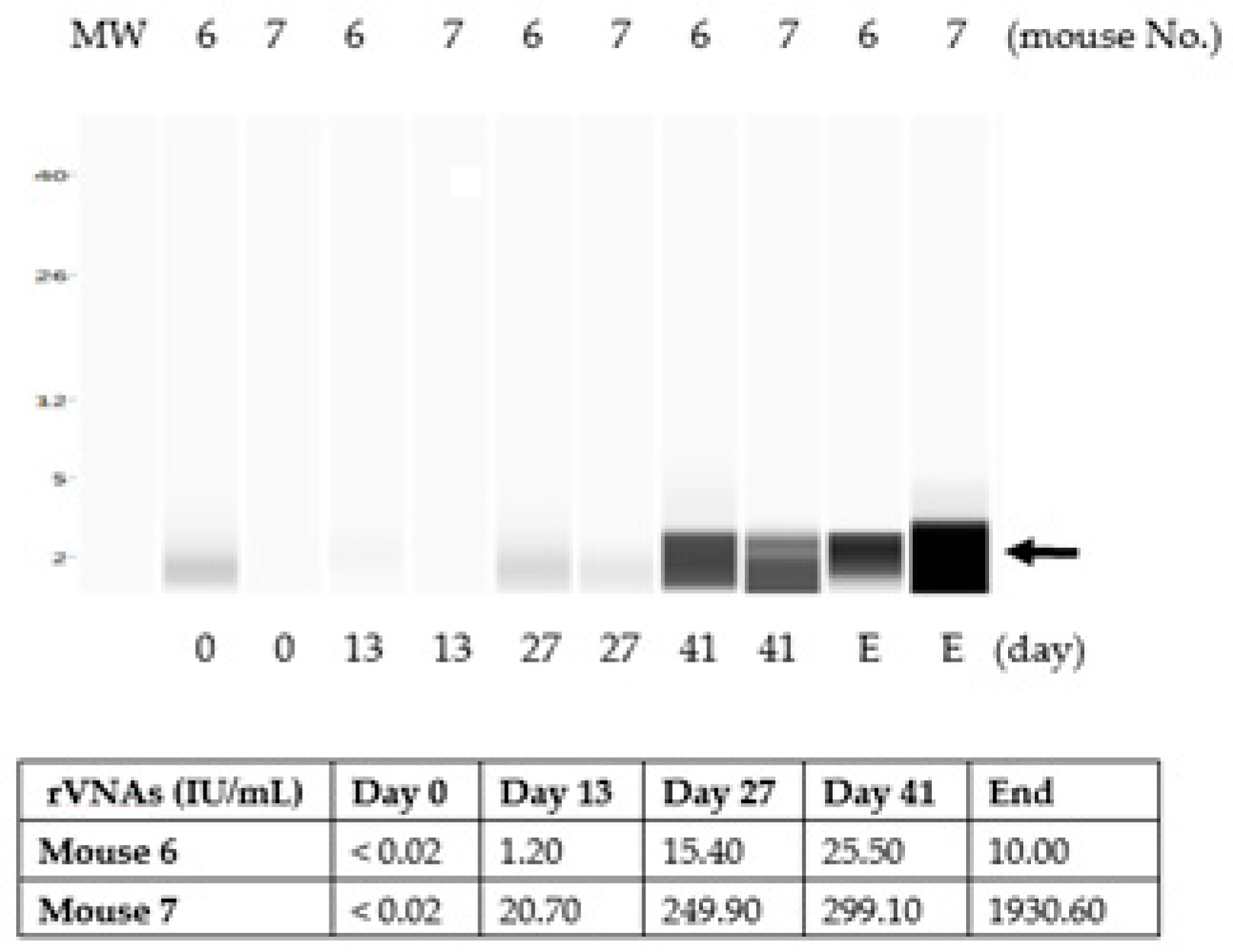
3.3. The Liquid Vaccine ERA-2GnRH Induced A Fast GnRH Antibody Response in Mice
3.4. The Chitosan Hydrogel ERA-2GnRH Vaccine Induced a Slow, but Sustained GnRH Immune Response in Mice
3.5. The GnRH Antibodies in Mice Were Not Sufficient to Prevent Pregnancy by One-Dose Vaccination
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Administration of Rabies Vaccination State Laws. Available online: https://www.avma.org/Advocacy/ StateAndLocal/Pages/rabies-vaccination.aspx (accessed on 4 June 2019).
