Adjuvantation of Pulmonary-Administered Influenza Vaccine with GPI-0100 Primarily Stimulates Antibody Production and Memory B Cell Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Vaccine Preparation
2.2. Immunization, Challenge and Sample Collection
2.3. ELISA
2.4. Virus Titration
2.5. ELISpot
2.6. Flow Cytometry
2.7. Statistical Analysis
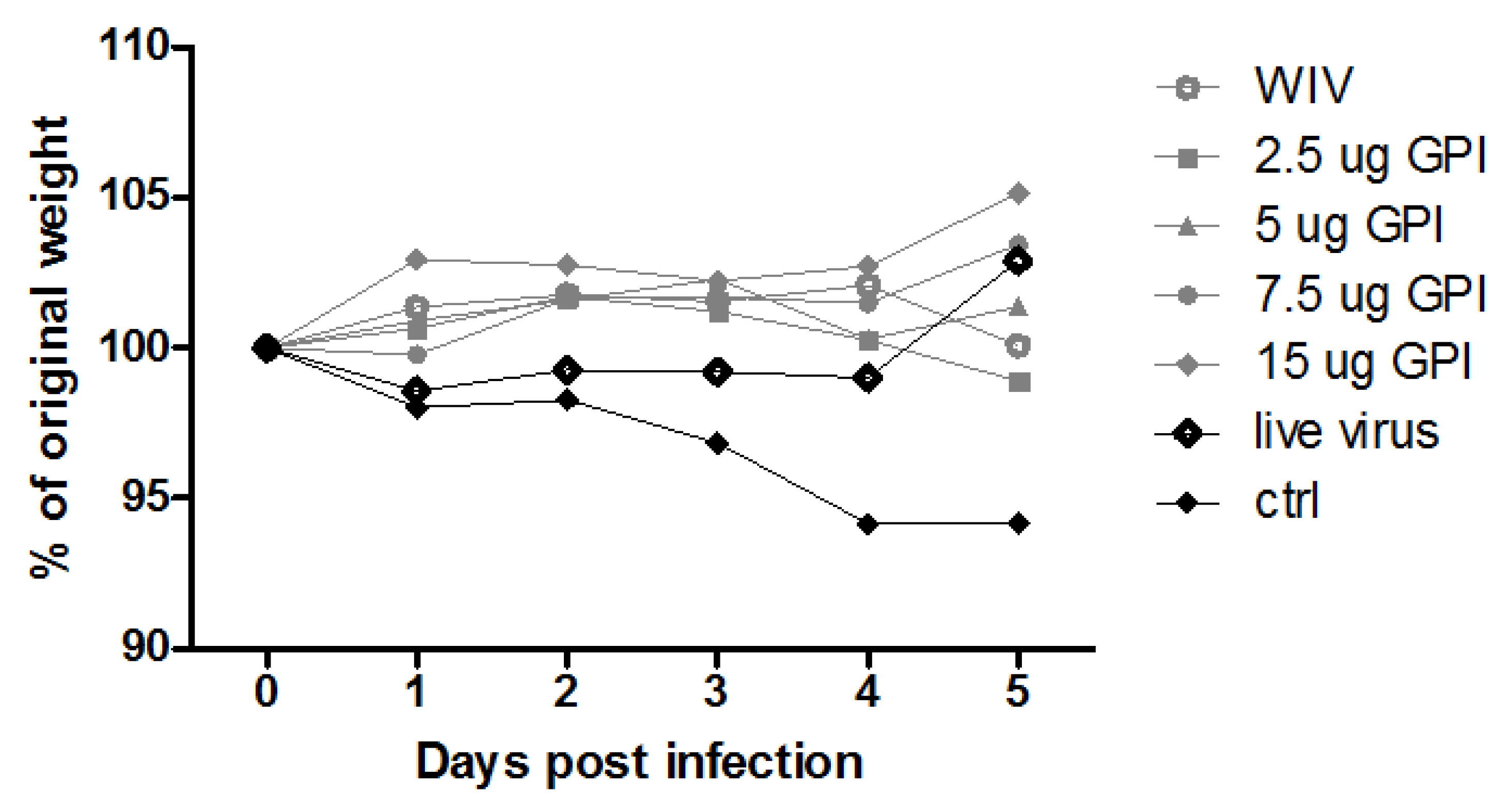
3. Results
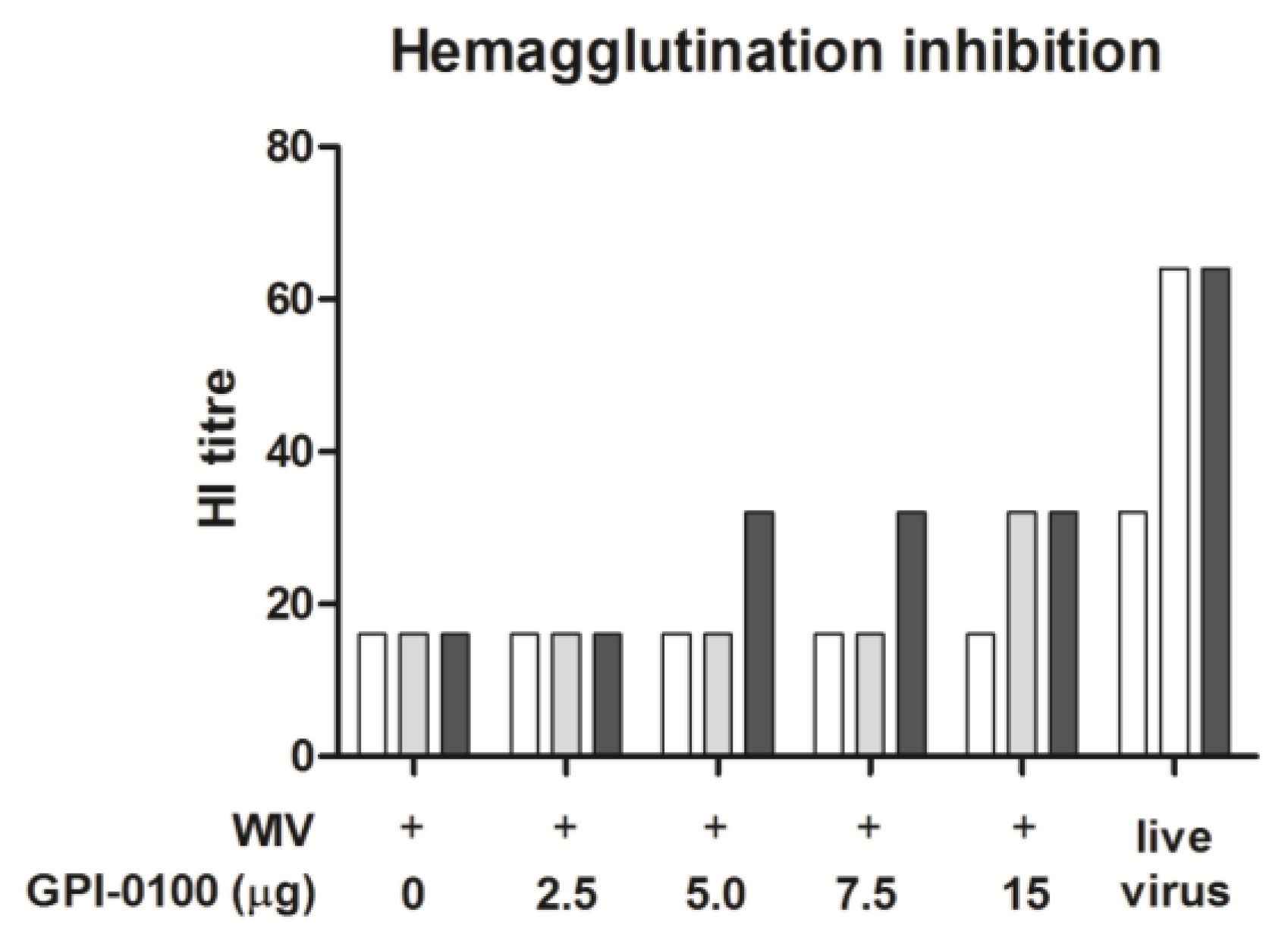
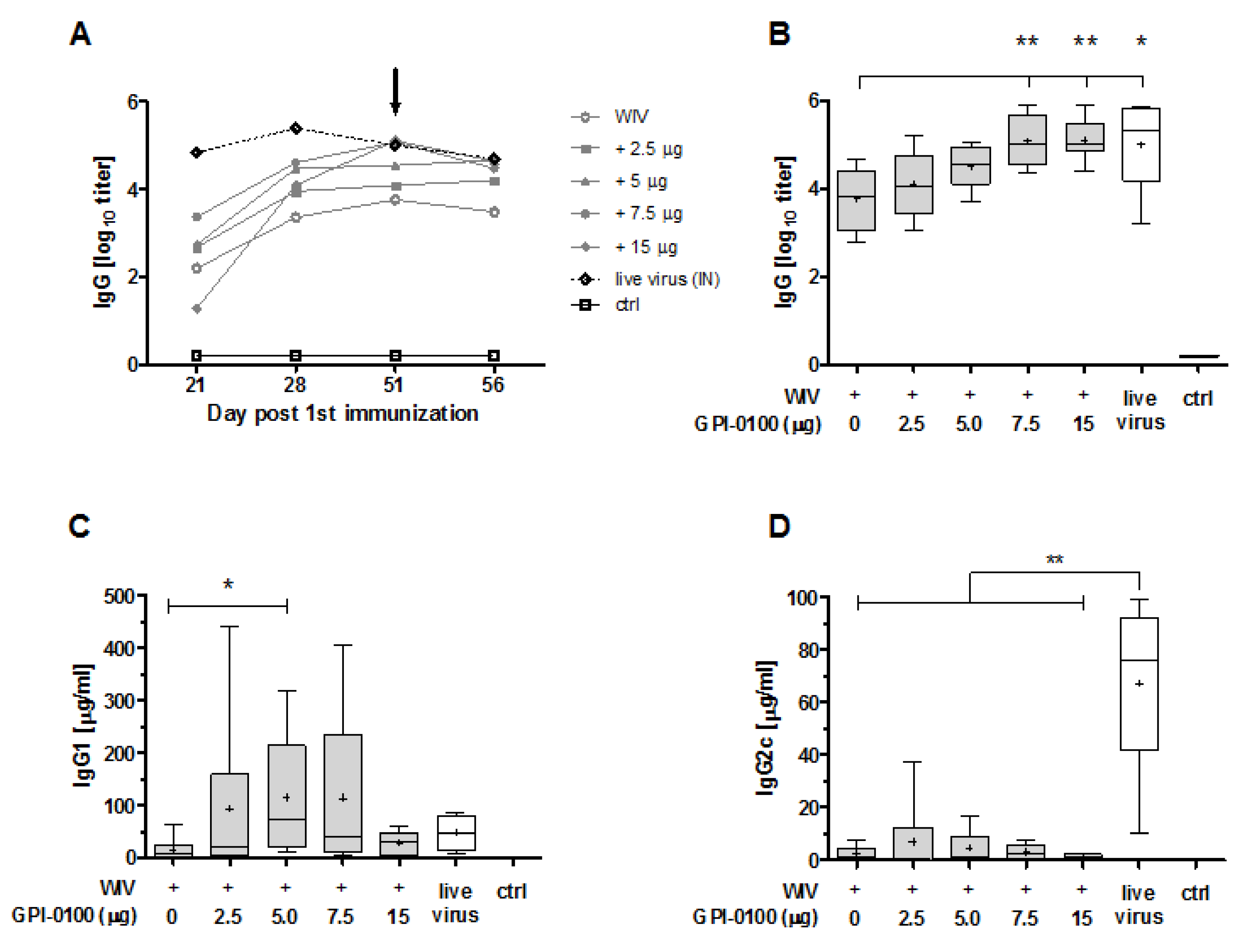
3.1. Systemic Antibody Responses
3.2. Mucosal Antibody Responses
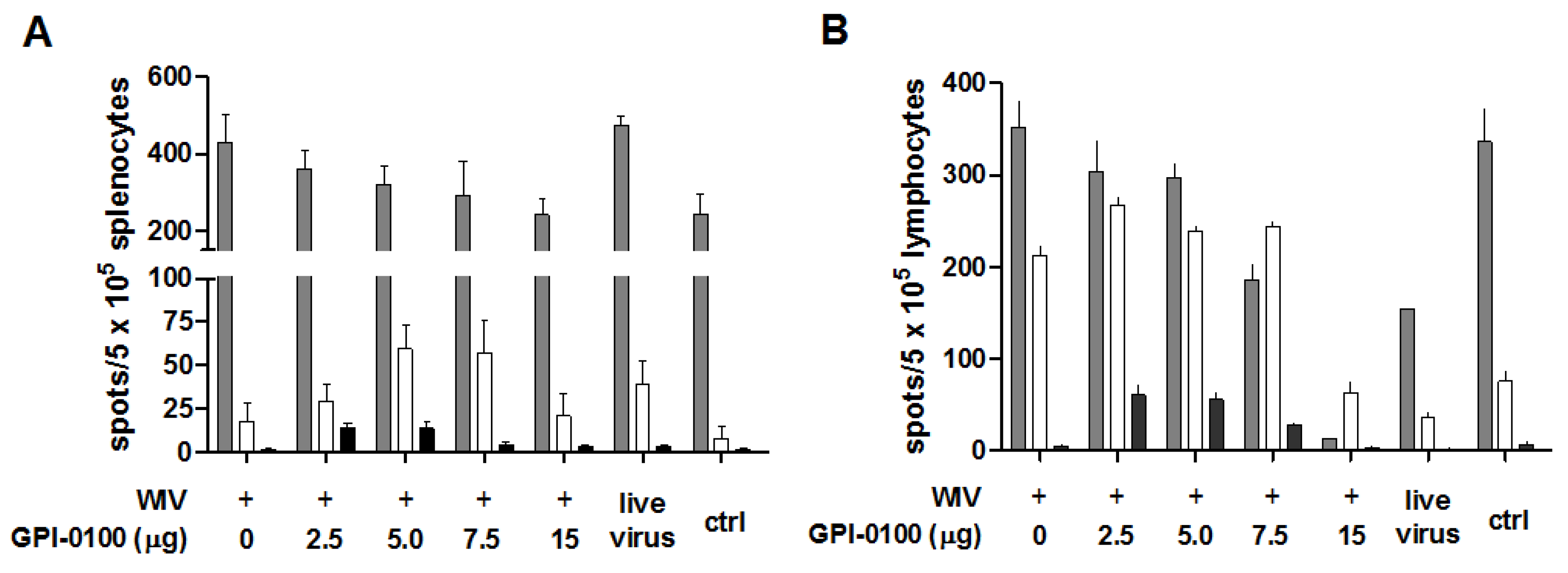
3.3. Analysis of B Cell Responses
3.4. Analysis of Cytokine Responses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Liu, H.; Bungener, L.; ter Veer, W.; Coller, B.-A.; Wilschut, J.; Huckriede, A. Preclinical evaluation of the saponin derivative GPI-0100 as an immunostimulating and dose-sparing adjuvant for pandemic influenza vaccines. Vaccine 2011, 29, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; de Vries-Idema, J.; Ter Veer, W.; Wilschut, J.; Huckriede, A. Influenza virosomes supplemented with GPI-0100 adjuvant: A potent vaccine formulation for antigen dose sparing. Med. Microbiol. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.J.; Shetty, A.; Lu, Y.; Ellis, P.R.; Mohlere, V.; Carnahan, N.; Low, P.S. A Phase I/Ib Study of Folate Immune (EC90 Vaccine Administered With GPI-0100 Adjuvant Followed by EC17) With Interferon-α and Interleukin-2 in Patients With Renal Cell Carcinoma. Immunotherapy 2014, 37, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Ragupathi, G.; Fernandez, C.; Jefferson, M.P.; Diani, M.; Wilton, A.S.; Powell, S.; Spassova, M.; Reis, C.; Clausen, H.; et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine 2005, 23, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J.; Press, J.B.; Reynolds, R.C.; Pathak, A.K.; Pathak, V.; Gundy, L.E.; Farmer, J.T.; Koratich, M.S.; May, R.D. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine 2000, 18, 3141–3151. [Google Scholar] [CrossRef]
- Liu, G.; Anderson, C.; Scaltreto, H.; Barbon, J.; Kensil, C.R. QS-21 structure/function studies: Effect of acylation on adjuvant activity. Vaccine 2002, 20, 2808–2815. [Google Scholar] [CrossRef]
- Marciani, D.J.; Reynolds, R.C.; Pathak, A.K.; Finley-Woodman, K.; May, R.D. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine 2003, 21, 3961–3971. [Google Scholar] [CrossRef]
- Tonnis, W.F.; Kersten, G.F.; Frijlink, H.W.; Hinrichs, W.L.J.; de Boer, A.H.; Amorij, J.-P. Pulmonary vaccine delivery: A realistic approach? J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Satti, I.; Meyer, J.; Harris, S.A.; Thomas, Z.R.M.; Griffiths, K.; Antrobus, R.D.; Rowland, R.; Ramon, R.L.; Smith, M.; Sheehan, S.; et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 939–946. [Google Scholar] [CrossRef]
- Shaikh, N.; Jadi, R.S.; Rajagopal, A.; Brown, K.E.; Brown, D.; Fink, J.B.; John, O.; Scott, P.; Balta, A.X.R.; Greco, M.; et al. A Randomized, Controlled Trial of an Aerosolized Vaccine against Measles. N. Engl. J. Med. 2015, 372, 1519–1529. [Google Scholar] [Green Version]
- Agarkhedkar, S.; Kulkarni, P.S.; Winston, S.; Sievers, R.; Dhere, R.M.; Gunale, B.; Powell, K.; Rota, P.A.; Papania, M. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase I clinical trial. Vaccine 2014, 32, 6791–6797. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.K.; Scheerlinck, J.