Abstract
The innate antiviral response is integral in protecting the host against virus infection. Many proteins regulate these signaling pathways including ubiquitin enzymes. The ubiquitin-activating (E1), -conjugating (E2), and -ligating (E3) enzymes work together to link ubiquitin, a small protein, onto other ubiquitin molecules or target proteins to mediate various effector functions. The tripartite motif (TRIM) protein family is a group of E3 ligases implicated in the regulation of a variety of cellular functions including cell cycle progression, autophagy, and innate immunity. Many antiviral signaling pathways, including type-I interferon and NF-κB, are TRIM-regulated, thus influencing the course of infection. Additionally, several TRIMs directly restrict viral replication either through proteasome-mediated degradation of viral proteins or by interfering with different steps of the viral replication cycle. In addition, new studies suggest that TRIMs can exert their effector functions via the synthesis of unconventional polyubiquitin chains, including unanchored (non-covalently attached) polyubiquitin chains. TRIM-conferred viral inhibition has selected for viruses that encode direct and indirect TRIM antagonists. Furthermore, new evidence suggests that the same antagonists encoded by viruses may hijack TRIM proteins to directly promote virus replication. Here, we describe numerous virus–TRIM interactions and novel roles of TRIMs during virus infections.
1. Introduction
Eukaryotes are constantly exposed to a variety of pathogens, including viruses. As with other environmental signals, viral invasion triggers tightly regulated intracellular signaling cascades to optimally respond to infection. Mammals enact both an innate and an adaptive immune response to identify an infecting pathogen, to clear the foreign agent, and to protect against subsequent invasion. A primary mechanism for fine-tuning molecular pathways is utilization of post-translational modifications. Altering the functional proteome influences protein interactions, transcriptional programs, translation, secretion, and cytoskeletal arrangement. A variety of molecules, including phosphates, sugars, lipids, or proteins, can be attached or removed enzymatically to modulate protein function. Post-translational modifications thus enable rapid and reversible regulation.
1.1. Ubiquitin
Ubiquitin is a key protein in orchestrating immune responses. Ubiquitin is a 76-amino-acid protein that associates either covalently or non-covalently with proteins [1,2,3]. Interaction of ubiquitin with its targets may influence subcellular localization, signaling, trafficking, protein stability, and cell cycle progression [1,2,3]. The addition of ubiquitin to a protein target is catalyzed by three classes of ubiquitin enzymes. The ubiquitin activating enzyme (E1) binds the C-terminal glycine of the ubiquitin molecule to its active-site cysteine in an ATP-dependent manner to form a high-energy thioester bond [1,2]. Interaction of the E1 with ubiquitin exposes a site to enable the recruitment of the next enzyme [4]. Via a trans-thiolation reaction, the ubiquitin is transferred from the E1 to the E2 active-site cysteine [1,2]. Once the ubiquitin is bound, an E3 ubiquitin ligase can interact with an E2-ubiquitin conjugase and assist in the transfer of the ubiquitin from the E2 onto a target protein [1,2]. When covalently attaching ubiquitin to a protein, most often the ubiquitin is covalently linked at the lysine (K) ε-amino group [1,2].
Since ubiquitin has seven lysine residues itself, K6, 11, 27, 29, 33, 48, and 63, the ubiquitin enzymes can coordinate the formation of covalently linked polyubiquitin (poly-Ub) chains [1,2]. In addition, ubiquitin molecules can be linked in a head-to-tail orientation in which the donor ubiquitin’s C-terminal glycine is linked to the acceptor ubiquitin’s methionine-1 amino group [1,2]. The E2 primarily determines the ubiquitin chain topology [4,5] while the E3 is more important in identification and recruitment of the appropriate target [4]. Ubiquitin can be covalently linked to a protein as a single ubiquitin at one site (monoubiquitination), single ubiquitin molecules at multiple lysine residues of the same target protein (multi-monoubiquitination), or a covalently linked chain of ubiquitin to a single lysine (poly-Ub) [1]. Additionally, ubiquitination enzymes can synthesize unanchored poly-Ub chains, which are not covalently attached to any other protein, and act as a ligand for proteins containing a ubiquitin-binding domain (UBD) [1,4,6,7]. In addition to the complexity of chain length and topology, phosphorylation and acetylation of ubiquitin has also been identified [8,9]. The unique combination of poly-Ub chain topologies, covalent or non-covalent modifications, and chain length variation allows for precise signaling regulation.
The host encodes a variety of mechanisms to enact, regulate, and interpret this complex ‘ubiquitin code.’ Ubiquitin modification can directly influence the target molecule through induction of a conformational change. Such changes may recruit other molecules, expose a subcellular localization sequence, or alter protein stability [1,3]. Recognition of ubiquitin by UBD-containing proteins is analogous to a receptor binding its ligand. The UBD-containing proteins may recognize a specific poly-Ub chain topology (e.g., UBD specifically recognizing K48-linked poly-Ub chains) and/or chain length (e.g., a UBD that interacts with monoubiquitin) and then recruit specific molecules to create a signaling complex [1,3,10]. Although some exceptions exist, different poly-Ub chain topologies are associated with particular effector responses. The best-characterized examples of chain topologies include K48-linked poly-Ub chains, which stereotypically target proteins for proteasome-mediated degradation, or K63-linked poly-Ub chains, which generally coordinate cell signaling complexes and subcellular localization [1,3]. Despite the canonical roles of these chain topologies, K48-linked poly-Ub chains have been noted to be involved in promoting signaling complex assembly [11] and K63-linked poly-Ub chains can mark protein targets for degradation [12]. To regulate the ubiquitin response, the host also encodes deubiquitinating enzymes (DUB) or ubiquitin specific proteases (USP), which can disassemble poly-Ub chains or coordinate the removal of ubiquitinated enzymes [1,3]. Overall, the host encodes the enzymes necessary to generate, respond to, and eliminate ubiquitin modifications.
1.2. Tripartite Motif (TRIM) Proteins
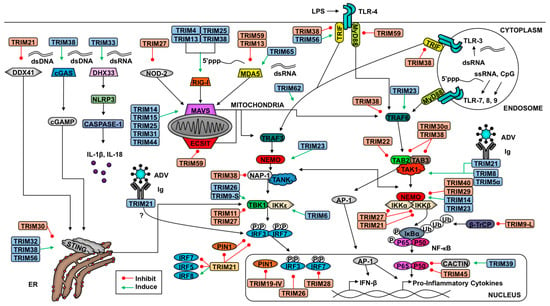
Tripartite motif proteins (TRIMs) are an E3 ligase family critical in many cellular functions, including the regulation and coordination of innate immunity and antiviral responses [6,7,13,14,15,16]. TRIMs are known to coordinate the ubiquitination of target proteins to assemble signaling complexes, mediate proteolytic degradation, alter subcellular localization, or modulate the host’s transcript or protein composition (reviewed in: [2,6,7,17]). TRIM proteins are such named due to their conserved RBCC domain. The RBCC domain includes a really interesting new gene (RING) E3 ligase domain (R), one or two B-box domains (B), and a coiled-coil domain (CC) [2,18]. Like other RING motif-containing E3 ligases, the RING domain of TRIMs usually mediates the interaction with ubiquitin-bound E2 via the zinc finger motifs [2,4,17]. These motifs are comprised of key cysteine and histidine residues that coordinate binding to two zinc ions and facilitate protein–protein interactions [2,4,17]. The function of the B-box is less well characterized, however studies suggest that this domain is important in coordinating TRIM self-association and protein–protein interactions, as well as promoting formation of higher-order TRIM oligomerization (reviewed in [17]). Since the B-box domain also contains a zinc finger motif, it has been suspected to confer E3 ubiquitin ligase activity to some TRIMs [19,20], although it does not appear to be a general feature of the TRIM family [21]. Some TRIMs include only a B-box 2 domain, while others encode a B-box 1 followed by a B-box 2 [17,18].
The coiled-coil domain is a hyper-helical structure that allows for dimerization and self-association of TRIMs [17,18,21,22]. Crystal structures and biochemical analyses of the coiled-coil regions of TRIM5α, TRIM20, TRIM25 and TRIM69 indicate that the TRIM dimers are formed by coiled-coil antiparallel helical structures [17,22,23,24,25], and bioinformatics analysis suggest that this could be a general characteristic of the TRIM family [22]. Often, dimerization of TRIMs via the coiled-coil domain and formation of higher order oligomerization through the B-box domain is critical for TRIM function and E3 ligase activity [17,21,23,26,27,28,29]. Furthermore, in some cases, the antiparallel nature of the coiled-coil dimers and the formation of higher order oligomers allows for dimerization of RING domains, which are also important for enzymatic activity [17,23]. However, the presence of one or two B-box domains, or the presence of different C-terminal regions, may affect dimerization and/or higher order oligomerization of TRIMs. In fact, the oligomerization mode of TRIM32 and TRIM25, which contain one and two B-box domains respectively, differs greatly and is critical for their function [21]. Prediction of TRIM structure and function is further complicated by differences in TRIM domain composition. For example, although TRIM members are characterized by the presence of an RBCC, some TRIM-like members, including TRIM14 and TRIM16, lack a RING domain within the RBCC [19,30].
The variable C-terminal region of TRIMs is responsible primarily for interaction with target proteins and subcellular localization [2,18,31]. To date, 11 classes of TRIM C-terminal domains have been characterized [7,17,18]. The most prevalent TRIM C-terminal domain is the B30.2, or PRY-SPRY, domain with approximately 40 members identified in humans [6,17,32]. TRIMs post-translational modifications [11,33,34,35,36,37], alternatively spliced isoforms [16,38,39], and heterodimerization [18,19] expand the diversity and functionality of this protein family. The expanding interest in TRIMs relates to the realization that these proteins play crucial roles in regulating responses to pathogens, autoimmune pathologies, and cancer.
The evolutionary history of TRIMs in vertebrates correlates with the role of this E3 ligase family in regulating responses to viral infection. Though present in invertebrates, the TRIM family is greatly diversified in vertebrates coinciding with the evolution of the interferon (IFN) pathway and adaptive immune system [32,38]. Further, several TRIMs are clustered within chromosomes [38,39,40]. The clustering of closely related TRIMs suggests they evolved due to gene duplication [38,39,40,41]. The close proximity of several TRIMs, particularly those with a B30.2 domain, near known immune genes such as HLA (Human Leukocyte Antigen) [38,39,40] supports immune regulation as a TRIM-mediated function. Additionally, the regulatory regions of several TRIMs include target sequences recognized by transcription factors involved in coordinating immune responses [42]. The species-specific expansion of B30.2-containing TRIMs [32,43] suggests that this group is specialized. In humans, the 10 TRIMs without murine orthologs all contain a B30.2 domain [32]. Additionally, the region encoding the C-terminus of many TRIMs is under positive selection [32,39,41,43,44]. The non-coding, transcriptional regulatory regions of more recently evolved TRIM genes are also under positive selection [42]. This species-specific pattern of positive selection of closely related TRIMs suggests that individual TRIMs play specific antiviral roles.
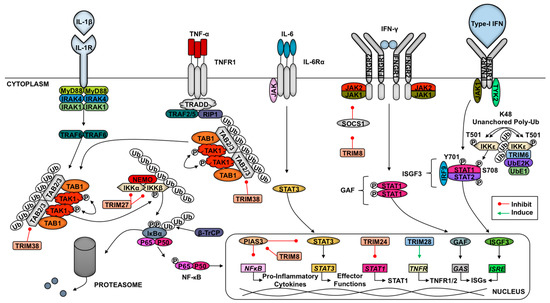
In addition to differential mRNA expression upon viral infection, several TRIM family members are intimately involved in the antiviral response. Type-I IFNs and other cytokines, such as pro-inflammatory cytokines induced via the NF-κB pathway, have been noted to differentially regulate the expression of a significant population of TRIMs [7,45,46,47,48,49,50,51]. Likewise, TRIM overexpression influences the transcription of type-I IFN, pro-inflammatory cytokines, and IFN-stimulated genes (ISGs) [7,16]. The roles of TRIMs in viral infection include intrinsic restriction of viral pathogens, positive regulation of immune pathways that promote viral clearance, and negative regulation of antiviral pathways to limit immunopathology [6,7,52]. The incorporation of TRIM antagonists into viral genomes exemplifies the importance of TRIMs in antiviral responses [53,54,55,56,57,58]. Here, we will focus on the role of TRIMs in the direct and indirect inhibition of viruses and novel mechanisms of viral-mediated antagonism and hijacking of TRIMs. Excellent reviews on the roles of TRIMs in autophagy, cancer, and other diseases have been recently published [59,60,61].
3. TRIM-Mediated Virus Inhibition
Despite the numerous host evasion mechanisms pathogens employ, a variety of host encoded molecules, such as TRIMs, are able to restrict viruses [2]. In addition to conferring an antiviral state indirectly by regulating cytokine production downstream of PRR signaling, TRIMs are capable of restricting the effectiveness of pathogens through direct interactions with viral proteins crucial to their entry, dissemination, or life cycle [6]. The categories of viral restriction include: inhibition of viral transcription, replication or translation, degradation or interference of viral proteins, and impairment of virus entry or exit. We next outline the various means by which hosts deploy TRIMs to counter and clear pathogens.
3.1. TRIMs and Retroviruses
3.1.1. TRIM5α and Retroviruses
TRIM5α is an example of a TRIM functioning as both a direct virus restriction factor as well as a pathogen-recognition receptor (Figure 3). In old-world monkeys, such as African green monkeys and rhesus macaques, TRIM5α enables natural resistance to HIV-1 while new-world monkeys, like owl monkeys, are protected from HIV-1 by fusion of TRIM5 with cyclophilin A (TCypA) [158,159,160,161]. Progress on the topic ranges from revealing the α isoform of TRIM5 as the restriction factor to discerning the role in restriction of each structural motif [158,159].
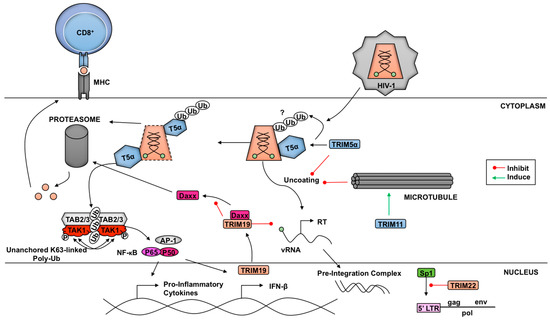
Figure 3.