- Frequently Asked Questions on Rabies. Available online: https://www.who.int/rabies/resources/ SEA_CD_278_FAQs_Rabies.pdf (accessed on 4 June 2019).
- Kurosawa, A.; Tojinbara, K.; Kadowaki, H.; Hampson, K.; Yamada, A.; Makita, K. The rise and fall of rabies in Japan: A quantitative history of rabies epidemics in Osaka Prefecture, 1914–1933. PLoS Negl. Trop. Dis. 2017, 11, e0005435. [Google Scholar] [CrossRef] [PubMed]
- Schildecker, S.; Millien, M.; Blanton, J.D.; Boone, J.; Emery, A.; Ludder, F.; Fenelon, N.; Crowdis, K.; Destine, A.; Etheart, M.; et al. Dog ecology and barriers to canine rabies control in the Republic of Haiti, 2014–2015. Transbound Emerg. Dis. 2016, 64, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.D.; Handel, I.G.; Shervell, K.; Roux, T.; Mayer, D.; Muyila, S.; Maruwo, G.B.; Nkhulungo, E.M.; Foster, R.A.; Chikungwa, P.; et al. The Vaccination of 35,000 dogs in 20 working days using combined static point and door-to-door methods in Blantyre, Malawi. PLoS Negl. Trop. Dis. 2016, 10, e0004824. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.D.; Mazeri, S.; Lohr, F.; Mayer, D.; Burdon Bailey, J.L.; Wallace, R.M.; Handel, I.G.; Shervell, K.; Bronsvoort, B.M.D.; Mellanby, R.J.; et al. One million dog vaccinations recorded on mHealth innovation used to direct teams in numerous rabies control campaigns. PLoS ONE 2018, 13, e0200942. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.J.O.; Jayme, S.I.; Amparo, A.C.B.; Cresencio, R.O.; Lopez, E.L.; Baquilod, M.S.; Hernandez, L.M.; Villalon, E.E.S., 3rd; Nel, L.D. World Rabies Day campaign in the Philippines. Trop. Dis. Travel Med. Vaccines 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Touihri, L.; Zaouia, I.; Elhili, K.; Dellagi, K.; Bahloul, C. Evaluation of mass vaccination campaign coverage against rabies in dogs in Tunisia. Zoonoses Public Health 2011, 58, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Wu, X.; Olson, V.; D’Souza, M.J. Characterization of rabies pDNA nanoparticulate vaccine in poloxamer 407 gel. Int. J. Pharm. 2018, 545, 318–328. [Google Scholar] [CrossRef]
- Wu, X.; Franka, R.; Svoboda, P.; Pohl, J.; Rupprecht, C.E. Development of combined vaccines for rabies and immunocontraception. Vaccine 2009, 27, 7202–7209. [Google Scholar] [CrossRef]
- Wu, X.; Smith, T.G.; Franka, R.; Wang, M.; Carson, W.C.; Rupprecht, C.E. The feasibility of rabies virus-vectored immunocontraception in a mouse model. Trials Vaccinol. 2014, 3, 11–18. [Google Scholar] [CrossRef]
- Lingappa, U.F.; Wu, X.; Macieik, A.; Yu, S.F.; Atuegbu, A.; Corpuz, M.; Francis, J.; Nichols, C.; Calayag, A.; Shi, H.; et al. Host-rabies virus protein-protein interactions as druggable antiviral targets. Proc. Natl. Acad. Sci. USA 2013, 110, E861–E868. [Google Scholar] [CrossRef]
- Smith, T.G.; Gilbert, A.T. Comparison of a micro-neutralization test with the rapid fluorescent focus inhibition test for measuring rabies virus neutralizing antibodies. Trop. Med. Infect. Dis. 2017, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lai, K.Y.; Ling, M.H.; Lin, C.W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018, 65, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Heuking, S.; Borchard, G. Toll-like receptor-7 agonist decoration enhances the adjuvanticity of chitosan-DNA nanoparticles. J. Pharm. Sci. 2012, 101, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.A.; Bramwell, V.W.; Somavarapu, S.; Alpar, H.O. Comparative immunomodulatory properties of a chitosan-MDP adjuvant combination following intranasal or intramuscular immunisation. Vaccine 2005, 23, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Crick, J.; Brown, F. Questions concerning the potency of rabies vaccine. Dev. Biol. Stand. 1978, 40, 179–182. [Google Scholar] [PubMed]