-P.Y.; Snibson, K.J.; Edwards, S.; Pearse, M.; Quinn, C.; Sutton, P. Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal Immunol. 2008, 1, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vujanic, A.; Snibson, K.J.; Wee, J.L.K.; Edwards, J.; Pearse, M.J.; Scheerlinck, J.Y.; Sutton, P.; Edwards, S.J. Long-Term Antibody and Immune Memory Response Induced by Pulmonary Delivery of the Influenza Iscomatrix Vaccine. Clin. Vaccine Immunol. 2012, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Patil, H.P.; de Vries-Idema, J.; Wilschut, J.; Huckriede, A. Enhancement of the immunogenicity and protective efficacy of a mucosal influenza subunit vaccine by the saponin adjuvant GPI-0100. PLoS ONE 2012, 7, e52135. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.P.; Murugappan, S.; de Vries-Idema, J.; Meijerhof, T.; de Haan, A.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.J.; Huckriede, A. Comparison of adjuvants for a spray freeze-dried whole inactivated virus influenza vaccine for pulmonary administration. Eur. J. Pharm. Biopharm. 2015, 93, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Audouy, S.A.L.; van der Schaaf, G.; Hinrichs, W.L.J.; Frijlink, H.W.; Wilschut, J.; Huckriede, A. Development of a dried influenza whole inactivated virus vaccine for pulmonary immunization. Vaccine 2011, 29, 4345–4352. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Murugappan, S.; ter Veer, W.; Meijerhof, T.; de Haan, A.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.J.; Huckriede, A. Evaluation of monophosphoryl lipid A as adjuvant for pulmonary delivered influenza vaccine. J. Control. Release 2013, 174, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, S.; Patil, H.P.; Kanojia, G.; Ter Veer, W.; Meijerhof, T.; Frijlink, H.W.; Huckriede, A.; Hinrichs, W.L.J. Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: Comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. Eur. J. Pharm. Biopharm. 2013, 85, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Budimir, N.; Huckriede, A.; Meijerhof, T.; Boon, L.; Gostick, E.; Price, D.A.; Wilschut, J.; de Haan, A. Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: the role of viral membrane fusion activity. PLoS ONE 2012, 7, e30898. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N.Y.; Coico, R. Measurement of immunoglobulin synthesis using the ELISPOT assay. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Foo, S.Y.; Phipps, S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010, 3, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Randall, T.D. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv. Immunol. 2010. [Google Scholar] [CrossRef]
- Schotsaert, M.; Ysenbaert, T.; Neyt, K.; Ibañez, L.I.; Bogaert, P.; Schepens, B.; Lambrecht, B.N.; Fiers, W.; Saelens, X. Natural and long-lasting cellular immune responses against influenza in the M2e-immune host. Mucosal Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ridderstad, A.; Tarlinton, D.M. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J. Immunol. 1998, 160, 4688–4695. [Google Scholar] [PubMed]
- Onodera, T.; Takahashi, Y.; Yokoi, Y.; Ato, M.; Kodama, Y.; Hachimura, S.; Kurosaki, T.; Kobayashi, K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 2012, 109, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.P.; Meijerhof, T.; Huckriede, A.; Department of Medical Microbiology, University of Groningen, The Netherlands. Unpublished data. 2014.