The role of TRIMs in retrovirus replication. TRIM5α oligomerization into a hexagonal lattice associates directly with HIV-1 capsids to promote premature uncoating. Recognition of HIV-1 capsid by TRIM5α also triggers NF-κB/AP-1-mediated innate immune signaling via synthesis of unanchored K63-linked poly-Ub chains that activate the TAK1 kinase. One potential mechanism of TRIM5α-mediated restriction could be involved ubiquitination of TRIM5α and proteasomal degradation of TRIM5α-capsid complexes. TRIM11 mobilizes cellular microtubule formation to prematurely uncoat HIV-1 and facilitate rapid release of the vRNA from the viral core, resulting in inhibition of virus replication. TRIM19 translocates to the cytoplasm and binds Daxx to prevent its degradation by the proteasome, allowing for Daxx-mediated disruption of HIV-1 reverse transcription (RT). TRIM22 inhibits Sp1, preventing LTR-mediated transcription. The connection between TRIM5α-mediated restriction and proteasomal-mediated degradation of HIV-1 requires further characterization. However, one potential model may involve improved proliferation of HIV-1-specific CD8+ T cells due to enhanced production of viral peptides from proteasome-mediated degradation of HIV-1 capsids.
Several components inherent to TRIM5α play a role in HIV-1 capsid restriction, including dimerization, oligomerization, and ubiquitination [162,163,164]. There have been different models proposed for the mechanism of restriction and all have been attributed to the interaction of the SPRY domain of TRIM5α with the retrovirus capsid. Binding of TRIM5α to viral cores serves to prematurely uncoat the viral particle and induces early release of the viral genome. Elucidation of the TRIM5α higher order structure has revealed the significant contributions made by each component of the TRIM5α protein [26,163,164,165,166]. Monomeric TRIM5α can dimerize in an antiparallel fashion through the coiled-coil domains, thereby generating the most fundamental component needed to bind the viral capsid [163,167]. Once bound to its target, TRIM5α can form higher-order structures resembling a hexagonal net [164,165,168,169]. TRIM5α and TRIM5 variants from several primate species, including rhesus macaques, African green monkeys, and owl monkeys, are capable of forming flexible, hexagonal frameworks encompassing HIV-1 capsid surfaces [164]. The hexagonal nets are formed from TRIM5α trimers and are dependent on the TRIM5α’s B-box domain [165,166]. These trimers have been shown to be flexible and may allow for ideal binding of the SPRY domains on the highly variable HIV-1 capsid [165,166]. The ability of rhesus macaque TRIM5α (rhTRIM5α) to form higher-order structures upon binding to the capsid also allows the formation of TRIM5α RING dimers, which enhances its E3-ubiquitin ligase activity and innate anti-HIV-1 activity [29]. As one potential mechanism of rhTRIM5α-mediated restriction it was proposed that as the linker 2 regions of the dimer change their conformation, the SPRY domains that are bound to the HIV-1 capsid in their hexagonal lattice formation disturb the viral structure [163]. The induction of this conformational change disrupts the integrity of the viral core and may be responsible for pre-mature viral uncoating.
Proteasomal-mediated degradation of viral components is a host restriction strategy characteristic of many TRIM family proteins involved in host innate immunity. However, the functional role of the proteasome in TRIM5α-mediated HIV-1 restriction has been difficult to discern and has been subject of debate. Early work with proteasomal inhibitors, like MG132, suggested a two phase restriction of HIV-1 by TRIM5α. In the proteasome-independent phase, TRIM5α inhibits nuclear entry of the reverse transcription (RT) products [170,171]. In the second phase, TRIM5α can inhibit late RT products in the presence of a functional proteasome, suggesting TRIM5α may utilize the proteasome to disrupt the viral RT complex [170,171]. While proteasome inhibition can block disassembly and/or degradation of viral core components, it does not appear to rescue infectivity, which has led some investigators to conclude that degradation of capsid by the proteasome is not the mechanism of TRIM5α-mediated restriction [172]. In addition, thus far no study has described ubiquitination sites on the HIV-1 capsids, which could potentially link degradation of the capsid with proteasomal function. However, additional studies have shown proteasomal involvement in TRIM5α restriction of HIV-1 by degrading TRIM5α itself [173]. TRIM5α’s E3 ligase activity can facilitate auto-ubiquitination, thereby signaling for its own destruction by the host proteasomal machinery [36,173]. These occurrences have led some to speculate on a potential model where TRIM5α interacts with the HIV-1 capsid leading to auto-ubiquitination, capsid uncoating, and delivery to the proteasome [173,174,175,176]. Despite numerous investigations centered around proteasomal involvement, controversy within the literature surrounding the exact model connecting TRIM5α, HIV-1 cores, and proteasomes remains, and proteasome-dependent and independent mechanism have been proposed [175,176].
Polyubiquitination through the E3 ligase activity of TRIM5α’s RING domain may also be a factor involved in inhibition of retroviral replication [177]. In addition to its restriction activity, TRIM5α can induce antiviral type-I IFNs. Multiple TRIM5 orthologs induce AP-1-mediated innate immune signaling [169,178]. In the presence of the HIV-1 capsid, the E3 ligase activity of TRIM5 facilitates the generation of unanchored K63-linked poly-Ub chains. These K63-linked chains promote TAK1 autophosphorylation and activation, resulting in the induction of AP-1- and NFκB-mediated transcription [169]. Collectively, these recent findings serve to better characterize the mechanisms through which TRIM5α achieves inhibition of HIV-1 replication.
The restriction benefits conferred by TRIM5α can be observed in the progression to disease upon infection with simian immunodeficiency virus (SIV) in rhesus macaques. Course of infection studies with either a TRIM5α-sensitive or -resistant strain of SIV revealed that inoculation with a TRIM5α susceptible strain of SIV provided higher survival rates for rhesus macaques. Also, delayed development of the pathology associated with TRIM5α-sensitive SIV compared to subjects treated with a TRIM5α-resistant strain was observed [179]. In addition to these benefits, the restriction of the TRIM5α sensitive SIV strain correlated with prolonged maintenance of CD4+ central memory T cells [179]. The preservation of CD4+ central memory T cells has been extensively characterized as important in resisting viremia and generating CTL (cytotoxic T lymphocytes) subsets [180,181,182]. The prolonged presence of these CD4+ T cells in the TRIM5α-susceptible SIV-infected treatment may be responsible for the higher survival rate [179]. In spite of this, TRIM5α escape mutants were present [179]. Half of the subjects that received the TRIM5α-sensitive strain of SIV still developed AIDS, and a third succumbed to infection at a similar time as the TRIM5α-resistant group [179]. Sequencing of the SIV capsids from these macaques revealed two mutations in the region encoding the gag protein, which resulted in nonsynonymous substitutions that mimicked the alterations made by the authors to generate their TRIM5α resistant strain [179]. Subversion of TRIMs is a central evolutionary strategy for viral innate immune evasion, as demonstrated by the high mutational rate of retroviruses.
Although first described as a viral restriction factor against HIV-1 in old-world monkeys, humans and other species are capable of utilizing TRIM5α to inhibit other retroviruses. Human TRIM5α is incapable of restricting HIV-1, yet a single amino acid mutation in its PRY-SPRY domain can confer resistance to the pathogen [183]. Instead, human TRIM5α can bind to and restrict N tropic mouse leukemia virus (N-MLV) [183]. Interestingly, the 13-amino-acid stretch in the PRY-SPRY domain of primate TRIM5α is under strong positive selection [44]. TRIM5α paralogues have been identified in bovine [184,185], ovine [186], and piscine [40] species, and have also been shown to restrict retroviruses. Overall, TRIM5α plays a convergent role in the recognition and restriction of retroviruses.
Aside from its role in non-human primates, TRIM5α has been described functioning in a cell-type specific manner [187,188,189]. In contrast to conventional DC-SIGN+ (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) DCs, Langerhans cells (LCs) benefit from the anti-retroviral capabilities of TRIM5α through an increased activity of the LC autophagocytic components [187]. Upon Langerin-mediated HIV-1 uptake, the presence of cellular autophagosomes increases as a result of TRIM5α-directed assembly, leading to targeting of the viral capsid for destruction [187]. Further evidence of cell-type specific HIV-1 restriction by TRIM5α was found in rhesus macaque DCs, which in contrast to macrophages appear to be permissive to HIV-1 infection [188]. The lack of TRIM5α-mediated restriction in both human and macaque conventional DCs may be due to SUMOylation of TRIM5α, which promotes its sequestration in nuclear bodies [189]. However, this lack of TRIM5α-mediated restriction in DCs provides an innate immune sensing advantage by allowing recognition of HIV-1 reversed transcribed DNA by cGAS, resulting in type-I IFN production [189]. It remains to be seen whether HIV-1 can actively promote or enhance SUMOylation of TRIM5α in DCs as mechanism to antagonize type-I IFN production.
The ability of TRIM5α to promote restriction of HIV-1 may go beyond direct intervention of the viral lifecycle. TRIM5α has also been linked to the activity of other components of cellular innate immunity including antigen presentation to cytotoxic T lymphocytes (CTL). TCypA and rhTRIM5α enhanced the ability of CD8+ T cells to identify HIV-1 infected cells, and promoted a HIV-1-specific immune response [174]. The presence of these TRIM5 orthologs was associated with increased associations between HIV-1 particles and the host proteasome [174]. It is speculated that this may facilitate improved HIV-1-specific CTL development, as amplified peptide concentrations could support enhanced antigen presentation to CD8+ T cells. The mechanism linking direct restriction of viral capsids with heightened CD8+ T cell activation warrants further investigation.
3.1.2. Other TRIMs and Retroviruses
A plethora of TRIM-mediated restriction mechanisms targeting HIV-1 and other retroviruses have been proposed [15], and have been reviewed previously [6]. More recent reports acknowledged TRIM11 as a potent host restriction factor of HIV-1 [114,190]. The mechanisms of TRIM11-mediated restriction include curbing the amount of viral reverse transcription products allowed to accumulate in the host. Through an interaction with the viral capsid-nucleocapsid protein (CA-NC) complexes, TRIM11 promotes premature uncoating and release of the viral genetic material, reducing transduction efficiency. Neither proteasomal nor lysosomal inhibitor treatments recovered viral p24 protein in the pellets of TRIM11 overexpressing cells, suggesting that ubiquitin-mediated degradation by the proteasome or lysosomal acidification is not required for TRIM11-mediated uncoating [190]. Furthermore, while rhTRIM5α-mediated inhibition of HIV-1 is rescued by proteasome inhibitors [170,171], TRIM11-mediated inhibition was not, indicating that TRIM11 and TRIM5α restrict HIV-1 by different mechanisms [114,190]. In contrast, using the microtubule dynamics inhibitors nocodazole and taxol, the authors were able to demonstrate a restoration of HIV-1 capsid levels in cells supplemented with exogenous TRIM11, suggesting that microtubules may contribute to TRIM11-mediated HIV-1 restriction [190]. Earlier work with the use of nocodazole and taxol in a study examining HIV-1 and TRIM5α also demonstrated recovered HIV-1 infectivity in the absence of functional microtubules [191].
Although the exact mechanism of TRIM11-mediated restriction of HIV-1 in the aforementioned study has yet to be determined, the authors demonstrated that purified TRIM11 associated with in vitro assembled HIV-1 capsids [190], indicating that TRIM11 interacts directly with the capsid and it probably does not require TRIM5α or other cellular proteins for promoting untimely capsid uncoating. Microtubule involvement in viral uncoating has been implicated as both a host restriction and a viral propagation mechanism [192,193,194,195]. Functional microtubules and their associated motor proteins, like dynein, have been suggested as key components in genome release for both IAV and HIV-1 [192,193,194,195]. Interestingly, in the case of IAV, unanchored poly-Ub chains contained in the virion are recognized by the host histone deacetylase 6 (HDAC6), a component of the aggresome-autophagy pathway, which interacts with dynein and microtubules to promote viral uncoating [192,195]. It will be interesting to examine whether HIV-1 utilizes a similar mechanism and whether this is mediated by TRIM11. Additionally, several members of the TRIM family (TRIMs 1, 9, 18, 36, 46, and 67) have been shown to possesses a C-terminal domain motif allowing for association with cytoskeletal elements like microtubules [31]. So far only TRIM1 (also called MID2) and TRIM18 (also called MID1) have been shown to be directly involved in microtubule stabilization [196], and TRIM1 has been implicated in restriction of N-MLV [159]. Whether TRIM1-mediated restriction is dependent on microtubules, or whether other microtubule-interacting TRIMs may have viral restriction activity by microtubule-dependent mechanisms, remains to be seen.
TRIM-mediated suppression of HIV-1 replication has revealed additional avenues through which TRIMs subdue pathogens. TRIM22 prevents normal viral transcription events by regulating the effectiveness of the transcription factor Sp1 to bind the HIV-1 long terminal repeat (LTR) promoter region [197]. This restriction was independent of TRIM22’s E3 ligase activity and did not involve direct interaction between TRIM22 and Sp1, implying that the observed reduction in HIV-1 LTR-mediated transcription requires additional unspecified factors [197]. TRIM37 exhibits anti-retroviral functions through interference of HIV-1 infection, replication, and transcription, possibly interfering with DNA synthesis [198]. However further investigation into TRIM-mediated transcriptional inhibition will be required to reveal additional pathways in which TRIMs play a significant role.
Another example of indirect inhibition of virus replication by TRIMs is illustrated by the promyelocytic leukemia protein (PML)/TRIM19. PML/TRIM19 interferes with HIV-1 infection in human and murine fibroblasts through the reduction of reverse transcriptase products [199,200]. This effect was dependent on two events; the translocation of PML/TRIM19 from the nucleus to the cytoplasm in the presence of HIV-1 infection, as well as association of the PML/TRIM19 cytoplasmic bodies (CB) with Daxx [201]. PML/TRIM19 interacts with Daxx in a protective fashion in order to prevent its degradation by the proteasome, thereby making PML/TRIM19 a necessary component for Daxx-mediated inhibition of HIV-1 RT [201]. The inhibition of HIV-1 by PML/TRIM19 and Daxx may be cell-type dependent, since studies using different cell types have shown different results [200,201].