- Habel, K. Habel test for potency. In Laboratory Techniques in Rabies, 3rd ed.; Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1973; pp. 276–277. [Google Scholar]
- Lazarowicz, M.; Kihm, U.; Bommeli, W.; Zutter, R. Potency testing of inactivated rabies vaccines in mice, dogs and cats. Comp. Immunol. Microbiol. Infect. Dis. 1982, 5, 233–235. [Google Scholar] [CrossRef]
- Cho, H.C.; Fenje, P.; Sparkes, J.D. Antibody and immunoglobulin response to antirabies vaccination in man. Infect. Immun. 1972, 6, 483–486. [Google Scholar]
- Pille, E.R.; Vagabov, M.A.; Karakuyumchan, M.K.; Matevosyan, K.S. Characteristics of cellular and humoral immune responses after immunization with different rabies vaccines. Acta Virol. 1985, 29, 137–142. [Google Scholar]
- Fu, Z.F. Rabies and rabies research: Past, present and future. Vaccine 1997, 15, S20–S24. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Koprowski, H. Rabies. Sci. Am. 1980, 242, 120–134. [Google Scholar] [CrossRef]
- Bahmanyar, M.; Fayaz, A.; Nour-Salehi, S.; Mohammadi, M.; Koprowski, H. Successful protection of humans exposed to rabies infection. Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA 1976, 236, 2751–2754. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Simani, S.; Janani, A.; Farahtaj, F.; Biglari, P.; Howeizi, N.; Eslami, N. Antibody persistence, 32 years after post-exposure prophylaxis with human diploid cell rabies vaccine (HDCV). Vaccine 2011, 29, 3742–3745. [Google Scholar] [CrossRef] [PubMed]
- Strady, A.; Lang, J.; Lienard, M.; Blondeau, C.; Jaussaud, R.; Plotkin, S.A. Antibody persistence following preexposure regimens of cell-culture rabies vaccines: 10-year follow-up and proposal for a new booster policy. J. Infect. Dis. 1998, 177, 1290–1295. [Google Scholar] [CrossRef]
- Briggs, D.J.; Schwenke, J.R. Longevity of rabies antibody titre in recipients of human diploid cell rabies vaccine. Vaccine 1992, 10, 125–129. [Google Scholar] [CrossRef]
- Suwansrinon, K.; Wilde, H.; Benjavongkulchai, M.; Banjongkasaena, U.; Lertjarutorn, S.; Boonchang, S.; Suttisri, R.; Khowplod, P.; Daviratanasilpa, S.; Sitprija, V. Survival of neutralizing antibody in previously rabies vaccinated subjects: A prospective study showing long lasting immunity. Vaccine 2006, 24, 3878–3880. [Google Scholar] [CrossRef]
- Thraenhart, O.; Kreuzfelder, E.; Hillebrandt, M.; Marcus, I.; Ramakrishnan, K.; Fu, Z.F.; Dietzschold, B. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin. Immunol. Immunopathol. 1994, 71, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Duttagupta, D.S.; Jadhav, V.M.; Kadam, V.J. Chitosan: A propitious biopolymer for drug delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Barber, X.; Sendra, E.; Perez-Alvarez, J.A.; Fernandez-Lopez, J. Effect of chitosan edible films added with Thymus moroderi and Thymus piperella essential oil on shelf-life of cooked cured ham. J. Food Sci. Technol. 2015, 52, 6493–6501. [Google Scholar] [CrossRef]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan: A promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccin. Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef]
- Dissen, G.A.; Lomniczi, A.; Boudreau, R.L.; Chen, Y.H.; Davidson, B.L.; Ojeda, S.R. Applying gene silencing technology to contraception. Reprod. Domest. Anim. 2012, 47, 381–386. [Google Scholar] [CrossRef]
- Freeman, C.; Coffey, D.S. Sterility in male animals induced by injection of chemical agents into the vas deferens. Fertil. Steril. 1973, 24, 884–890. [Google Scholar] [CrossRef]
