Distinctive Responses in an In Vitro Human Dendritic Cell-Based System upon Stimulation with Different Influenza Vaccine Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccines
2.2. Generation of DC Derived from the MUTZ-3 Cell Line
2.3. Generation of Immature Mo-DCs from Primary Cells
2.4. Treatments
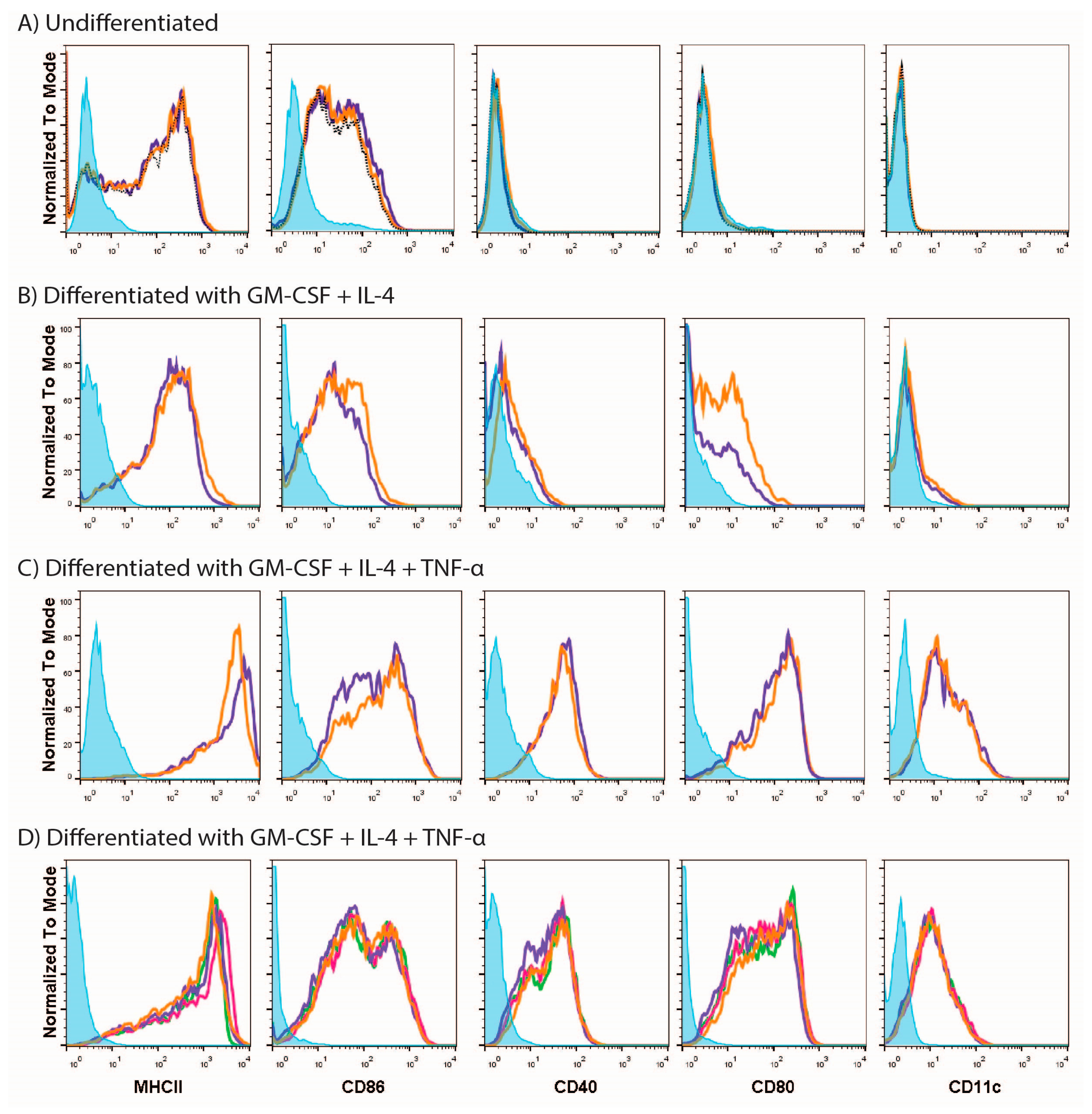
2.4.1. Undifferentiated MUTZ
2.4.2. MUTZ-3-Derived DCs
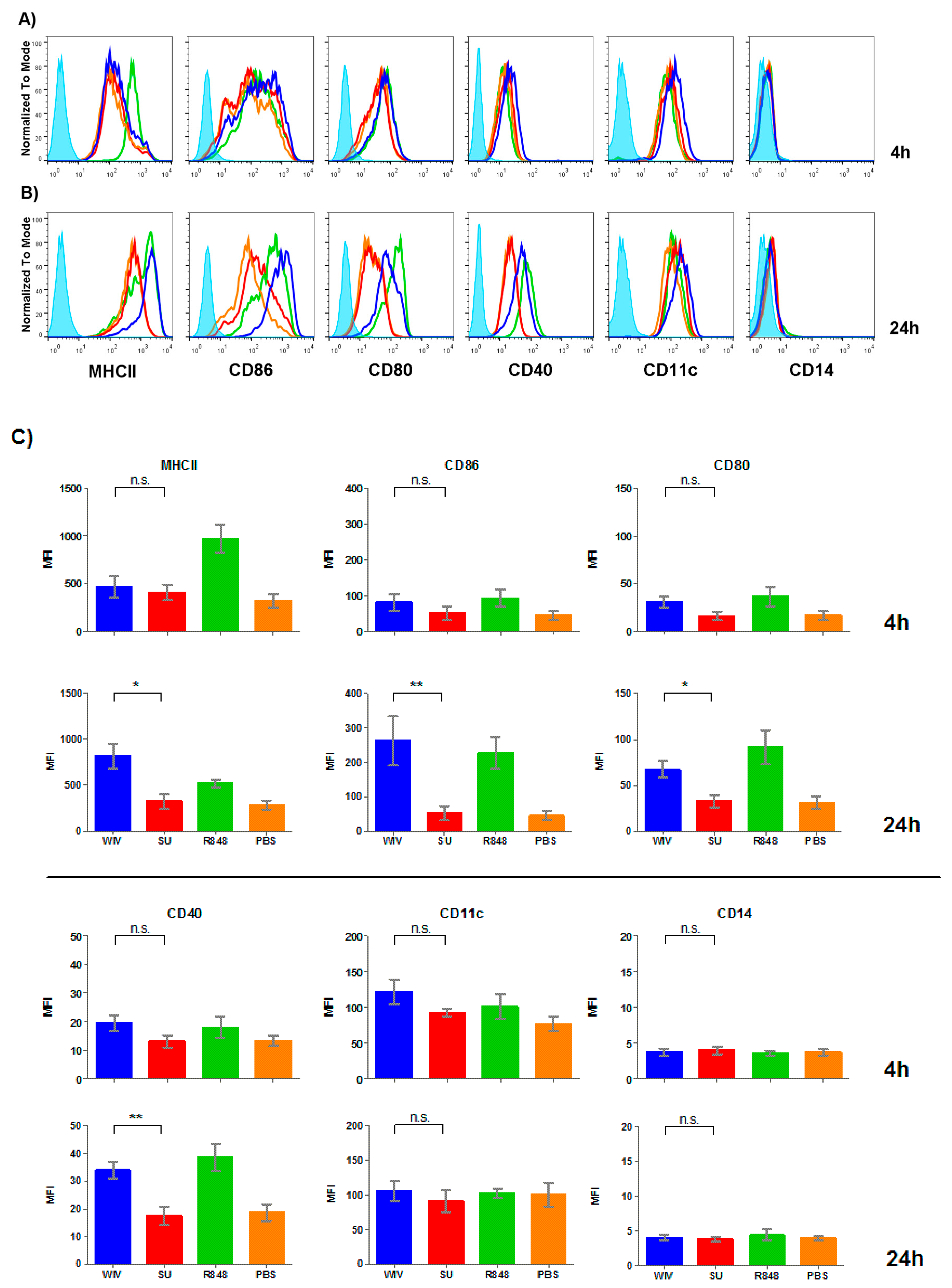
2.4.3. Monocyte-Derived DCs
2.5. Surface Marker Staining and Flow Cytometry Analysis
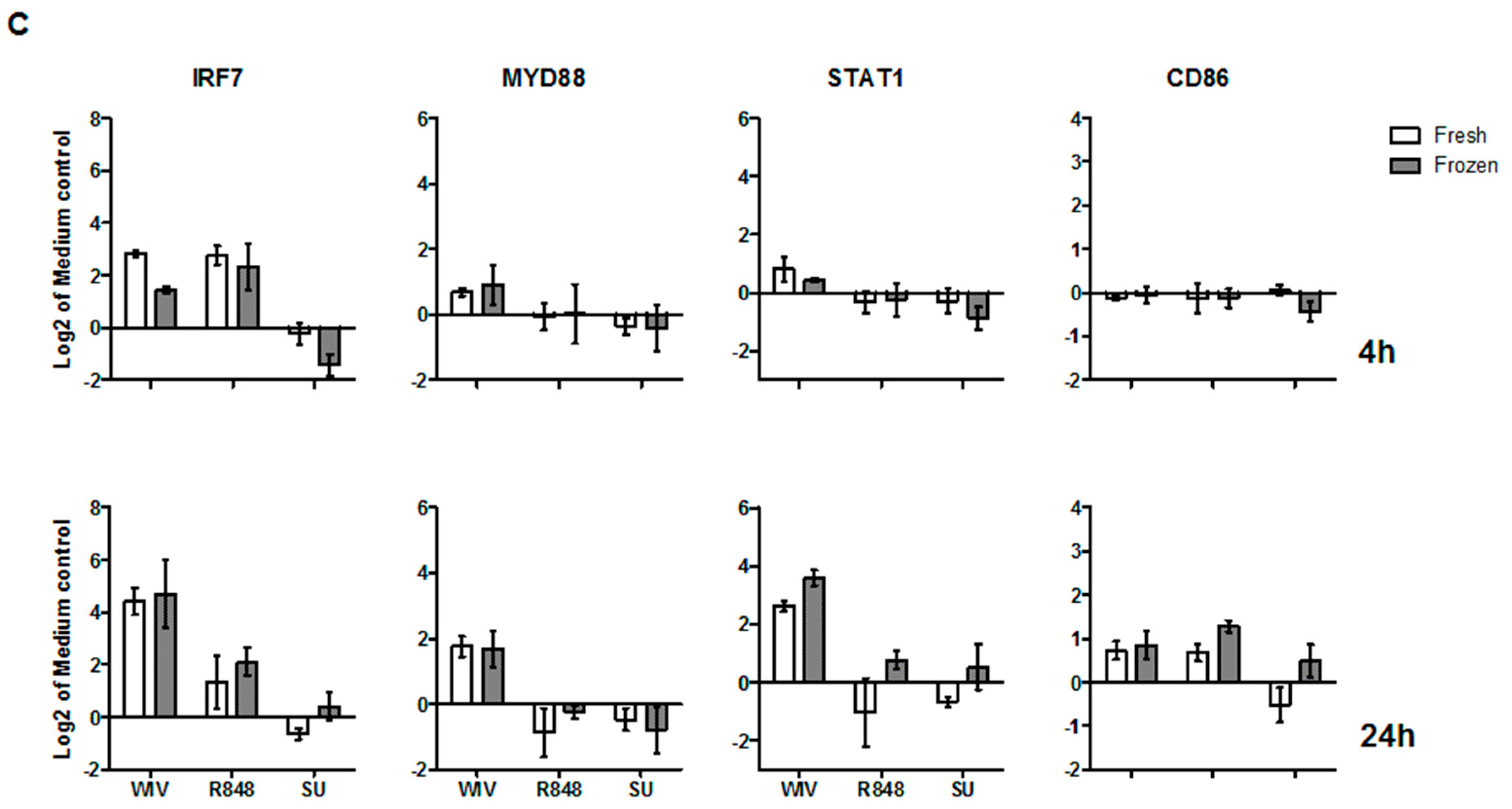
2.6. RNA Isolation and qPCR
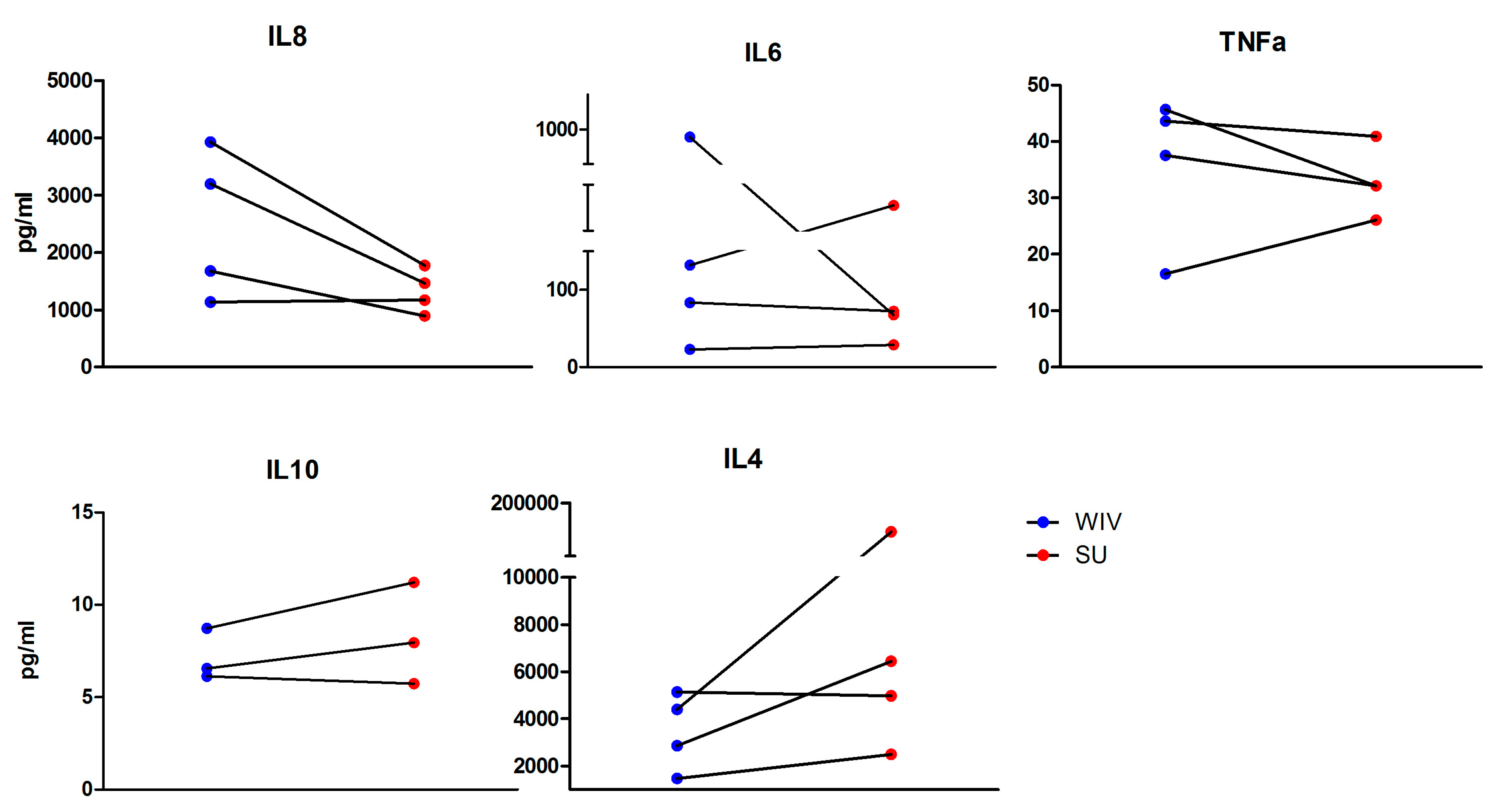
2.7. Cytokine Quantification by Multiplex Immunoassay
2.8. Statistics
3. Results
3.1. MUTZ-3 Cells Are Restricted in Their Ability to Respond to Different Stimuli
3.2. Mo-DCs Do Respond to External Stimuli
3.3. Mo-DCs Derived from Freshly Isolated and Frozen/Thawed PBMCs Are Similar in Their Capacity to Respond to Stimuli
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Days of Differentiation | IL-4 (U/mL) | GM-CSF (U/mL) | TNF-α (U/mL) |
|---|---|---|---|
| 5 | 250 | 500 | 0 |
| 250 | |||
| 1000 | 0 | ||
| 250 | |||
| 6 | 250 | 500 | 0 |
| 250 | |||
| 1000 | 0 | ||
| 250 | |||
| 7 | 250 | 500 | 0 |
| 250 | |||
| 1000 | 0 | ||
| 250 |
References
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T.A.; et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.I.; Burton, D.R.; Kallas, E.G.; Moore, J.P.; Koff, W.C. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 2008, 14, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.-Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.; Hoefnagel, M.M.N. Dendritic cell-based in vitro assays for vaccine immunogenicity. Hum. Vaccin. Immunother. 