3.2. TRIM21 and Antibody-Dependent Intracellular Neutralization of Viruses
Another well characterized TRIM involved in pathogen recognition is TRIM21, which has been shown to detect intracellular antibody-opsonized viruses (reviewed previously in [6,7]). Some immunoglobulin-coated non-enveloped viruses are internalized into the host cell. Within most cells, a high affinity antibody receptor, TRIM21, binds to the highly conserved Fc region of virion-bound immunoglobulin (Ig)G, IgM, or IgA [7,202], and targets the virus for degradation by the proteasome before the virus can transcribe its genes [203]. TRIM21’s PRY-SPRY domain interacts with Fc residues conserved across mammalian species [204]. TRIM21 binding with an immunoglobulin-virion (Ig-V) complex triggers tightly regulated intracellular antibody neutralization and pro-inflammatory pathways [7,202]. Upon engagement of TRIM21 with the Ig-V complex within the cytoplasm, the E2 Ube2W (Ubiquitin Conjugating Enzyme E2 W) monoubiquitinates TRIM21, which promotes Ube2N/Ube2V2 E2 complex recruitment to TRIM21 for K63-linked poly-Ub [12]. Following poly-Ub, TRIM21 is recruited to the proteasome to initiate degradation of the antibody-bound virus. Concurrently, with proteasome-mediated degradation, Poh1 de-ubiquitinates TRIM21 [12]. This antibody-dependent intracellular neutralization appears to be limited to non-enveloped DNA and RNA viruses that can enter the host cytosol with immunoglobulin attached to the virion surface [205]. The de-ubiquitinated TRIM21 is then able to promote both IFN induction and NF-κB activation [12,205,206], hypothesized to result from the release of the viral genome into the cytoplasm for PRR-mediated recognition [207]. However, some antiviral cytokines are induced independent of PRRs [207], but the pathway is not well characterized. In addition, it has also been proposed that recognition of the Ig-V complex in the cytoplasm by TRIM21 triggers the synthesis of unanchored K63-linked poly-Ub chains that activate NF-κB, AP-1 and IRF3 pathways [205]. Notably, when the affinity of TRIM21 for the Fc portion of an antibody is decreased, the viral neutralization efficiency is maintained while the activation of cytokine signaling is diminished [208]. This suggests that the association of TRIM21 with Ig-V complexes is important for triggering antiviral responses. TRIM21-deficient mice infected with mouse adenovirus 1 experienced lethal disease while wild-type mice were protected, exemplifying the importance of TRIM21 in viral neutralization in vivo [209]. Most likely, these mechanisms are shared in other species due to the highly conserved Fc and TRIM21 interacting residues, however the activity of TRIM21 as an intracellular antibody receptor in non-human, non-murine models has only been demonstrated in pig cells infected with foot-and-mouth disease virus [210].
3.3. TRIMs and Negative Sense RNA Viruses
TRIM family proteins can target several components of pathogens ranging from structural elements to factors essential for transcription and replication. A variety of influenza A virus (IAV) and influenza B virus (IBV) proteins are targets of TRIM-mediated inhibition of virus replication (Figure 4). Despite being a target of IAV-NS1 (nonstructural protein 1)-dependent IFN antagonism, TRIM25 interacts with the N-terminus of IBV-NS1 preventing the viral protein’s C-terminal component from binding viral RNA [211]. Preventing IBV-NS1 from interacting with RIG-I allows signaling through this RLR pathway to proceed [211].
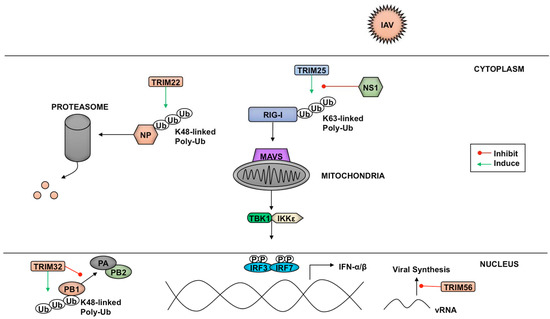
Figure 4.
Effects of TRIMs on influenza virus components and IFN antagonism. The TRIM25-binding domain of the viral NS1 protein prevents TRIM25-mediated activation of RIG-I. TRIM56 has been proposed to target viral 5’ triphosphate RNA and inhibit vRNA synthesis. TRIM22 targets the viral nucleoprotein, while TRIM32 prevents association of PB1 to the viral polymerase complex through K48-linked polyubiquitin chains that target the viral components for proteasome-mediated degradation.
Another TRIM-mediated anti-IAV mechanism is polyubiquitination of IAV nucleoprotein (NP) by TRIM22, which leads to NP proteasome-mediated degradation and reduced virus replication [212]. The polymerase basic protein 1 (PB1) of IAV is also a target of TRIM-mediated inhibition. PB1 is one of the three components that make up the RNA-dependent RNA-polymerase responsible for transcribing the eight segments of the IAV genome [213]. TRIM32 facilitates K48-linked polyubiquitination of PB1, resulting in enhanced turnover of this viral subunit, and a reduction in viral titers [214].
Aside from targeting pathogen components for proteasomal degradation via ubiquitination, some members of the TRIM family have been shown to enact their restriction factor capabilities through other means. The TRIM56 C-terminal domain, rather than its E3 ligase activity, is required to reduce IAV and IBV replication. The mechanism TRIM56 employs to diminish vRNA levels of both influenza viruses is currently unknown, although inhibition of translation through direct interaction between TRIM56 and the vRNAs has been proposed [215].
3.4. TRIMs and Positive Sense RNA Viruses
TRIMs have also been reported to mediate restriction against Flaviviruses (Figure 5). The Flavivirus genus comprises more than 70 viruses including a number of important human pathogens such as Dengue virus (DENV), Zika virus (ZIKV), West Nile virus (WNV), tick-borne encephalitis virus (TBEV), Japanese encephalitis virus (JEV), hepatitis C virus (HCV), and yellow fever virus (YFV) [216]. Flaviviruses are small enveloped viruses hosting a positive-sense single-stranded RNA genome. Several flaviviral proteins are associated with viral persistence, immune system evasion, or viral replication [217].
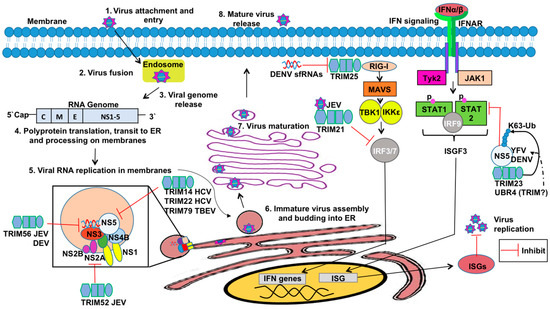
Figure 5.
The roles of TRIMs in flavivirus infection and innate immune antagonism. Flaviviruses are internalized by receptor-mediated endocytosis and by clathrin-mediated endocytosis (1). Fusion of virus membrane with host endosomal membrane (2). RNA genome is released into the cytoplasm (3). The positive-sense genomic ssRNA is translated into a polyprotein, which is cleaved into all structural and non-structural proteins (4). Replication takes place at the surface of endoplasmic reticulum in cytoplasmic viral factories (5). In this step, the TRIMs restrict virus replication, degrading viral proteins such as NS2A in Japanese encephalitis virus (JEV) by TRIM52 and viral RNA inhibition in JEV and Dengue virus (DENV) by TRIM56. TRIM22 and TRIM79 degrade NS5 protein in hepatitis C virus (HCV) and tick-borne encephalitis virus (TBEV), respectively. Virus assembly occurs at the endoplasmic reticulum. The virion buds at the endoplasmic reticulum and is transported to the Golgi apparatus (6). The prM protein is cleaved in the Golgi, thereby maturing the virion, which is fusion competent (7). Release of new virions by exocytosis (8). TRIMs and antagonism function also are used by flaviviruses. TRIM21 inhibits IFN-β production during JEV infection. TRIM23 promotes yellow fever virus replication. DENV short noncoding sfRNAs bind TRIM25 to inhibit IFN expression.
HCV encodes a nonstructural protein, NS5A, which inhibits the phosphorylation and nuclear translocation of STAT1 in the IFN-α2-induced JAK/STAT pathway via their IFN sensitivity-determining region [218,219]. TRIM14’s SPRY domain specifically interacts with NS5 of HCV and induces NS5A degradation [220], which is an example of TRIM-mediated IFN-independent inhibition. TRIM22 specifically binds the NS5A-D1 protein (Domain 1) via its SPRY domain and utilizes its E3 ubiquitin ligase activity to target NS5A for removal [221].
Wenchun and colleagues have shown that TRIM52 interacts with the NS2A protein of JEV and targets the protein for proteasome-mediated destruction [222]. NS2A is a small, hydrophobic transmembrane protein involved in the virus life cycle and subversion of host antiviral responses [223,224], including inhibition of the double-stranded RNA-activated protein kinase PKR during JEV infection [225]. TRIM52-dependent inhibition of JEV NS2A protein occurs in BHK-21 and 293T cells and is therefore important for restricting JEV replication [222].
Recent evidence also suggests that the RING and C-terminal domains of TRIM56 may be important in the restriction of other Flaviviruses, including YFV and DENV [226], but the mechanism of action remains elusive. TRIM56 might modulate post-translational modification of one or more viral proteins and/or host factors to suppress viral replication [227]. Although TRIM56 fails to restrict HCV replication when overexpressed in human hepatoma Huh7 cells [228], TRIM56 overexpression in HEK293 cells support some selectable HCV RNA replicons at very low efficiencies [227]. Perhaps TRIM56 facilitates degradation of viral proteins similar to mechanisms observed with TRIMs 14 and 22 against HCV and TRIM52 against JEV. Further studies are needed to elucidate the underlying molecular mechanisms of TRIM56-mediated restriction of DENV and YFV. Interestingly, TRIM56 is capable of binding to the protease Npro of bovine diarrheal virus (BVDV), a Pestivirus of the Flaviviridae family [228]. The restriction of this viral protein is critical considering that Npro is capable of mediating IRF3 degradation, thus impairing production of IFN-β [228]. TRIM56-mediated restriction of BVDV is specific, as closely related viruses are not likewise impaired in their replication [228].
TRIM79α, also known as TRIM30-3 or TRIM30D, is present only in rodents. TRIM79α is highly expressed in the spleen, lymph node, and bone marrow in a type-I IFN-dependent manner, and is required for effective restriction of TBEV replication [229]. TRIM79α is an important mediator of the innate cellular response to restrict Langat virus (a member of the TBEV serogroup) infection by targeting the viral RNA polymerase and major IFN antagonist, NS5 [229]. NS5 has a methyltransferase and RNA-dependent RNA polymerase activity that associates with NS3 and NS2B to form the viral replication complex. NS5 inhibits IFN-α/β-dependent responses by preventing JAK-STAT signaling and thus suppresses IFN-stimulated gene (ISG) expression [229,230,231,232]. Taylor and colleagues demonstrated that TRIM79α interacts with NS5 from LGTV and TBEV and blocks the replication of these viruses via a lysosomal-targeting mechanism. Despite NS5 being the most conserved of the flaviviral proteins, TRIM79α did not target NS5 from WNV, nor could it inhibit WNV replication [232].
Besides Flaviviruses, other positive-sense RNA viruses have been reported to be inhibited by TRIM-mediated mechanisms and can occur through both direct and indirect means. For example, TRIM25 acts as a cofactor for the Zinc-finger antiviral protein (ZAP), a member of the poly(ADP-ribose) polymerase family that is known to bind and promote viral RNA degradation [233]. TRIM25 enhances ZAPs’ ability to inhibit Sindbis virus translation [233]. However, although TRIM25 is capable of promoting K48- and K63-linked polyubiquitination of ZAP isoforms, ubiquitination does not appear to affect ZAP antiviral activity [233]. It will be of interest to elucidate what other factors may be ubiquitinated by TRIM25 that could affect ZAP antiviral activity. TRIM19-IV has been shown to confer resistance to encephalomyocarditis virus (EMCV) hampering viral replication and protein synthesis [234]. This ability was shown to originate from TRIM19-IV’s C-terminus, specifically through an interaction with the viral 3D polymerase (3Dpol), leading to nuclear body sequestration of the viral polymerase [234]. Additionally, SUMOylation of TRIM19-IV was required to facilitate restriction of EMCV [234].
3.5. TRIMs and DNA Viruses
TRIMs play an additional role in restricting DNA virus replication. Eight different TRIM proteins (TRIM5, 6, 11, 14, 25, 26, 31, and 41) were identified to inhibit hepatitis B virus (HBV) [235]. In particular, TRIM41 was the only TRIM from this group of eight that specifically reduced both the enhancers I and II components of HBV. This inhibition of viral transcription required TRIM41’s RING and PRY/SPRY domains, implicating its E3 ligase activity [235]. TRIM22 is associated with HBV clearance in acutely infected chimpanzees, and possesses anti-HBV activity under physiological conditions [236]. Gao and colleagues also reported that TRIM22 was one of the most strongly induced TRIM family molecules in human hepatoma HepG2 cells after treatment with IFNs [237].
Epstein–Barr virus (EBV) replication and transcription activator (Rta) protein activates EBV lytic genes for proliferation [238]. TRIM5α promotes the ubiquitination of Rta upon interaction, thereby blocking the EBV lytic cycle [238]. The involvement of TRIM5α in a non-retroviral innate immune response implies TRIMs possess diverse, situation-specific functions that have yet to be characterized. TRIMs may also operate as recognition receptors for other components of host immunity. TRIM19 isoforms are able to capture varicella-zoster virus nucleoproteins to impede nuclear egress and thus block the release of new virions [239]. Comprehension of the multiple roles TRIM-family proteins play may become critical in discerning the impact that they have on the innate immune response.
4. Viral Antagonism of TRIMs
As we have described, TRIM family proteins play an important role in innate immunity and counter pathogens [7]. In retaliation to this evolutionary pressure, pathogens have adapted to antagonize TRIMs. Mechanisms of viral-mediated TRIMs restriction range from impairment of TRIM-promoted innate immune signaling complex assembly to direct hindrance of the TRIM proteins. Interfering with TRIMs, either directly or indirectly, undermines their intended function, and enables viruses to gain an early advantage over the host [58]. The following section highlights several recent examples that elucidate viral manipulation of TRIMs.
4.1. Antagonism of TRIMs by RNA Viruses
A broad range of RNA viruses have evolved effective evasion strategies to manipulate host antiviral immunity. Influenza viruses employ well-studied examples of TRIM antagonism (Figure 4). The nonstructural protein 1 (NS1) of influenza A and B virus (IAV/IBV) has been characterized as a viral antagonist of host innate immunity through interactions with TRIM25 [57,100,240,241]. As described above, TRIM25 plays a critical role in the activation of the RLR pathways [57,242,243]. The NS1 protein from IAV directly interacts with the coiled-coil domain of TRIM25 to impede its multimerization [57,244]. Since dimerization is required for TRIM25 E3 ligase activity, NS1 binding to TRIM25 leads to impaired ubiquitination of the RIG-I and downstream signaling, resulting in a reduced antiviral response [57,244].