- Kutzler, M.; Wood, A. Non-surgical methods of contraception and sterilization. Theriogenology 2006, 66, 514–525. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, M.J.; Perkins, E.G.; Asa, C.; Skinner, D.C. Contraception has gone to the coyotes (Canis latrans). J. Zoo Wildl. Med. 2013, 44, S4–S8. [Google Scholar] [CrossRef]
- Munks, M.W. Progress in development of immunocontraceptive vaccines for permanent non-surgical sterilization of cats and dogs. Reprod. Domest. Anim. 2012, 47, 223–227. [Google Scholar] [CrossRef]
- Rhodes, L. New approaches to non-surgical sterilization for dogs and cats: Opportunities and challenges. Reprod. Domest. Anim. 2017, 52, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Bulldan, A.; Shihan, M.; Goericke-Pesch, S.; Scheiner-Bobis, G. Signaling events associated with gonadotropin releasing hormone-agonist-induced hormonal castration and its reversal in canines. Mol. Reprod. Dev. 2016, 83, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Han, X.F.; Li, J.L.; Zhou, Y.Q.; Ren, X.H.; Liu, G.C.; Cao, X.H.; Du, X.G.; Zeng, X.Y. Active immunization with GnRH-tandem-dimer peptide in young male rats reduces serum reproductive hormone concentrations, testicular development and spermatogenesis. Asian J. Androl 2016, 18, 485–491. [Google Scholar] [CrossRef]
- Hsu, C.T.; Ting, C.Y.; Ting, C.J.; Chen, T.Y.; Lin, C.P.; Whang-Peng, J.; Hwang, J. Vaccination against gonadotropin-releasing hormone (GnRH) using toxin receptor-binding domain-conjugated GnRH repeats. Cancer Res. 2000, 60, 3701–3705. [Google Scholar]
- Jung, M.J.; Moon, Y.C.; Cho, I.H.; Yeh, J.Y.; Kim, S.E.; Chang, W.S.; Park, S.Y.; Song, C.S.; Kim, H.Y.; Park, K.K.; et al. Induction of castration by immunization of male dogs with recombinant gonadotropin-releasing hormone (GnRH)-canine distemper virus (CDV) T helper cell epitope p35. J. Vet. Sci. 2005, 6, 21–24. [Google Scholar] [CrossRef]
- Lucas, X. Clinical use of deslorelin (GnRH agonist) in companion animals: A review. Reprod. Domest. Anim. 2014, 49, 64–71. [Google Scholar] [CrossRef]
- Miller, L.A.; Gionfriddo, J.P.; Fagerstone, K.A.; Rhyan, J.C.; Killian, G.J. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: comparison of several GnRH preparations. Am. J. Reprod. Immunol. 2008, 60, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Sad, S.; Chauhan, V.S.; Arunan, K.; Raghupathy, R. Synthetic gonadotrophin-releasing hormone (GnRH) vaccines incorporating GnRH and synthetic T-helper epitopes. Vaccine 1993, 11, 1145–1150. [Google Scholar] [CrossRef]
- Liu, I.K.; Bernoco, M.; Feldman, M. Contraception in mares heteroimmunized with pig zonae pellucidae. J. Reprod. Fertil. 1989, 85, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.B.; Schulman, M.L.; Botha, A.E.; Human, A.M.; Roth, R.; Crampton, M.C.; Bertschinger, H.J. Serum antibody immunoreactivity and safety of native porcine and recombinant zona pellucida vaccines formulated with a non-Freund’s adjuvant in horses. Vaccine 2019, 37, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Shideler, S.E.; Stoops, M.A.; Gee, N.A.; Howell, J.A.; Lasley, B.L. Use of porcine zona pellucida (PZP) vaccine as a contraceptive agent in free-ranging tule elk (Cervus elaphus nannodes). Reprod. Suppl. 2002, 60, 169–176. [Google Scholar]
- Sacco, A.G. Immunocontraception: consideration of the zona pellucida as a target antigen. Obstet. Gynecol. Annu. 1981, 10, 1–26. [Google Scholar] [PubMed]
- Notice of Registration for ZonaStat-H. Available online: https://www3.epa.gov/pesticides/chem_search/ ppls/086833-00001-20120130.pdf (accessed on 4 June 2019).