2012, 8, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Gibbs, J.S.; Lu, X.; Brooke, C.B.; Roy, D.; Modlin, R.L.; Bennink, J.R.; Yewdell, J.W. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood 2012, 119, 3128–3131. [Google Scholar] [CrossRef] [PubMed]
- Memel, T.R.; Hendrickson, S.E.; von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Charleston, B.; Stephens, S.A.; Sopp, P.; Hope, J.C. The role of dendritic cells in shaping the immune response. Anim. Health Res. Rev. 2007, 5, 191–195. [Google Scholar] [CrossRef]
- Rasaiyaah, J.; Noursadeghi, M.; Kellam, P.; Chain, B. Transcriptional and functional defects of dendritic cells derived from the MUTZ-3 leukaemia line. Immunology 2009, 127, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Marshak, D.; Greenwalt, D. Differentiating primary human cells in rapid throughput discovery applications. Methods Mol. Biol. 2007, 356, 121–128. [Google Scholar] [PubMed]
- Li, Y.; Oosting, M.; Deepen, P.; Ricaño-Ponce, I.; Smeekens, S.; Jaeger, M.; Matzaraki, V.; Swertz, M.A.; Xavier, R.J.; Franke, L.; et al. Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi. Nat. Med. 2016, 22, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, A.; Gaugler, B.; Sainty, D.; Lafage-Pochitaloff, M.; Olive, D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur. J. Immunol. 1999, 29, 2567–2578. [Google Scholar] [CrossRef]
- Choudhury, A.; Liang, J.C.; Thomas, E.K.; Flores-Romo, L.; Xie, Q.S.; Agusala, K.; Sutaria, S.; Sinha, I.; Champlin, R.E.; Claxton, D.F. Dendritic Cells Derived In Vitro From Acute Myelogenous Leukemia Cells Stimulate Autologous, Antileukemic T-Cell Responses. Blood 1999, 93, 780–786. [Google Scholar] [PubMed]