The TRIM25 interaction with IAV NS1 is species-specific. Human TRIM25 interacts with IAV strains isolated from many species while chicken TRIM25 binds only NS1 from avian strains and murine TRIM25 did not bind any NS1 [100]. Riplet, a close relative of TRIM25, lacks a B-box domain but shares homologous RING and SPRY domains. Its predicted coiled-coil structure also binds NS1 to inhibit RIG-I signaling in mice and humans [100]. Aside from interacting with TRIM25, IAV NS1 also associates with host RIG-I directly [244]. As seen with IAV, the N-terminal domain of IBV NS1 is also able to block the Lys63-linked ubiquitination of RIG-I and subsequent antiviral signaling downstream of the RLR pathway [211].
Members of the Coronaviridae, Flaviviridae, and Bunyaviridae families also encode viral proteins that inhibit TRIM-mediated regulation of RLR signaling. Severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus (SARS/MERS-CoV) are large, positive-sense single-stranded RNA viruses. Akin to the influenza virus NS1 protein, the nucleoprotein of SARS-CoV was demonstrated to interact with the SPRY domain of TRIM25, preventing the necessary interaction and subsequent ubiquitination of the RIG-I CARD domains [245]. A similar loss of RIG-I-induced IFN-β is achieved when MERS-CoV nucleoprotein associates with TRIM25 [245]. For DENV, Manokaran et al. compared two viral sequences (PR1 and PR-2B) and identified mutations that resulted in the increased production of subgenomic flavivirus non-coding RNAs (sfRNAs) by the PR-2B strain [55] (Figure 5). The PR-2B sfRNAs were capable of binding to host TRIM25 and prevented USP15-mediated deubiquitination [55], which is crucial for activation of RIG-I [94]. This data provides unique molecular insight into the epidemiological fitness of DENV, suggesting that DENV sfRNAs can bind to host proteins to promote viral evasion of innate immunity [55]. The NSs protein of severe fever with thrombocytopenia syndrome virus (SFTSV), a negative-sense RNA virus in the family Bunyaviridae, interacts directly with TRIM25, and indirectly with RIG-I and TBK1 to isolate these signaling molecules from associating with MAVS [246]. As with the other viral protein-TRIM25 interactions described above, downstream activation of IRF3 and subsequent IFN-β production are impaired [246]. TRIM25 is a common target of a diverse group of RNA viruses, suggesting that other pathogens may also impair TRIM25-mediated stimulation of the RLR pathway.
Similar to IAV, HIV-1 encodes multiple proteins that restrict TRIM function (Figure 3). For example, HIV-1 Vpr protein has been suggested to play a role in TRIM manipulation [114]. Interestingly, protein expression levels of TRIM11 have shown to be under the control of Vpr in a dose-dependent manner, where the presence of TRIM11 was decreased when the concentration of Vpr was low [114]. Currently, the mechanism HIV-1 Vpr employs to antagonize TRIM11 is unknown. Following infection of human neural precursor cells (hNPCs) with HIV-1, TRIM32 becomes upregulated, primarily due to the viral trans-activator of transcription protein (Tat) [247]. This Tat-mediated upregulation of TRIM32 induces proliferation arrest in hNPCs, eventually leading to neurodegeneration [247].
Flaviviruses also encode viral proteins that antagonize TRIM-mediated innate immunity (Figure 5). YFV and DENV NS5 protein have 10 amino acid residues on the N-terminus, which are essential for antagonism of type-I IFN signaling [54,248]. YFV NS5 binds STAT2 only after IFN treatment, and appears to inactivate ISGF3 within the nucleus [54]. Morrison et al. found in DENV NS5 a glycine and a threonine residue within the N-terminus that are required for binding with UBR4 (Ubiquitin Protein Ligase E3 Component N-Recognin 4) to mediate STAT2 degradation [249]. UBR4, a member of the N-recognin family, is a potential E3 ligase that recognizes and degrades proteins containing destabilizing N termini. [250]. UBR4 interacts preferentially with proteolytically-processed DENV NS5, but not with YFV NS5 or WNV NS5 [249]. Although UBR4 does not belong to the TRIM family, it is possible that TRIM members may also be involved in STAT2 degradation by NS5. For example, TRIM23 was identified as an essential factor in YFV replication due to its interaction and poly-Ub of residue K6 on YFV-NS5, promoting binding with STAT2 and inhibition of type-I IFN signaling [54]. Another flavivirus, Japanese encephalitis virus (JEV), induces expression of TRIM21 in human microglial cells, which attenuates JEV-induced antiviral signaling [53]. The study by Manocha et al. demonstrated that TRIM21 overexpression suppressed phosphorylation of IRF3 and activation of IFN-β, while silencing TRIM21 permitted efficient type-I IFN responses in JEV-infected human microglial cells [53]. This study provides evidence that JEV suppress the IFN-I response due to induction of TRIM21.
Finally, we recently showed that Nipah virus (NiV), a single-stranded negative-sense highly pathogenic RNA virus (Paramyxoviridae family, genus Henipavirus) that causes fatal diseases in humans [251], can inhibit TRIM6-mediated type-I IFN responses [56] (Figure 6). Mechanistically, the NiV matrix (NiV-M) structural protein, which is required for virus assembly and budding [252,253], targets TRIM6 for degradation. Interestingly, NiV budding requires trafficking of NiV-M from the cytoplasm to the nucleus before reaching the cell membrane for virus assembly [252,253]. The reason for NiV-M trafficking to the nucleus is still unclear, however, a lysine residue (K258) in the NiV-M bipartite nuclear localization signal that is conserved in divergent henipaviruses and is required for trafficking, is critical for the IFN antagonist function [56]. Consistent with this, the matrix proteins of Ghana, Hendra and Cedar viruses were also able to inhibit IFN-β induction [56]. It is currently unknown whether TRIM6 E3-ligase activity affects NiV-M trafficking or whether it directly interferes with NiV-replication. NiV-M-induced TRIM6 degradation did not appear to require the proteasome, however. Although the precise mechanism of TRIM6 degradation remains to be elucidated, inhibitors that recover TRIM6 protein levels could potentially be used as therapeutic drugs against NiV infections. These findings highlight the importance of TRIM6 as an antiviral factor of the type-I IFN system.
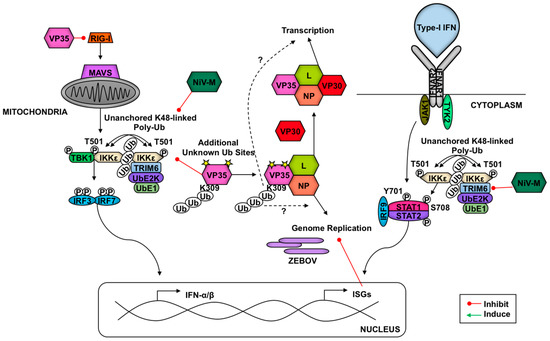
Figure 6.
TRIM6 is targeted by Nipah and Ebola viruses to enhance virus replication. Ebola virus (EBOV) inhibits type-I IFN production by multiple mechanisms. EBOV VP35 binds and inhibits RIG-I, IKKε, and TBK-1 to inhibit IFN production. TRIM6 ubiquitinates VP35 on K309 and promotes VP35 activity as the cofactor of the viral polymerase and enhances virus replication. Additional unidentified ubiquitination sites of VP35 exist. Whether TRIM6 enhances EBOV replication by promoting viral genome replication or viral gene transcription is not known. In Nipah virus infection, the viral matrix protein (NiV-M) promotes TRIM6 degradation, resulting in reduced synthesis of K48-linked unanchored polyubiquitin chains, IKKε oligomerization, IKKε-T501 autophosphorylation, IRF3 phosphorylation, and reduced IFN induction. These combined losses confer an impaired host-antiviral response.
4.2. Antagonism of TRIMs by DNA Viruses
A variety of DNA viruses also encode viral proteins that interfere with TRIMs. Alternative methods for subverting TRIM-induced innate immune responses can be noted through alterations made to host transcription by hepatitis B virus (HBV). The HBV-encoded protein X (HBx) methylates a single CpG in TRIM22’s promoter region [254]. This viral-mediated epigenetic modification blocks IFN-α/γ-induced IRF1, a transcriptional activator, from binding the TRIM22 promoter and consequently facilitates viral proliferation and escape [255]. In Epstein–Barr virus (EBV), KAP1 (KRAB [Kruppel-Associated Box Domain]-Associated Protein 1)/TRIM28 regulates activation of the viral lytic cycle [256]. In cells undergoing lytic stage activation, KAP1/TRIM28 becomes phosphorylated at serine residue 824 by ataxia telangiectasia mutated (ATM). This post-translational modification impairs KAP1/TRIM28’s restriction factor function and allows EBV to transition from latency to lytic stage [256]. Additionally, the anti-malarial drug chloroquine acts as an activator for ATM by phosphorylating KAP1/TRIM28, which ultimately leads to promotion of the EBV lytic cycle and escape of the virus particles [256].
Mutations in gene expression serve as an additional focal point highlighting the role that TRIMs play in a dysfunctional antiviral response. When a population of 765 HBV-infected individuals was screened, chronic HBV infection was found to correlate to a single T to C silent mutation single nucleotide polymorphism (SNP) in the TRIM22 RING domain [257]. Although the implications to patient outcomes are considerable, additional investigations into the basic biological mechanisms are warranted to discern the importance of these SNPs in TRIM-mediated innate immunity.
In addition to impairing TRIM gene expression, DNA viruses can mimic host proteins to prohibit TRIM function. The immediate early protein (ICP0) of herpes simplex virus 1 (HSV-1) possesses a RING domain and facilitates the degradation of TRIM27 through ubiquitination [258]. This destruction of TRIM27 is facilitated through the host’s proteasome [258], mimicking the numerous instances of TRIM-mediated restriction. Gammaherpesvirus MHV-68 similarly targets TRIM19 for proteasome-mediated degradation [239]. The IE1 proteins of human cytomegalovirus (HCMV) mimics the coiled-coil domain of TRIMs to recruit and sequester TRIM19 from nuclear bodies thus impairing the activation of IFN responses [239].
6. Potential Roles of TRIM-mediated Autophagy during Virus Infections
Autophagy (self-eating) is a highly conserved catabolic mechanism through which eukaryotic cells deliver dispensable, or potentially dangerous, cytoplasmic material to lysosomes for degradation [264]. The process is characterized by the formation of autophagosomes, which sequester the cytoplasmic structures targeted for destruction. Autophagy has been linked to a wide range of physiological processes, including cell differentiation and development, the degradation of aberrant structures and turnover of damaged organelles, as well as innate and adaptive immunity [265,266]. A growing number of studies indicate that several TRIMs are linked to autophagy and recent excellent reviews are available on the emerging roles of TRIMs in autophagy [59,60,267,268].
A good example of the potential role of TRIMs in autophagy and virus infections is rhesus TRIM5α, which acts both as a regulator of autophagy by providing a platform for the assembly of activated ULK1 and Beclin 1 (key components of the autophagy regulatory complexes) and as a receptor for selective autophagy [269,270]. In its role as an autophagic cargo receptor, TRIM5α directly recognizes viral capsid sequences via its SPRY domain [158,269,270,271]. This is an example of selective autophagy in mammalian cells, which could occur via direct substrate recognition by TRIMs and connects autophagy with a role in defense against viral pathogens. The rhesus TRIM5 can execute precision autophagy of the HIV-1 capsid. In contrast, the weak affinity of human TRIM5 for the HIV-1 capsid precludes effective precision autophagy. As a consequence, rhesus TRIM5, but not human TRIM5, could contribute to defense against HIV-1 through precision autophagy. Since this recognition depends on the C-terminal region of TRIMs (e.g., SPRY domain of TRIM5α), other types of C-terminal domains on TRIMs could selectively recognize diverse protein targets. Thus, TRIM proteins, as a group, could comprise a class of broad-repertoire, high-fidelity, selective autophagic receptors. Given the breadth of the role of TRIMs in various diseases, it will be important to explore precision autophagy—in addition to bulk autophagy—as a therapeutic target against viral infections.
The proposed role of TRIMs in autophagy raise questions. For example, how many TRIMs act as autophagic receptors and what are their specific targets? Do TRIM proteins function as hubs connecting different signaling pathways or different systems? How is the autophagic role of TRIMs integrated with the other functions of TRIMs, including regulation of gene expression and pro-inflammatory signaling? What is the interplay between the E3 ligase activity of TRIMs and precision autophagy? It is important to determine inhibitory compounds of TRIM proteins for their use as therapeutic tools in infectious diseases. However, because some TRIM proteins have simultaneous dual functions in carcinogenesis and the immune response, it should be considered that putative drugs (inhibitors of some TRIM proteins) for cancer therapy may affect immunological reactions as a side effect. Further detailed analysis of TRIM proteins is needed for their use as novel therapeutics with minimal side effects.
7. Conclusions and Future Perspectives
This review highlighted the roles of TRIMs in virus–host interactions. Extensive reports detail TRIM involvement in immune signaling and direct virus restriction. In addition, viral antagonism of TRIMs exemplifies the importance of this protein family in antiviral responses. Despite these advancements, many TRIMs have yet to be characterized. Additionally, the molecular mechanisms underlying TRIM-mediated virus restriction or viral protein-mediated TRIM inhibition are not fully elucidated. The role of TRIMs in regulating poly-Ub chain topology is also of interest. In several examples, including TRIMs 5 [169], 6 [11], 21 [12], and 25 [77], TRIMs facilitate the synthesis of unanchored poly-Ub chains. The relative contribution of TRIMs in synthesizing specifically unanchored poly-Ub chains, versus covalently linked poly-Ub chains, is unclear. Classically, the E2 is considered more important in determining poly-Ub chain characteristics [4,5], but post-translational modifications may influence the decision between covalent and non-covalent linkage [12] as may the choice of the partnering E3. Perhaps TRIMs play a unique role in the synthesis of unanchored chains. In the future, evaluating the factors that regulate E2-TRIM pairing may add an additional layer of complexity to TRIM-mediated regulation and the ubiquitin code. Although one study addressed this question by testing interactions between TRIMs and the E2-conjugases and a few TRIM-E2 pairs were identified [272], the complexity of potential transient interactions, and the possibility of cell-type specific expression for the combination of TRIMs and E2-conjugases makes this task a huge challenge.