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.L.; Lee, K.S.; Bottomly, K. Site of antigen delivery can influence T cell priming: Pulmonary environment promotes preferential Th2-type differentiation. Eur. J. Immunol. 2000, 30, 840–847. [Google Scholar] [CrossRef]
- Wakeham, J.; Wang, J.U.N.; Xing, Z.; Wang, J.U.N. Genetically Determined Disparate Innate and Adaptive Cell-Mediated Immune Responses to Pulmonary Mycobacterium bovis BCG Infection in C57BL/6 and BALB/c Mice Genetically Determined Disparate Innate and Adaptive Cell-Mediated Immune Responses to Pulmon. Infect. Immun. 2000, 68, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Ashtekar, A.R.; Katz, J.; Xu, Q.; Michalek, S.M. A mucosal subunit vaccine protects against lethal respiratory infection with Francisella tularensis LVS. PLoS ONE 2012, 7, e50460. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [PubMed]
- Bienenstock, J.; Mcdermott, M.R. Bronchus- and nasal-associated lymphoid tissues. Immunol. Rev. 2005, 206, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S. Nasal vaccines. Adv. Drug Deliv. Rev. 2001, 51, 21–42. [Google Scholar] [CrossRef]
- Yoshida, M.; Claypool, S.M.; Wagner, J.S.; Mizoguchi, E.; Mizoguchi, A.; Roopenian, D.C.; Lencer, W.I.; Blumberg, R.S.; Street, M.; Harbor, B. Human Neonatal Fc Receptor Mediates Transport of IgG into Luminal Secretions for Delivery of Antigens to Mucosal Dendritic Cells. Immunity 2004, 20, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Ozaki, Y.A.; Yoshikawa, T.; Hasegawa, H.; Sato, Y.; Suzuki, Y.; Inoue, R.; Morishima, T.; Kondo, N.; Sata, T.; et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 