- Masterson, A.J.; Sombroek, C.C.; de Gruijl, T.D.; Graus, Y.M.F.; van der Vliet, H.J.J.; Lougheed, S.M.; van den Eertwegh, A.J.; Pinedo, H.M.; Scheper, R.J. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood 2002, 100, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.N.; Portsmouth, D.; Ehrlich, H.J. Accelerated Biopharmaceutical Development: Vero Cell Technology and Pandemic Influenza Vaccine Production. In Modern Biopharmaceuticals Recent Success Stories; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Geeraedts, F.; Bungener, L.; Pool, J.; ter Veer, W.; Wilschut, J.; Huckriede, A. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respir. Viruses 2008, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Budimir, N.; de Haan, A.; Meijerhof, T.; Waijer, S.; Boon, L.; Gostick, E.; Price, D.A.; Wilschut, J.; Huckriede, A. Critical Role of TLR7 Signaling in the Priming of Cross-Protective Cytotoxic T Lymphocyte Responses by a Whole Inactivated Influenza Virus Vaccine. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Edwards, D.C.; Brand, C.; Heath, T. Formation of virosomes from Influenza subunits and liposomes. Lancet 1975, 306, 899–901. [Google Scholar] [CrossRef]
- Van Boxtel, R.A.J.; Verdijk, P.; de Boer, O.J.; van Riet, E.; Mensinga, T.T.; Luytjes, W. Safety and immunogenicity of influenza whole inactivated virus vaccines: A phase I randomized clinical trial. Hum. Vaccin. Immunother. 2015, 11, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; de Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior Immunogenicity of Inactivated Whole Virus H5N1 Influenza Vaccine is Primarily Controlled by Toll-like Receptor Signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef] [PubMed]