- GonaCon™—Birth Control for Deer: Questions and Answers. Available online: https://www.aphis.usda.gov/wildlife_damage/nwrc/downloads/faq_gonacon_07.pdf (accessed on 4 June 2019).
- Levy, J.K.; Friary, J.A.; Miller, L.A.; Tucker, S.J.; Fagerstone, K.A. Long-term fertility control in female cats with GonaCon, a GnRH immunocontraceptive. Theriogenology 2011, 76, 1517–1525. [Google Scholar] [CrossRef]
- Miller, L.A.; Fagerstone, K.A.; Eckery, D.C. Twenty years of immunocontraceptive research: lessons learned. J. Zoo Wildl. Med. 2013, 44, S84–S96. [Google Scholar] [CrossRef]
- Michelson Prize and Grants. Available online: https://www.acc-d.org/research-innovation/michelson-prize-grants (accessed on 4 June 2019).
- Hiby, E.; Tasker, L. Qualitative Evaluation of the Five-Year ’Red Collar’ Campaign to End Inhumane Culling of Dogs as a Method of Rabies Control. Vet. Sci 2018, 5. [Google Scholar] [CrossRef]
- Morters, M.K.; Restif, O.; Hampson, K.; Cleaveland, S.; Wood, J.L.; Conlan, A.J. Evidence-based control of canine rabies: A critical review of population density reduction. J. Anim. Ecol. 2013, 82, 6–14. [Google Scholar] [CrossRef]
- Tenzin, T.; Ahmed, R.; Debnath, N.C.; Ahmed, G.; Yamage, M. Free-roaming dog population estimation and status of the dog population management and rabies control program in Dhaka City, Bangladesh. PLoS Negl. Trop. Dis. 2015, 9, e0003784. [Google Scholar] [CrossRef]
- Totton, S.C.; Wandeler, A.I.; Ribble, C.S.; Rosatte, R.C.; McEwen, S.A. Stray dog population health in Jodhpur, India in the wake of an animal birth control (ABC) program. Prev. Vet. Med. 2011, 98, 215–220. [Google Scholar] [CrossRef]
- Li, J.; Olvera, A.I.; Akbari, O.S.; Moradian, A.; Sweredoski, M.J.; Hess, S.; Hay, B.A. Vectored antibody gene delivery mediates long-term contraception. Curr. Biol. 2015, 25, R820–R822. [Google Scholar] [CrossRef][Green Version]


| Measurement | ERA-2GnRH | Chitosan |
|---|---|---|
| Titer/Concentration | 1.0 × 108 ffu/mL | 1.2% |
| Room Temperature | Stable in lyophilized form | 60 min |
| 37 °C | Not tested | 5–10 min |
| 4 °C | Stable | Overnight |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, Y.; Kling, C.; Seigler, L.; Gallardo-Romero, N.F.; Martin, B.E.; Smith, T.G.; Olson, V.A. Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception. Vaccines 2019, 7, 73. https://doi.org/10.3390/vaccines7030073
Wu X, Yang Y, Kling C, Seigler L, Gallardo-Romero NF, Martin BE, Smith TG, Olson VA. Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception. Vaccines. 2019; 7(3):73. https://doi.org/10.3390/vaccines7030073
Chicago/Turabian StyleWu, Xianfu, Yong Yang, Chantal Kling, Laurie Seigler, Nadia F. Gallardo-Romero, Brock E. Martin, Todd G. Smith, and Victoria A. Olson. 2019. "Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception" Vaccines 7, no. 3: 73. https://doi.org/10.3390/vaccines7030073
APA StyleWu, X., Yang, Y., Kling, C., Seigler, L., Gallardo-Romero, N. F., Martin, B. E., Smith, T. G., & Olson, V. A. (2019). Inactivated Rabies Virus-Vectored Immunocontraceptive Vaccine in a Thermo-Responsive Hydrogel Induces High and Persistent Antibodies against Rabies, but Insufficient Antibodies against Gonadotropin-Releasing Hormone for Contraception. Vaccines, 7(3), 73. https://doi.org/10.3390/vaccines7030073