Importantly, another question is how poly-Ub chains of unconventional linkages may affect virus replication. For example, unanchored poly-Ub chains can be incorporated into the IAV virion to enable hijacking of the host’s aggresomal pathway to facilitate viral replication [192]. The E2 and E3 enzymes responsible for synthesizing these poly-Ub chains have not been identified, but perhaps IAV hijacks a TRIM to make these poly-Ub chains critical in efficient infection. Therefore, unanchored poly-Ub chains may have both pro-viral and antiviral functions [195] and elucidating how to tip the balance towards antiviral responses may help develop novel antiviral therapies. Additionally, IAV replication via host ubiquitin and aggresome systems relies on the host’s cytoskeletal network [192]. This may further implicate the role of TRIMs in similar pathways as several TRIMs associate with microtubules [18,31]. Many TRIMs assemble into cytoplasmic bodies, which can be dynamic structures as described with TRIM32 [35]. The exact role of cytoplasmic bodies is not well characterized. Possible TRIM-cytoplasmic body functions may include facilitation of signaling complex assembly [11], regulation of active TRIM solubility, and/or generation of autophagosome-like complexes to target proteins for degradation. Viruses can also disrupt or reorganize TRIM-cytoplasmic bodies, further supporting a role in antiviral responses. Understanding how TRIM sub-cellular localization influences activity will also benefit the field.
The applicability of the information garnered from studying TRIM–virus interactions may facilitate the identification of novel antiviral targets. Further studies will advance our understanding of the complexities involved with TRIM signaling such as isoform-specific roles, cell-type specific activity, cytoplasmic body assembly and function, and post-transcriptional modification-induced TRIM function. Finally, future studies will need to address whether other TRIMs, in addition to TRIM6, may be hijacked by viruses to directly enhance replication, acting as pro-viral factors.
Acknowledgments
This work was supported by funding from the Institute for Human Infections and Immunity (IHII) and the University of Texas Medical Branch (UTMB), to RR. No funds were received for covering the costs to publish in open access.
Author Contributions
S.v.T., A.H., M.I.G., P.B., and R.R. wrote the manuscript. A.H., M.G., and S.v.T. prepared the figures. R.R., S.v.T., and A.H. provided critical review and revised the final document.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yau, R.; Rape, M. The increasing complexity of the ubiquitin code. Nat. Cell Biol. 2016, 18, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.; Versteeg, G.A.; Ikeda, F. Ubiquitin enzymes in the regulation of immune responses. Crit. Rev. Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Husnjak, K.; Dikic, I. Ubiquitin-binding proteins: Decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 2012, 81, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Rape, M. Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 2009, 10, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, B.; Zhu, W.; Zhang, R.; Li, L.; Hao, X.; Chen, S.; Hou, F. Ube2d3 and ube2n are essential for RIG-I-mediated mavs aggregation in antiviral innate immunity. Nat. Commun. 2017, 8, 15138. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Garcia-Sastre, A.; Versteeg, G.A. Trimmunity: The roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J. Mol. Biol. 2014, 426, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Benke, S.; Garcia-Sastre, A.; Rajsbaum, R. Intrimsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014, 25, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, F.; Saeki, Y.; Sakamoto, K.; Ohtake, K.; Nishikawa, H.; Tsuchiya, H.; Ohta, T.; Tanaka, K.; Kanno, J. Ubiquitin acetylation inhibits polyubiquitin chain elongation. EMBO Rep. 2015, 16, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Hicke, L.; Schubert, H.L.; Hill, C.P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 2005, 6, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Versteeg, G.A.; Schmid, S.; Maestre, A.M.; Belicha-Villanueva, A.; Martinez-Romero, C.; Patel, J.R.; Morrison, J.; Pisanelli, G.; Miorin, L.; et al. Unanchored k48-linked polyubiquitin synthesized by the e3-ubiquitin ligase TRIM6 stimulates the interferon-ikkepsilon kinase-mediated antiviral response. Immunity 2014, 40, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.; Mallery, D.L.; Watkinson, R.E.; Dickson, C.F.; James, L.C. Sequential ubiquitination and deubiquitination enzymes synchronize the dual sensor and effector functions of TRIM21. Proc. Natl. Acad. Sci. USA 2015, 112, 10014–10019. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Uchil, P.D.; Hinz, A.; Siegel, S.; Coenen-Stass, A.; Pertel, T.; Luban, J.; Mothes, W. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J. Virol. 2013, 87, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Uchil, P.D.; Quinlan, B.D.; Chan, W.T.; Luna, J.M.; Mothes, W. TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 2008, 4, e16. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Rajsbaum, R.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Valdiviezo, J.; Shi, M.; Inn, K.S.; Fernandez-Sesma, A.; Jung, J.; Garcia-Sastre, A. The E3-ligase trim family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity 2013, 38, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Koliopoulos, M.G.; Rittinger, K. Structural determinants of TRIM protein function. Biochem. Soc. Trans. 2017, 45, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Reymond, A.; Meroni, G.; Fantozzi, A.; Merla, G.; Cairo, S.; Luzi, L.; Riganelli, D.; Zanaria, E.; Messali, S.; Cainarca, S.; et al. The tripartite motif family identifies cell compartments. EMBO J. 2001, 20, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.L.; Malyukova, A.; Holien, J.K.; Koach, J.; Parker, M.W.; Kavallaris, M.; Marshall, G.M.; Cheung, B.B. TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS ONE 2012, 7, e37470. [Google Scholar] [CrossRef] [PubMed]
- Massiah, M.A.; Simmons, B.N.; Short, K.M.; Cox, T.C. Solution structure of the RBCC/TRIM B-box1 domain of human mid1: B-box with a ring. J. Mol. Biol. 2006, 358, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, M.G.; Esposito, D.; Christodoulou, E.; Taylor, I.A.; Rittinger, K. Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity. EMBO J. 2016, 35, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.G.; Okreglicka, K.; Chandrasekaran, V.; Welker, J.M.; Sundquist, W.I.; Pornillos, O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proc. Natl. Acad. Sci. USA 2014, 111, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Dawidziak, D.M.; Sanchez, J.G.; Wagner, J.M.; Ganser-Pornillos, B.K.; Pornillos, O. Structure and catalytic activation of the TRIM23 ring E3 ubiquitin ligase. Proteins 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Wu, W.; Zhuo, W.; Liu, W.; Zhang, Y.; Cheng, M.; Chen, Y.G.; Gao, N.; Yu, H.; et al. Structural insights into the TRIM family of ubiquitin E3 ligases. Cell Res. 2014, 24, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Weinert, C.; Morger, D.; Djekic, A.; Grutter, M.G.; Mittl, P.R. Crystal structure of TRIM20 c-terminal coiled-coil/b30.2 fragment: Implications for the recognition of higher order oligomers. Sci. Rep. 2015, 5, 10819. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Griffero, F.; Qin, X.-r.; Hayashi, F.; Kigawa, T.; Finzi, A.; Sarnak, Z.; Lienlaf, M.; Yokoyama, S.; Sodroski, J. A b-box 2 surface patch important for TRIM5α self-association, capsid binding avidity, and retrovirus restriction. J. Virol. 2009, 83, 10737–10751. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sodroski, J. The TRIM5alpha b-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J. Virol. 2008, 82, 11495–11502. [Google Scholar] [CrossRef] [PubMed]
- Streich, F.C., Jr.; Ronchi, V.P.; Connick, J.P.; Haas, A.L. Tripartite motif ligases catalyze polyubiquitin chain formation through a cooperative allosteric mechanism. J. Biol. Chem. 2013, 288, 8209–8221. [Google Scholar] [CrossRef] [PubMed]
- Yudina, Z.; Roa, A.; Johnson, R.; Biris, N.; de Souza Aranha Vieira, D.A.; Tsiperson, V.; Reszka, N.; Taylor, A.B.; Hart, P.J.; Demeler, B.; et al. Ring dimerization links higher-order assembly of TRIM5alpha to synthesis of k63-linked polyubiquitin. Cell Rep. 2015, 12, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jia, X.; Xue, Q.; Dou, Z.; Ma, Y.; Zhao, Z.; Jiang, Z.; He, B.; Jin, Q.; Wang, J. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-i-like receptor-mediated innate immune response. Proc. Natl. Acad. Sci. USA 2014, 111, E245–E254. [Google Scholar] [CrossRef] [PubMed]
- Short, K.M.; Cox, T.C. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J. Biol. Chem. 2006, 281, 8970–8980. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Cairo, S.; Fontanella, B.; Ballabio, A.; Meroni, G. Genomic analysis of the TRIM family reveals two groups of genes with distinct evolutionary properties. BMC Evol. Biol. 2008, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Cho, H.; Inn, K.S.; Yang, A.; Zhao, Z.; Liang, Q.; Versteeg, G.A.; Amini-Bavil-Olyaee, S.; Wong, L.Y.; Zlokovic, B.V.; et al. Negative regulation of NF-κB activity by brain-specific tripartite motif protein 9. Nat. Commun. 2014, 5, 4820. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S.; Suita, N.; Okazaki, I.M.; Shibayama, S.; Honjo, T. TRIM28 prevents autoinflammatory T cell development in vivo. Nat. Immunol. 2012, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Taoka, M.; Shoji, I.; Kato, H.; Sato, T.; Hatakeyama, S.; Isobe, T.; Hachiya, N. 14-3-3 proteins sequester a pool of soluble TRIM32 ubiquitin ligase to repress autoubiquitylation and cytoplasmic body formation. J. Cell Sci. 2013, 126, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Griffero, F.; Li, X.; Javanbakht, H.; Song, B.; Welikala, S.; Stremlau, M.; Sodroski, J. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 2006, 349, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T. The cellular level of TRIM31, an RBCC protein overexpressed in gastric cancer, is regulated by multiple mechanisms including the ubiquitin-proteasome system. Cell Biol. Int. 2011, 35, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G. Genomics and evolution of the trim gene family. Adv. Exp. Med. Biol. 2012, 770, 1–9. [Google Scholar] [PubMed]
- Han, K.; Lou, D.I.; Sawyer, S.L. Identification of a genomic reservoir for new TRIM genes in primate genomes. PLoS Genet. 2011, 7, e1002388. [Google Scholar] [CrossRef] [PubMed]
- Boudinot, P.; van der Aa, L.M.; Jouneau, L.; Du Pasquier, L.; Pontarotti, P.; Briolat, V.; Benmansour, A.; Levraud, J.P. Origin and evolution of TRIM proteins: New insights from the complete trim repertoire of zebrafish and pufferfish. PLoS ONE 2011, 6, e22022. [Google Scholar] [CrossRef] [PubMed]
- Malfavon-Borja, R.; Sawyer, S.L.; Wu, L.I.; Emerman, M.; Malik, H.S. An evolutionary screen highlights canonical and noncanonical candidate antiviral genes within the primate TRIM gene family. Genome Biol. Evol. 2013, 5, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- He, D.D.; Lu, Y.; Gittelman, R.; Jin, Y.; Ling, F.; Joshua, A. Positive selection of the TRIM family regulatory region in primate genomes. Proc. Biol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.L.; Emerman, M.; Malik, H.S. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007, 3, e197. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.L.; Wu, L.I.; Emerman, M.; Malik, H.S. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. USA 2005, 102, 2832–2837. [Google Scholar] [CrossRef] [PubMed]
- Carthagena, L.; Bergamaschi, A.; Luna, J.M.; David, A.; Uchil, P.D.; Margottin-Goguet, F.; Mothes, W.; Hazan, U.; Transy, C.; Pancino, G.; et al. Human trim gene expression in response to interferons. PLoS ONE 2009, 4, e4894. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Xu, W.; Wang, Y.; Zhong, L.; Xiong, S. Induction of TRIM22 by IFN-gamma involves jak and pc-plc/pkc, but not mapks and PI3K/AKT/mtor pathways. J. Interferon Cytokine Res. 2013, 33, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Sjostrand, M.; Ambrosi, A.; Brauner, S.; Sullivan, J.; Malin, S.; Kuchroo, V.K.; Espinosa, A.; Wahren-Herlenius, M. Expression of the immune regulator tripartite-motif 21 is controlled by IFN regulatory factors. J. Immunol. 2013, 191, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Type I interferon-dependent and -independent expression of tripartite motif proteins in immune cells. Eur. J. Immunol. 2008, 38, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Xie, X.Q.; Yang, Q.; Liao, C.Y.; Ye, W.; Lin, H.; Shu, H.B. TRIM38 negatively regulates TLR3/4-mediated innate immune and inflammatory responses by two sequential and distinct mechanisms. J. Immunol. 2015, 195, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.; Waggoner, L.; Pham, S.T.; Hendricks, G.L.; Waggoner, S.N.; Conlon, J.; Wang, J.P.; Fitzgerald, K.A.; Kang, J. TRIM13 is a negative regulator of MDA5-mediated type I interferon production. J. Virol. 2014, 88, 10748–10757. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.X.; Hong, X.; Liao, B.B.; Shi, S.Z.; Lai, X.F.; Zheng, H.Y.; Xie, L.; Wang, Y.; Wang, X.L.; Xin, H.B.; et al. Expression profiling of TRIM protein family in thp1-derived macrophages following TLR stimulation. Sci. Rep. 2017, 7, 42781. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.W.; Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Tripartite-motif proteins and innate immune regulation. Curr. Opin. Immunol. 2011, 23, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Manocha, G.D.; Mishra, R.; Sharma, N.; Kumawat, K.L.; Basu, A.; Singh, S.K. Regulatory role of TRIM21 in the type-I interferon pathway in japanese encephalitis virus-infected human microglial cells. J. Neuroinflamm. 2014, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martinez-Romero, C.; tenOever, B.R.; et al. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Manokaran, G.; Finol, E.; Wang, C.; Gunaratne, J.; Bahl, J.; Ong, E.Z.; Tan, H.C.; Sessions, O.M.; Ward, A.M.; Gubler, D.J.; et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 2015, 350, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bharaj, P.; Wang, Y.E.; Dawes, B.E.; Yun, T.E.; Park, A.; Yen, B.; Basler, C.F.; Freiberg, A.N.; Lee, B.; Rajsbaum, R. The matrix protein of nipah virus targets the E3-ubiquitin ligase TRIM6 to inhibit the ikkepsilon kinase-mediated type-I IFN antiviral response. PLoS Pathog. 2016, 12, e1005880. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.S.; Huang, I.C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; Garcia-Sastre, A. Influenza a virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA Sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Garcia-Sastre, A. Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways. Trends Microbiol. 2013, 21, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. TRIM family proteins: Roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Mandell, M.; Deretic, V. Precision autophagy directed by receptor regulators––Emerging examples within the TRIM family. J. Cell Sci. 2016, 129, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hatakeyama, S. TRIM proteins and diseases. J. Biochem. 2017, 161, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Z.J. Expanding role of ubiquitination in NF-κb signaling. Cell Res. 2010, 21, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J. Convergence of the NF-κb and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007, 18, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Runge, S.; Sparrer, K.M.; Lassig, C.; Hembach, K.; Baum, A.; Garcia-Sastre, A.; Soding, J.; Conzelmann, K.K.; Hopfner, K.P. In vivo ligands of MDA5 and RIG-I in measles virus-infected cells. PLoS Pathog. 2014, 10, e1004081. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzozka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5’-triphosphate rna is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Naslund, T.I.; Liljestrom, P.; Weber, F.; Reis e Sousa, C. Rig-i-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Gack, M.U. RIG-I-like receptor regulation in virus infection and immunity. Curr. Opin. Virol. 2015, 12, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kinch, L.N.; Brautigam, C.A.; Chen, X.; Du, F.; Grishin, N.V.; Chen, Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 2012, 36, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Peisley, A.; Richards, C.; Yao, H.; Zeng, X.; Lin, C.; Chu, F.; Walz, T.; Hur, S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 2013, 152, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of mavs, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Peisley, A.; Wu, B.; Yao, H.; Walz, T.; Hur, S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell 2013, 51, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Nistal-Villan, E.; Inn, K.S.; Garcia-Sastre, A.; Jung, J.U. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010, 84, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Miyashita, M.; Inoue, N.; Okabe, M.; Matsumoto, M.; Seya, T. The ubiquitin ligase riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe 2010, 8, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Sun, L.; Jiang, X.; Chen, X.; Hou, F.; Adhikari, A.; Xu, M.; Chen, Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010, 141, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.; Takahashi, H.; Hishiki, T.; Konishi, H.; Fujita, T.; Shimotohno, K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. USA 2007, 104, 7500–7505. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.; Konishi, H.; Shimotohno, K. Ubch8 regulates ubiquitin and ISG15 conjugation to RIG-I. Mol. Immunol. 2008, 45, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Sun, L.; Zheng, H.; Skaug, B.; Jiang, Q.X.; Chen, Z.J. Mavs forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011, 146, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, J.; Cai, X.; Wu, J.; Chen, X.; Wu, Y.T.; Sun, L.; Chen, Z.J. Mavs recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2013, 2, e00785. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Traf molecules in cell signaling and in human diseases. J. Mol. Signal. 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the iκb kinase complex by traf6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Kanayama, A.; Seth, R.B.; Sun, L.; Ea, C.K.; Hong, M.; Shaito, A.; Chiu, Y.H.; Deng, L.; Chen, Z.J. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 2004, 15, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.P.; Sun, L.; Chen, X.; Pineda, G.; Jiang, X.; Adhikari, A.; Zeng, W.; Chen, Z.J. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 2009, 461, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.L.; Gioia, R.; Gatot, J.S.; Patrascu, F.; Carpentier, I.; Chapelle, J.P.; O’Neill, L.; Beyaert, R.; Piette, J.; Chariot, A. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem. Sci. 2008, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.T.; Lowther, W.; Kellum, M.; Au, W.C.; Lin, R.; Hiscott, J.; Pitha, P.M. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 1998, 95, 9837–9842. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Tang, T.; Jin, T.; Ding, C.; Zhou, R.; Jiang, W. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. J. Exp. Med. 2017, 214, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Q.; Mao, A.P.; Hu, M.M.; Shu, H.B. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for k63-linked ubiquitination. J. Mol. Cell Biol. 2014, 6, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Shin, Y.C.; Joo, C.H.; Urano, T.; Liang, C.; Sun, L.; Takeuchi, O.; Akira, S.; Chen, Z.; Inoue, S.; et al. TRIM25 ring-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007, 446, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsumoto, M.; Seya, T. Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I. J. Biochem. 2011, 151, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Pauli, E.K.; Chan, Y.K.; Davis, M.E.; Gableske, S.; Wang, M.K.; Feister, K.F.; Gack, M.U. The ubiquitin-specific protease usp15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25. Sci. Signal. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Shu, H.B. Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol. Immunol. 2017, 14, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Liao, C.Y.; Yang, Q.; Xie, X.Q.; Shu, H.B. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 2017, 214, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Watanabe, M.; Hatakeyama, S. TRIM59 interacts with ecsit and negatively regulates NF-κB and IRF-3/7-mediated signal pathways. Biochem. Biophys. Res. Commun. 2012, 422, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Evensen, O.; Munang’andu, H.M. De novo transcriptome analysis shows that sav-3 infection upregulates pattern recognition receptors of the endosomal toll-like and RIG-I-like receptor signaling pathways in macrophage/dendritic like to-cells. Viruses 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Miranzo-Navarro, D.; Magor, K.E. Activation of duck rig-i by TRIM25 is independent of anchored ubiquitin. PLoS ONE 2014, 9, e86968. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Albrecht, R.A.; Wang, M.K.; Maharaj, N.P.; Versteeg, G.A.; Nistal-Villan, E.; Garcia-Sastre, A.; Gack, M.U. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza a virus ns1 protein. PLoS Pathog. 2012, 8, e1003059. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Q.; Cheng, Y.; Yang, H.L.; Zhu, Q.; Yu, D.; Liu, Y.P. Molecular characterization, tissue distribution and expression analysis of TRIM25 in gallus gallus domesticus. Gene 2015, 561, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Blaine, A.H.; Miranzo-Navarro, D.; Campbell, L.K.; Aldridge, J.R.; Webster, R.G.; Magor, K.E. Duck TRIM27-l enhances mavs signaling and is absent in chickens and turkeys. Mol. Immunol. 2015, 67, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Castanier, C.; Zemirli, N.; Portier, A.; Garcin, D.; Bidere, N.; Vazquez, A.; Arnoult, D. Mavs ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 2012, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, M.; Chu, H.; Zhang, H.; Wu, H.; Song, G.; Wang, P.; Zhao, K.; Hou, J.; Wang, X.; et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor mavs through lys63-linked polyubiquitination. Nat. Immunol. 2016, 18, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, J.; Wang, Y.; Zhou, H.; Wu, X.; Tian, Z.; Sun, B. Novel function of TRIM44 promotes an antiviral response by stabilizing visa. J. Immunol. 2013, 190, 3613–3619. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Q.; Tian, S.; Xie, W.; Cui, J.; Wang, R.F. TRIM9 short isoform preferentially promotes DNA and RNA virus-induced production of type i interferon by recruiting GSK3β to TBK1. Cell Res. 2016, 26, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Zevini, A.; Olagnier, D.; Hiscott, J. Crosstalk between cytoplasmic RIG-I and sting sensing pathways. Trends Immunol. 2017, 38, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, B.; Park, C.; Kwon, K.S. TRIM11 negatively regulates ifnβ production and antiviral activity by targeting TBK1. PLoS ONE 2013, 8, e63255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, L.; Zhang, M.; Wang, P.; Yuan, C.; Qi, J.; Meng, H.; Gao, C. Tripartite motif-containing protein 38 negatively regulates TLR3/4- and RIG-I-mediated IFN-β production and antiviral response by targeting NAP1. J. Immunol. 2012, 188, 5311–5318. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.; Funami, K.; Saeki, Y.; Tanaka, K.; Okawa, K.; Takeuchi, O.; Akira, S.; Murakami, Y.; Shimotohno, K. Polyubiquitin conjugation to nemo by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc. Natl. Acad. Sci. USA 2010, 107, 15856–15861. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Zhang, J.; Liu, L.L.; Pan, Z.Y.; Nie, Y.; Zhang, H.Y.; Wang, Y.Y. Autoubiquitination of TRIM26 links TBK1 to nemo in RLR-mediated innate antiviral immune response. J. Mol. Cell Biol. 2015, 8, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Wynne, C.; Lazzari, E.; Smith, S.; McCarthy, E.M.; Ni Gabhann, J.; Kallal, L.E.; Higgs, R.; Greco, A.; Cryan, S.A.; Biron, C.A.; et al. TRIM68 negatively regulates IFN-β production by degrading TRK fused gene, a novel driver of IFN-β downstream of anti-viral detection systems. PLoS ONE 2014, 9, e101503. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Deng, W.; Bi, E.; Mao, K.; Ji, Y.; Lin, G.; Wu, X.; Tao, Z.; Li, Z.; Cai, X.; et al. Trim30 α negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat. Immunol. 2008, 9, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yao, W.; Huang, F.; Sun, B.; Yang, R. The human antiviral factor TRIM11 is under the regulation of HIV-1 VPR. PLoS ONE 2014, 9, e104269. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Weng, L.; Yuan, B.; Wang, Z.; Jia, L.; Jin, R.; Lu, H.; Li, X.C.; Liu, Y.J.; Zhang, Z. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract. Nat. Immunol. 2016, 17, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Watanabe, M.; Nakamaru, Y.; Takagi, D.; Takahashi, H.; Fukuda, S.; Hatakeyama, S. TRIM39 negatively regulates the NFκB-mediated signaling pathway through stabilization of cactin. Cell Mol. Life Sci. 2015, 73, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. Sting is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. Sting regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. Sting is a direct innate immune sensor of cyclic di-gmp. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Yi, G.; Watts, T.; Kao, C.C.; Li, P. Structure of sting bound to cyclic di-gmp reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat. Struct. Mol. Biol. 2012, 19, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Omura, H.; Oikawa, D.; Nakane, T.; Kato, M.; Ishii, R.; Ishitani, R.; Tokunaga, F.; Nureki, O. Structural and functional analysis of ddx41: A bispecific immune receptor for DNA and cyclic dinucleotide. Sci. Rep. 2016, 6, 34756. [Google Scholar] [CrossRef] [PubMed]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase ddx41 recognizes the bacterial secondary messengers cyclic di-gmp and cyclic di-amp to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bao, M.; Lu, N.; Weng, L.; Yuan, B.; Liu, Y.J. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat. Immunol. 2012, 14, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lian, Q.; Yang, B.; Yan, S.; Zhou, H.; He, L.; Lin, G.; Lian, Z.; Jiang, Z.; Sun, B. TRIM30α is a negative-feedback regulator of the intracellular DNA and DNA virus-triggered response by targeting sting. PLoS Pathog. 2015, 11, e1005012. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Zou, J.; Saitoh, T.; Kumar, H.; Abe, T.; Matsuura, Y.; Kawai, T.; Akira, S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 2010, 33, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, M.M.; Wang, Y.Y.; Shu, H.B. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting mita/sting protein for k63-linked ubiquitination. J. Biol. Chem. 2012, 287, 28646–28655. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors--from microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Ni Gabhann, J.; Ben Larbi, N.; Breen, E.P.; Fitzgerald, K.A.; Jefferies, C.A. The E3 ubiquitin ligase ro52 negatively regulates IFN-β production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J. Immunol. 2008, 181, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Lazzari, E.; Wynne, C.; Ni Gabhann, J.; Espinosa, A.; Wahren-Herlenius, M.; Jefferies, C.A. Self protection from anti-viral responses--ro52 promotes degradation of the transcription factor IRF7 downstream of the viral toll-like receptors. PLoS ONE 2010, 5, e11776. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, E.; Korczeniewska, J.; Ni Gabhann, J.; Smith, S.; Barnes, B.J.; Jefferies, C.A. Tripartite motif 21 (TRIM21) differentially regulates the stability of interferon regulatory factor 5 (IRF5) isoforms. PLoS ONE 2014, 9, e103609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, L.; Zhang, M.; Yuan, C.; Gao, C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. J. Immunol. 2012, 188, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhou, Z.; Lei, X.; Liu, X.; He, B.; Wang, J.; Hung, T. TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting trif for degradation. PLoS ONE 2012, 7, e46825. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, N.L.; Wang, J.; Liu, B.; Lester, S.; Li, K. TRIM56 is an essential component of the TLR3 antiviral signaling pathway. J. Biol. Chem. 2012, 287, 36404–36413. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Forman, M.; Arav-Boger, R. Activation of nucleotide oligomerization domain 2 (NOD2) by human cytomegalovirus initiates innate immune responses and restricts virus replication. PLoS ONE 2014, 9, e92704. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 signaling in infection and inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Mitoma, H.; Trichot, C.; Bao, M.; Liu, Y.; Zhang, Z.; Liu, Y.J. The E3 ubiquitin ligase tripartite motif 33 is essential for cytosolic RNA-induced NLRP3 inflammasome activation. J. Immunol. 2014, 193, 3676–3682. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mao, K.; Zeng, Y.; Chen, S.; Tao, Z.; Yang, C.; Sun, S.; Wu, X.; Meng, G.; Sun, B. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J. Immunol. 2010, 185, 7699–7705. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, B.; Huai, W.; Yu, Z.; Wang, W.; Zhao, J.; Han, L.; Jiang, G.; Zhang, L.; Gao, C.; et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016, 7, 13727. [Google Scholar] [CrossRef] [PubMed]