- Budimir, N.; Huckriede, A.; Meijerhof, T.; Boon, L.; Gostick, E.; Price, D.A.; Wilschut, J.; de Haan, A. Induction of Heterosubtypic Cross-Protection against Influenza by a Whole Inactivated Virus Vaccine: The Role of Viral Membrane Fusion Activity. PLoS ONE 2012, 7, e30898. [Google Scholar] [CrossRef] [PubMed]
- Stoel, M.; Pool, J.; de Vries-Idema, J.; Zaaraoui-Boutahar, F.; Bijl, M.; Andeweg, A.C.; Wilschut, J.; Huckriede, A. Innate Responses Induced by Whole Inactivated Virus or Subunit Influenza Vaccines in Cultured Dendritic Cells Correlate with Immune Responses In Vivo. PLoS ONE 2015, 10, e0125228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.G.; Tyrrell, D.A.J.; Harrison, P.; Potter, C.W.; Jennings, R.; Clark, A.; Schild, G.C.; Wood, J.M.; Yetts, R.; Seagroatt, V.; et al. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: Serological responses and clinical reactions. J. Biol. Stand. 1979, 7, 123–136. [Google Scholar] [CrossRef]
- Stephenson, I.; Nicholson, K.G.; Glück, R.; Mischler, R.; Newman, R.W.; Palache, A.M.; Verlander, N.Q.; Warburtonet, F.; Wood, J.M.; Zambon, M.C. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: Phase I randomised trial. Lancet 2003, 362, 1959–1966. [Google Scholar] [CrossRef]
- Nelissen, I.; Selderslaghs, I.; Van Den Heuvel, R.; Witters, H.; Verheyen, G.R.; Schoeters, G. MUTZ-3-derived dendritic cells as an in vitro alternative model to CD34+ progenitor-derived dendritic cells for testing of chemical sensitizers. Toxicol. In Vitro 2009, 23, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Lindstedt, M.; Borrebaeck, C.A. Functional and transcriptional profiling of MUTZ-3, a myeloid cell line acting as a model for dendritic cells. Immunology 2006, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, S.F.G.; van Leeuwen, F.N.; Figdor, C.G. Human and murine model cell lines for dendritic cell biology evaluated. Immunol. Lett. 2008, 117, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.A.M.; van den Eertwegh, A.J.M.; van de Loosdrecht, A.A.; Scheper, R.J.; de Gruijl, T.D. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J. Leukoc. Biol. 2008, 84, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, M.H.N.; Vermeulen, J.P.; Scheper, R.J.; Vandebriel, R.J. Response of MUTZ-3 dendritic cells to the different components of the Haemophilus influenzae type B conjugate vaccine: Towards an in vitro assay for vaccine immunogenicity. Vaccine 2011, 29, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Albrekt, A.-S.; Nelissen, I.; Santegoets, S.; de Gruijl, T.D.; Gibbs, S.; Lindstedt, M. Transcriptional profiling of human dendritic cell populations and models—Unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS ONE 2013, 8, e52875. [Google Scholar] [CrossRef] [PubMed]
- Hoonakker, M.E.; Verhagen, L.M.; Hendriksen, C.F.M.; van Els, C.A.C.M.; Vandebriel, R.J.; Sloots, A.; Han, W.G. In vitro innate immune cell based models to assess whole cell Bordetella pertussis vaccine quality: A proof of principle. Biologicals 2015, 43, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, S.; Noh, Y.; Kim, J.; Paik, S.; Yang, Y.; Kim, K., II; Lim, J.S. Impaired responses of leukemic dendritic cells derived from a human myeloid