- Zurek, B.; Schoultz, I.; Neerincx, A.; Napolitano, L.M.; Birkner, K.; Bennek, E.; Sellge, G.; Lerm, M.; Meroni, G.; Soderholm, J.D.; et al. TRIM27 negatively regulates NOD2 by ubiquitination and proteasomal degradation. PLoS ONE 2012, 7, e41255. [Google Scholar] [CrossRef] [PubMed]
- Tomar, D.; Sripada, L.; Prajapati, P.; Singh, R.; Singh, A.K. Nucleo-cytoplasmic trafficking of TRIM8, a novel oncogene, is involved in positive regulation of TNF induced NF-κB pathway. PLoS ONE 2012, 7, e48662. [Google Scholar] [CrossRef] [PubMed]
- Okumura, F.; Matsunaga, Y.; Katayama, Y.; Nakayama, K.I.; Hatakeyama, S. TRIM8 modulates STAT3 activity through negative regulation of PIAS3. J. Cell Sci. 2010, 123, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Yang, Q.; Zhang, J.; Liu, S.M.; Zhang, Y.; Lin, H.; Huang, Z.F.; Wang, Y.Y.; Zhang, X.D.; Zhong, B.; et al. TRIM38 inhibits TNFα- and IL-1β-triggered NF-κB activation by mediating lysosome-dependent degradation of TAB2/3. Proc. Natl. Acad. Sci. USA 2014, 111, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hou, J.; Zhou, Y.; Yang, Y.; Xie, B.; Cao, X. Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res. 2015, 25, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Huang, Y.; Dai, X.; Liu, Y.; Liu, Z.; Wang, N.; Zhang, P. Tripartite motif-containing 28 bridges endothelial inflammation and angiogenic activity by retaining expression of TNFR-1 and -2 and VEGFR2 in endothelial cells. FASEB J. 2017, 31, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferons and viral infections. Biofactors 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, J.; Khetchoumian, K.; Thibault, C.; Dembele, D.; Chambon, P.; Losson, R. Tripartite motif 24 (TRIM24/TIF1α) tumor suppressor protein is a novel negative regulator of interferon (IFN)/signal transducers and activators of transcription (STAT) signaling pathway acting through retinoic acid receptor α (RARα) inhibition. J. Biol. Chem. 2011, 286, 33369–33379. [Google Scholar] [CrossRef] [PubMed]
- Toniato, E.; Chen, X.P.; Losman, J.; Flati, V.; Donahue, L.; Rothman, P. TRIM8/gerp ring finger protein interacts with socs-1. J. Biol. Chem. 2002, 277, 37315–37322. [Google Scholar] [CrossRef] [PubMed]
- Tenoever, B.R.; Ng, S.L.; Chua, M.A.; McWhirter, S.M.; Garcia-Sastre, A.; Maniatis, T. Multiple functions of the IKK-related kinase ikkepsilon in interferon-mediated antiviral immunity. Science 2007, 315, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.L.; Friedman, B.A.; Schmid, S.; Gertz, J.; Myers, R.M.; Tenoever, B.R.; Maniatis, T. Iκb kinase ε (IKKε) regulates the balance between type I and type II interferon responses. Proc. Natl. Acad. Sci. USA 2011, 108, 21170–21175. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, A.; Mechti, N. Control of foxo4 activity and cell survival by TRIM22 directs TLR3-stimulated cells toward IFN type I gene induction or apoptosis. J. Interferon Cytokine Res. 2015, 35, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Gongora, C.; Tissot, C.; Cerdan, C.; Mechti, N. The interferon-inducible STAF50 gene is downregulated during T cell costimulation by CD2 and CD28. J. Interferon Cytokine Res. 2000, 20, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Obad, S.; Olofsson, T.; Mechti, N.; Gullberg, U.; Drott, K. Regulation of the interferon-inducible p53 target gene TRIM22 (STAF50) in human T lymphocyte activation. J. Interferon Cytokine Res. 2007, 27, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, J.; Okoye, I.S.; Guidi, R.; Kannan, Y.; Coomes, S.M.; Czieso, S.; Mengus, G.; Davidson, I.; Wilson, M.S. T-cell-intrinsic TIF1α/TRIM24 regulates IL-1r expression on Th2 cells and Th2 cell-mediated airway allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E568–E576. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Srivastava, S.; Sun, Y.; Li, Z.; Wu, H.; Zuvela-Jelaska, L.; Li, J.; Salamon, R.S.; Backer, J.M.; Skolnik, E.Y. Tripartite motif containing protein 27 negatively regulates CD4 T cells by ubiquitinating and inhibiting the class II PI3K-c2β. Proc. Natl. Acad. Sci. USA 2011, 108, 20072–20077. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Hur, J.Y.; Lee, M.S.; Zhang, Q.; Choi, W.Y.; Kim, L.K.; Lee, W.B.; Oh, G.T.; Kim, Y.J. Tripartite motif-containing protein 30 modulates TCR-activated proliferation and effector functions in CD4+ T cells. PLoS ONE 2014, 9, e95805. [Google Scholar] [CrossRef] [PubMed]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in old world monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.W.; Nisole, S.; Lynch, C.; Stoye, J.P. TRIM5α protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 2004, 101, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Nisole, S.; Lynch, C.; Stoye, J.P.; Yap, M.W. A TRIM5-cyclophilin a fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA 2004, 101, 13324–13328. [Google Scholar] [CrossRef] [PubMed]
- Sayah, D.M.; Sokolskaja, E.; Berthoux, L.; Luban, J. Cyclophilin a retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature 2004, 430, 569. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.; Christensen, D.E.; Nelson, C.; Tan, C.P.; Schaller, T.; Lehner, P.J.; Sundquist, W.I.; Towers, G.J. TRIM5α requires ube2w to anchor Lys63-linked ubiquitin chains and restrict reverse transcription. EMBO J. 2015, 34, 2078–2095. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, R.; Mukherjee, S.; Smolin, N.; Pauszek, R.F.; Bradley, M.; Sastri, J.; Robia, S.L.; Millar, D.; Campbell, E.M. Dynamic conformational changes in the rhesus TRIM5α dimer dictate the potency of HIV-1 restriction. Virology 2017, 500, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Chandrasekaran, V.; Carter, S.D.; Woodward, C.L.; Christensen, D.E.; Dryden, K.A.; Pornillos, O.; Yeager, M.; Ganser-Pornillos, B.K.; Jensen, G.J. Primate TRIM5 proteins form hexagonal nets on HIV-1 capsids. Elife 2016, 5, e16269. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Roganowicz, M.D.; Skorupka, K.; Alam, S.L.; Christensen, D.; Doss, G.; Wan, Y.; Frank, G.A.; Ganser-Pornillos, B.K.; Sundquist, W.I. Mechanism of B-box 2 domain-mediated higher-order assembly of the retroviral restriction factor TRIM5α. Elife 2016, 5, e16309. [Google Scholar] [CrossRef] [PubMed]
- Keown, J.R.; Goldstone, D.C. Crystal structure of the TRIM5α bbox2 domain from rhesus macaques describes a plastic oligomerisation interface. J. Struct. Biol. 2016, 195, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Sastri, J.; Campbell, E.M. Recent insights into the mechanism and consequences of TRIM5α retroviral restriction. AIDS Res. Hum. Retroviruses 2011, 27, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ganser-Pornillos, B.K.; Chandrasekaran, V.; Pornillos, O.; Sodroski, J.G.; Sundquist, W.I.; Yeager, M. Hexagonal assembly of a restricting TRIM5αprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Pertel, T.; Hausmann, S.; Morger, D.; Züger, S.; Guerra, J.; Lascano, J.; Reinhard, C.; Santoni, F.A.; Uchil, P.D.; Chatel, L. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 2011, 472, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Campbell, E.M.; Wu, X.; Vandegraaff, N.; Engelman, A.; Hope, T.J. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J. Virol. 2006, 80, 9754–9760. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Anderson, J.L.; Campbell, E.M.; Joseph, A.M.; Hope, T.J. Proteasome inhibitors uncouple rhesus TRIM5α restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA 2006, 103, 7465–7470. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Perez-Caballero, D.; Bieniasz, P.D. Fates of retroviral core components during unrestricted and TRIM5-restricted infection. PLoS Pathog. 2013, 9, e1003214. [Google Scholar] [CrossRef] [PubMed]
- Rold, C.J.; Aiken, C. Proteasomal degradation of TRIM5α during retrovirus restriction. PLoS Pathog. 2008, 4, e1000074. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Moyano, E.; Ruiz, A.; Kløverpris, H.N.; Rodriguez-Plata, M.T.; Peña, R.; Blondeau, C.; Selwood, D.L.; Izquierdo-Useros, N.; Moris, A.; Clotet, B. Nonhuman TRIM5 variants enhance recognition of HIV-1-infected cells by CD8+ T cells. J. Virol. 2016, 90, 8552–8562. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ramos, M.; Stoye, J.P. Capsid-binding retrovirus restriction factors: Discovery, restriction specificity and implications for the development of novel therapeutics. J. Gen. Virol. 2013, 94, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Grütter, M.G.; Luban, J. TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr. Opin. Virol. 2012, 2, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Weingart, J.; Sette, P.; Opp, S.; Sastri, J.; O’Connor, S.K.; Talley, S.; Diaz-Griffero, F.; Hirsch, V.; Bouamr, F. TRIM5α-mediated ubiquitin chain conjugation is required for inhibition of HIV-1 reverse transcription and capsid destabilization. J.Virol. 2016, 90, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Lascano, J.; Uchil, P.D.; Mothes, W.; Luban, J. TRIM5 retroviral restriction activity correlates with the ability to induce innate immune signaling. J. Virol. 2016, 90, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ourmanov, I.; Riddick, N.; Matsuda, K.; Whitted, S.; Plishka, R.J.; Buckler-White, A.; Starost, M.F.; Hirsch, V.M. TRIM5α restriction affects clinical outcome and disease progression in simian immunodeficiency virus-infected rhesus macaques. J. Virol. 2015, 89, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Letvin, N.L.; Mascola, J.R.; Sun, Y.; Gorgone, D.A.; Buzby, A.P.; Xu, L.; Yang, Z.-y.; Chakrabarti, B.; Rao, S.S.; Schmitz, J.E. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 2006, 312, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Bevan, M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003, 300, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.W.; Nisole, S.; Stoye, J.P. A single amino acid change in the spry domain of human TRIM5αleads to HIV-1 restriction. Curr. Biol. 2005, 15, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Vandegraaff, N.; O’Huigin, C.; Song, B.; Yuan, W.; Xu, C.; Perron, M.; Li, X.; Marasco, W.A.; Engelman, A.; et al. Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc. Natl. Acad. Sci. USA 2006, 103, 7454–7459. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, L.M.; Keckesova, Z.; Webb, B.L.; Gifford, R.J.; Smith, T.P.; Towers, G.J. Isolation of an active lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals. J. Virol. 2006, 80, 7332–7338. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, P.; Crespo, H.; Glaria, I.; Lujan, L.; Contreras, A.; Rosati, S.; de Andres, D.; Amorena, B.; Towers, G.J.; Reina, R. Ovine TRIM5α can restrict visna/maedi virus. J. Virol. 2012, 86, 9504–9509. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Sarrami-Forooshani, R.; Setiawan, L.C.; Zijlstra-Willems, E.M.; van Hamme, J.L.; Tigchelaar, W.; van der Wel, N.N.; Kootstra, N.A.; Gringhuis, S.I.; Geijtenbeek, T.B. Receptor usage dictates HIV-1 restriction by human TRIM5α in dendritic cell subsets. Nature 2016, 540, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Arhel, N.J.; Nisole, S.; Carthagena, L.; Coutant, F.; Souque, P.; Brussel, A.; Estaquier, J.; Charneau, P. Lack of endogenous TRIM5α-mediated restriction in rhesus macaque dendritic cells. Blood 2008, 112, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Portilho, D.M.; Fernandez, J.; Ringeard, M.; Machado, A.K.; Boulay, A.; Mayer, M.; Müller-Trutwin, M.; Beignon, A.-S.; Kirchhoff, F.; Nisole, S. Endogenous TRIM5α function is regulated by sumoylation and nuclear sequestration for efficient innate sensing in dendritic cells. Cell Rep. 2016, 14, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yao, W.; Tokunaga, K.; Yang, R.; Sun, B. An HIV-1 capsid binding protein TRIM11 accelerates viral uncoating. Retrovirology 2016, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Pawlica, P.; Le Sage, V.; Poccardi, N.; Tremblay, M.J.; Mouland, A.J.; Berthoux, L. Functional evidence for the involvement of microtubules and dynein motor complexes in TRIM5α-mediated restriction of retroviruses. J. Virol. 2014, 88, 5661–5676. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Miyake, Y.; Nobs, S.P.; Schneider, C.; Horvath, P.; Kopf, M.; Matthias, P.; Helenius, A.; Yamauchi, Y. Influenza a virus uses the aggresome processing machinery for host cell entry. Science 2014, 346, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Lukic, Z.; Dharan, A.; Fricke, T.; Diaz-Griffero, F.; Campbell, E.M. HIV-1 uncoating is facilitated by dynein and kinesin 1. J. Virol. 2014, 88, 13613–13625. [Google Scholar] [CrossRef] [PubMed]
- Pawlica, P.; Berthoux, L. Cytoplasmic dynein promotes HIV-1 uncoating. Viruses 2014, 6, 4195–4211. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Garcia-Sastre, A. Virology. Unanchored ubiquitin in virus uncoating. Science 2014, 346, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hara, Y.; Takagi, C.; Yamamoto, T.S.; Ueno, N. MID1 and MID2 are required for xenopus neural tube closure through the regulation of microtubule organization. Development 2010, 137, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Marelli, S.; Kajaste-Rudnitski, A.; Lusic, M.; Lint, C.; Das, A.T.; Harwig, A.; Berkhout, B.; Vicenzi, E. HIV-1 transcriptional silencing caused by TRIM22 inhibition of sp1 binding to the viral promoter. Retrovirology 2015, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.A.; Tardif, K.; Mansky, L.M. Anti-HIV-1 activity of TRIM 37. J. Gen. Virol. 2014, 95, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Masroori, N.; Merindol, N.; Berthoux, L. The interferon-induced antiviral protein pml (TRIM19) promotes the restriction and transcriptional silencing of lentiviruses in a context-specific, isoform-specific fashion. Retrovirology 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Kahle, T.; Volkmann, B.; Eissmann, K.; Herrmann, A.; Schmitt, S.; Wittmann, S.; Merkel, L.; Reuter, N.; Stamminger, T.; Gramberg, T. TRIM19/pml restricts HIV infection in a cell type-dependent manner. Viruses 2015, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Dutrieux, J.; Maarifi, G.; Portilho, D.M.; Arhel, N.J.; Chelbi-Alix, M.K.; Nisole, S. Pml/TRIM19-dependent inhibition of retroviral reverse-transcription by daxx. PLoS Pathog. 2015, 11, e1005280. [Google Scholar] [CrossRef] [PubMed]
- Bidgood, S.R.; Tam, J.C.; McEwan, W.A.; Mallery, D.L.; James, L.C. Translocalized IGA mediates neutralization and stimulates innate immunity inside infected cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13463–13468. [Google Scholar] [CrossRef] [PubMed]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar] [CrossRef] [PubMed]
- Keeble, A.H.; Khan, Z.; Forster, A.; James, L.C. TRIM21 is an igg receptor that is structurally, thermodynamically, and kinetically conserved. Proc. Natl. Acad. Sci. USA 2008, 105, 6045–6050. [Google Scholar] [CrossRef] [PubMed]
- McEwan, W.A.; Tam, J.C.; Watkinson, R.E.; Bidgood, S.R.; Mallery, D.L.; James, L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the fc receptor TRIM21. Nat. Immunol. 2013, 14, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, R.E.; Tam, J.C.; Vaysburd, M.J.; James, L.C. Simultaneous neutralization and innate immune detection of a replicating virus by TRIM21. J. Virol. 2013, 87, 7309–7313. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, R.E.; McEwan, W.A.; Tam, J.C.; Vaysburd, M.; James, L.C. TRIM21 promotes cgas and RIG-I sensing of viral genomes during infection by antibody-opsonized virus. PLoS Pathog. 2015, 11, e1005253. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.; Watkinson, R.E.; Grevys, A.; McAdam, M.B.; Bern, M.; Hoydahl, L.S.; Dalhus, B.; Michaelsen, T.E.; Sandlie, I.; James, L.C.; et al. TRIM21 immune signaling is more sensitive to antibody affinity than its neutralization activity. J. Immunol. 2016, 196, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Vaysburd, M.; Watkinson, R.E.; Cooper, H.; Reed, M.; O’Connell, K.; Smith, J.; Cruickshanks, J.; James, L.C. Intracellular antibody receptor TRIM21 prevents fatal viral infection. Proc. Natl. Acad. Sci. USA 2013, 110, 12397–12401. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, D.; Qian, P.; Qian, S.; Wu, M.; Chen, H.; Li, X. Swine TRIM21 restricts fmdv infection via an intracellular neutralization mechanism. Antiviral Res. 2016, 127, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Fan, W.; Zheng, W.; Yu, M.; Chen, C.; Sun, L.; Bi, Y.; Ding, C.; Gao, G.F. Robust lys63-linked ubiquitination of RIG-I promotes cytokine eruption in early influenza b virus infection. J. Virol. 2016, 90, 6263–6275. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Kajaste-Rudnitski, A.; Oteiza, A.; Nicora, L.; Towers, G.J.; Mechti, N.; Vicenzi, E. TRIM22 inhibits influenza a virus infection by targeting the viral nucleoprotein for degradation. J. Virol. 2013, 87, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Pflug, A.; Guilligay, D.; Reich, S.; Cusack, S. Structure of influenza a polymerase bound to the viral RNA promoter. Nature 2014, 516, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wang, L.; Ding, H.; Schwamborn, J.C.; Li, S.; Dorf, M.E. TRIM32 senses and restricts influenza a virus by ubiquitination of pb1 polymerase. PLoS Pathog. 2015, 11, e1004960. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, N.L.; Shen, Y.; Bao, X.; Fabrizio, T.; Elbahesh, H.; Webby, R.J.; Li, K. The C-terminal tail of TRIM56 dictates antiviral restriction of influenza A and B viruses by impeding viral RNA synthesis. J. Virol. 2016, 90, 4369–4382. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, M.R. Historical perspectives on flavivirus research. Viruses 2017. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, M.D.; Mazzon, M.; Jacobs, M.; Amara, A. Pathogenesis of flavivirus infections: Using and abusing the host cell. Cell Host Microbe 2009, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Qashqari, H.; Al-Mars, A.; Chaudhary, A.; Abuzenadah, A.; Damanhouri, G.; Alqahtani, M.; Mahmoud, M.; El Sayed Zaki, M.; Fatima, K.; Qadri, I. Understanding the molecular mechanism(s) of hepatitis C virus (HCV) induced interferon resistance. Infect. Genet. Evol. 2013, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Sakuma, I.; Asahina, Y.; Kurosaki, M.; Murakami, T.; Yamamoto, C.; Ogura, Y.; Izumi, N.; Marumo, F.; Sato, C. Mutations in the nonstructural protein 5a gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 1996, 334, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Li, C.; Wu, Y.; Guo, L.; Peng, C.; Huang, Y.; Cheng, G.; Qin, F.X. TRIM14 inhibits hepatitis C virus infection by spry domain-dependent targeted degradation of the viral NS5a protein. Sci. Rep. 2016, 6, 32336. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, X.; Sun, D.; Yang, L.; Chong, C.; Pan, Y.; Chi, X.; Gao, Y.; Wang, M.; Shi, X.; et al. Interferon α(ifnα)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5a. Cell Mol. Immunol. 2016, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wu, M.; Qian, S.; Zhou, Y.; Chen, H.; Li, X.; Qian, P. TRIM52 inhibits japanese encephalitis virus replication by degrading the viral NS2A. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Davidson, A.; Hibbert, L.; Gruenwald, P.; Schlaak, J.; Ball, S.; Foster, G.R.; Jacobs, M. Dengue virus inhibits α interferon signaling by reducing stat2 expression. J. Virol. 2005, 79, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L.; Sanchez-Burgos, G.G.; Laurent-Rolle, M.; Garcia-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.C.; Yu, C.Y.; Liang, J.J.; Lin, E.; Liao, C.L.; Lin, Y.L. Blocking double-stranded RNA-activated protein kinase PKR by japanese encephalitis virus nonstructural protein 2a. J. Virol. 2012, 86, 10347–10358. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, N.L.; Wang, J.; Shi, P.Y.; Wang, T.; Miller, M.A.; Li, K. Overlapping and distinct molecular determinants dictating the antiviral activities of TRIM56 against flaviviruses and coronavirus. J. Virol. 2014, 88, 13821–13835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, H.; Xu, C.; Chang, J.; Gu, B.; Wang, L.; Block, T.M.; Guo, J.T. Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J. Virol. 2008, 82, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, B.; Wang, N.; Lee, Y.M.; Liu, C.; Li, K. TRIM56 is a virus- and interferon-inducible E3 ubiquitin ligase that restricts pestivirus infection. J. Virol. 2011, 85, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Lubick, K.J.; Freedman, B.A.; Carmody, A.B.; Best, S.M. Tick-borne flaviviruses antagonize both IRF-1 and type I IFN signaling to inhibit dendritic cell function. J. Immunol. 2014, 192, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S. Mechanisms of evasion of the type I interferon antiviral response by flaviviruses. J. Interferon Cytokine Res. 2009, 29, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Morris, K.L.; Shannon, J.G.; Robertson, S.J.; Mitzel, D.N.; Park, G.S.; Boer, E.; Wolfinbarger, J.B.; Bloom, M.E. Inhibition of interferon-stimulated jak-stat signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 2005, 79, 12828–12839. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.T.; Lubick, K.J.; Robertson, S.J.; Broughton, J.P.; Bloom, M.E.; Bresnahan, W.A.; Best, S.M. TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 2011, 10, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Lau, Z.; Cheung, P.; Aguilar, E.G.; Schneider, W.M.; Bozzacco, L.; Molina, H.; Buehler, E.; Takaoka, A.; Rice, C.M. TRIM25 enhances the antiviral action of zinc-finger antiviral protein (zap). PLoS Pathog. 2017, 13, e1006145. [Google Scholar] [CrossRef] [PubMed]
- Maroui, M.A.; Pampin, M.; Chelbi-Alix, M.K. Promyelocytic leukemia isoform iv confers resistance to encephalomyocarditis virus via the sequestration of 3d polymerase in nuclear bodies. J. Virol. 2011, 85, 13164–13173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, J.-T.; Wu, J.Z.; Yang, G. Identification and characterization of multiple trim proteins that inhibit hepatitis B virus transcription. PLoS ONE 2013, 8, e70001. [Google Scholar] [CrossRef] [PubMed]
- Hattlmann, C.J.; Kelly, J.N.; Barr, S.D. TRIM22: A diverse and dynamic antiviral protein. Mol. Biol. Int. 2012, 2012, 153415. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Duan, Z.; Xu, W.; Xiong, S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located ring domain. Hepatology 2009, 50, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Chen, C.-S.; Wang, W.-H.; Hsu, S.-W.; Tsai, H.-H.; Liu, S.-T.; Chang, L.-K. TRIM5α promotes ubiquitination of RTA from epstein–barr virus to attenuate lytic progression. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Stamminger, T. Emerging role of PML nuclear bodies in innate immune signaling. J. Virol. 2016, 90, 5850–5854. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Wolff, T. Influenza A virus TRIMs the type I interferon response. Cell Host Microbe 2009, 5, 420–421. [Google Scholar]
- Heikel, G.; Choudhury, N.R.; Michlewski, G. The role of TRIM25 in development, disease and RNA metabolism. Biochem. Soc. Trans. 2016, 44, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.; Yamauchi, Y. Ubiquitin in influenza virus entry and innate immunity. Viruses 2016, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, H.; Wang, A.; Sun, L.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; Wu, Y. TRIM25 identification in the chinese goose: Gene structure, tissue expression profiles, and antiviral immune responses in vivo and in vitro. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aparicio, M.T.; Ayllon, J.; Leo-Macias, A.; Wolff, T.; Garcia-Sastre, A. Subcellular localizations of RIG-I, TRIM25, and MAVS complexes. J. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, W.; Gao, T.; Cui, Y.; Jin, Y.; Li, P.; Ma, Q.; Liu, X.; Cao, C. The severe acute respiratory syndrome coronavirus nucleocapsid inhibits type I interferon production by interfering with TRIM25-mediated RIG-I ubiquitination. J. Virol. 2017, 91, e02143-16. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.W.; Covaleda, L.M.; Sanchez-Aparicio, M.T.; Silvas, J.A.; Diaz-Vizarreta, A.C.; Patel, J.R.; Popov, V.; Yu, X.J.; Garcia-Sastre, A.; Aguilar, P.V. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the inhibition of type I interferon responses. J. Virol. 2014, 88, 4572–4585. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Kumari, R.; Schwamborn, J.C.; Mahadevan, A.; Shankar, S.; Raja, R.; Seth, P. Tripartite containing motif 32 modulates proliferation of human neural precursor cells in HIV-1 neurodegeneration. Cell Death Differ. 2016, 23, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.Y.; Garcia-Sastre, A. Ns5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Laurent-Rolle, M.; Maestre, A.M.; Rajsbaum, R.; Pisanelli, G.; Simon, V.; Mulder, L.C.; Fernandez-Sesma, A.; Garcia-Sastre, A. Dengue virus co-opts ubr4 to degrade Stat2 and antagonize type I interferon signaling. PLoS Pathog. 2013, 9, e1003265. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Mulder, L.C.; Iwamatsu, A.; Lee, M.J.; Davydov, I.V.; Varshavsky, A.; Muesing, M.; Kwon, Y.T. A family of mammalian E3 ubiquitin ligases that contain the ubr box motif and recognize N-degrons. Mol. Cell Biol. 2005, 25, 7120–7136. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.F. Hendra and nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, M.; Vashisht, A.A.; Lester, T.; Voros, T.; Beaty, S.M.; Park, A.; Wang, Y.E.; Yun, T.E.; Freiberg, A.N.; Wohlschlegel, J.A.; et al. Evidence for ubiquitin-regulated nuclear and subnuclear trafficking among paramyxovirinae matrix proteins. PLoS Pathog. 2015, 11, e1004739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Park, A.; Lake, M.; Pentecost, M.; Torres, B.; Yun, T.E.; Wolf, M.C.; Holbrook, M.R.; Freiberg, A.N.; Lee, B. Ubiquitin-regulated nuclear-cytoplasmic trafficking of the nipah virus matrix protein is important for viral budding. PLoS Pathog. 2010, 6, e1001186. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E. Hepatitis b virus x protein: Trimming antiviral defences in hepatocytes. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-H.; Park, E.-S.; Kim, D.H.; Cho, K.C.; Kim, K.P.; Park, Y.K.; Ahn, S.H.; Park, S.H.; Kim, K.-H.; Kim, C.W. Suppression of interferon-mediated anti-hbv response by single CpG methylation in the 5′-utr of TRIM22. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Burton, E.M.; Bhaduri-McIntosh, S. Chloroquine triggers epstein-barr virus replication through phosphorylation of kap1/TRIM28 in burkitt lymphoma cells. PLoS Pathog. 2017, 13, e1006249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, X.-L.; Gu, Q.-H.; Huang, F.; Zheng, W.; Li, Z.-W. Tripartite motif-containing 22 gene-364t/c polymorphism associated with hepatitis B virus infection in chinese han population. Hepat. Mon. 2014. [Google Scholar] [CrossRef] [PubMed]
- Conwell, S.E.; White, A.E.; Harper, J.W.; Knipe, D.M. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 icp0. J. Virol. 2015, 89, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Bharaj, P.; Atkins, C.; Luthra, P.; Giraldo, M.I.; Dawes, B.E.; Miorin, L.; Johnson, J.R.; Krogan, N.J.; Basler, C.F.; Freiberg, A.N.; et al. The host E3-ubiquitin ligase TRIM6 ubiquitinates the ebola virus VP35 protein and promotes virus replication. J. Virol. 2017, 91, e00833-17. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, W.B.; Loo, Y.M.; Gale, M., Jr.; Hartman, A.L.; Kimberlin, C.R.; Martinez-Sobrido, L.; Saphire, E.O.; Basler, C.F. Ebola virus VP35 protein binds double-stranded RNA and inhibits α/β interferon production induced by RIG-I signaling. J. Virol. 2006, 80, 5168–5178. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.; Ramanan, P.; Mire, C.E.; Weisend, C.; Tsuda, Y.; Yen, B.; Liu, G.; Leung, D.W.; Geisbert, T.W.; Ebihara, H.; et al. Mutual antagonism between the ebola virus VP35 protein and the RIG-I activator pact determines infection outcome. Cell Host Microbe 2013, 14, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.C.; Cardenas, W.B.; Basler, C.F. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases ikkepsilon and TBK-1. J. Virol. 2009, 83, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Muhlberger, E. Filovirus replication and transcription. Future Virol. 2007, 2, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in leukocytes and other cells: Mechanisms, subsystem organization, selectivity, and links to innate immunity. J. Leukoc. Biol. 2016, 100, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Jain, A.; Choi, S.W.; Mandell, M.A.; Johansen, T.; Deretic, V. TRIM-directed selective autophagy regulates immune activation. Autophagy 2017, 13, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Jain, A.; Arko-Mensah, J.; Chauhan, S.; Kimura, T.; Dinkins, C.; Silvestri, G.; Munch, J.; Kirchhoff, F.; Simonsen, A.; et al. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev. Cell 2014, 30, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Kimura, T.; Jain, A.; Johansen, T.; Deretic, V. TRIM proteins regulate autophagy: TRIM5 is a selective autophagy receptor mediating HIV-1 restriction. Autophagy 2014, 10, 2387–2388. [Google Scholar] [CrossRef] [PubMed]
- Stremlau, M.; Perron, M.; Lee, M.; Li, Y.; Song, B.; Javanbakht, H.; Diaz-Griffero, F.; Anderson, D.J.; Sundquist, W.I.; Sodroski, J. Specific recognition and accelerated uncoating of retroviral capsids by the trim5α restriction factor. Proc. Natl. Acad. Sci. USA 2006, 103, 5514–5519. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.M.; Jaffray, E.G.; Hay, R.T.; Meroni, G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem. J. 2011, 434, 309–319. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).