cell line to LPS stimulation. Exp. Mol. Med. 2006, 28, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Hitzler, M.; Bergert, A.; Luch, A.; Peiser, M. Evaluation of selected biomarkers for the detection of chemical sensitization in human skin: A comparative study applying THP-1, MUTZ-3 and primary dendritic cells in culture. Toxicol. In Vitro 2013, 27, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Kastenmuller, W.; Drexler, I.; Ludwig, H.; Erfle, V.; Peschel, C.; Bernhard, H.; Sutter, G. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 2006, 350, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Nájera, J.L.; González, J.M.; López-Fernández, L.A.; Climent, N.; Gatell, J.M.; Gallart, T.; Esteban, M. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J. Virol. 2007, 81, 8707–8721. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Kan-Mitchell, J.; Kuhen, K.L.; Mukai, T.; Wong-Staal, F. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood 2000, 96, 1327–1333. [Google Scholar] [PubMed]
- Gardner, J.P.; Frolov, I.; Perri, S.; Ji, Y.; MacKichan, M.L.; zur Megede, J.; Chen, M.; Belli, B.A.; Driver, D.A.; Sherrill, S.; et al. Infection of Human Dendritic Cells by a Sindbis Virus Replicon Vector Is Determined by a Single Amino Acid Substitution in the E2 Glycoprotein. J. Virol. 2000, 74, 11849–11857. [Google Scholar] [CrossRef] [PubMed]
- Hunsawong, T.; Sunintaboon, P.; Warit, S.; Thaisomboonsuk, B.; Jarman, R.G.; Yoon, I.-K.; Ubol, S.; Fernandez, S. Immunogenic Properties of a BCG Adjuvanted Chitosan Nanoparticle-Based Dengue Vaccine in Human Dendritic Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003958. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, E.; Remoli, M.E.; Gafa, V.; Pardini, M.; Fattorini, L.; Coccia, E.M. IFN-β improves BCG immunogenicity by acting on DC maturation. J. Leukoc. Biol. 2009, 85, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.K.; Wieczorek, L.; Kijak, G.; Lombardi, K.; Currier, J.; Wesberry, M.; Kappes, J.C.; Ngauy, V.; Marovich, M.; Michael, N.; et al. The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS ONE 2012, 7, e29454. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, L.; Brown, B.K.; DelSarto Macedo, C.; Wesberry-Schmierer, M.; Ngauy, V.; Rosa Borges, A.; Michael, N.L.; Marovich, M.A.; Montefiori, D.C.; Polonis, V.R. Mitigation of variation observed in a peripheral blood mononuclear cell (PBMC) based HIV-1 neutralization assay by donor cell pooling. Virology 2013, 447, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Calle, G.; Stoel, M.; De Vries-Idema, J.; Huckriede, A. Distinctive Responses in an In Vitro Human Dendritic Cell-Based System upon Stimulation with Different Influenza Vaccine Formulations. Vaccines 2017, 5, 21. https://doi.org/10.3390/vaccines5030021
Tapia-Calle G, Stoel M, De Vries-Idema J, Huckriede A. Distinctive Responses in an In Vitro Human Dendritic Cell-Based System upon Stimulation with Different Influenza Vaccine Formulations. Vaccines. 2017; 5(3):21. https://doi.org/10.3390/vaccines5030021
Chicago/Turabian StyleTapia-Calle, Gabriela, Maaike Stoel, Jacqueline De Vries-Idema, and Anke Huckriede. 2017. "Distinctive Responses in an In Vitro Human Dendritic Cell-Based System upon Stimulation with Different Influenza Vaccine Formulations" Vaccines 5, no. 3: 21. https://doi.org/10.3390/vaccines5030021