2003, 21, 2362–2371. [Google Scholar] [CrossRef]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2012, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Bergqvist, P.; Stensson, A.; Holmberg, A.; Mattsson, J.; Lycke, N.Y. A unique role of the cholera toxin A1-DD adjuvant for long-term plasma and memory B cell development. J. Immunol. 2011, 186, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Hartson, L.; Kusser, K.; Tighe, M.P.; Klonowski, K.D.; Lefrancois, L.; Cauley, L.S.; Harmsen, A.G.; Lund, F.E.; et al. Persistence and Responsiveness of Immunologic Memory in the Absence of Secondary Lymphoid Organs. Immunity 2006, 25, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Milpied, P.J.; McHeyzer-Williams, M.G. High-affinity IgA needs TH17 cell functional plasticity. Nat. Immunol. 2013, 14, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kiyono, H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012, 2, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; de la Luz Garcia-Hernandez, M.; Reome, J.B.; Misra, S.K.; Strutt, T.M.; McKinstry, K.K.; Cooper, A.M.; Swain, S.L.; Dutton, R.W. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009, 182, 3469–3481. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, K.K.; Strutt, T.M.; Buck, A.; Curtis, J.D.; Dibble, J.P.; Huston, G.; Tighe, M.; Hamada, H.; Sell, S.; Dutton, R.W.; et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J. Immunol. 2009, 182, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Maroof, A.; Yorgensen, Y.M.; Li, Y.; Evans, J.T. Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection. PLoS Pathog. 2014, 10, e1003875. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Rangel-Moreno, J.; Fallert Junecko, B.A.; Mallon, D.J.; Chen, K.; Pociask, D.A.; Connell, T.D.; Reinhart, T.A.; Alcorn, J.F.; Ross, T.M.; et al. Mucosal Pre-Exposure to Th17-Inducing Adjuvants Exacerbates Pathology after Influenza Infection. Am. J. Pathol. 2014, 184, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gueders, M.M.; Paulissen, G.; Crahay, C.; Quesada-Calvo, F.; Hacha, J.; Van Hove, C.; Tournoy, K.; Louis, R.; Foidart, J.M.; Noël, A.; et al. Mouse models of asthma: A comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 2009, 58, 845–854. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, H.P.; Herrera Rodriguez, J.; De Vries-Idema, J.; Meijerhof, T.; Frijlink, H.W.; Hinrichs, W.L.J.; Huckriede, A. Adjuvantation of Pulmonary-Administered Influenza Vaccine with GPI-0100 Primarily Stimulates Antibody Production and Memory B Cell Proliferation. Vaccines 2017, 5, 19. https://doi.org/10.3390/vaccines5030019
Patil HP, Herrera Rodriguez J, De Vries-Idema J, Meijerhof T, Frijlink HW, Hinrichs WLJ, Huckriede A. Adjuvantation of Pulmonary-Administered Influenza Vaccine with GPI-0100 Primarily Stimulates Antibody Production and Memory B Cell Proliferation. Vaccines. 2017; 5(3):19. https://doi.org/10.3390/vaccines5030019
Chicago/Turabian StylePatil, Harshad P., José Herrera Rodriguez, Jacqueline De Vries-Idema, Tjarko Meijerhof, Henderik W. Frijlink, Wouter L. J. Hinrichs, and Anke Huckriede. 2017. "Adjuvantation of Pulmonary-Administered Influenza Vaccine with GPI-0100 Primarily Stimulates Antibody Production and Memory B Cell Proliferation" Vaccines 5, no. 3: 19. https://doi.org/10.3390/vaccines5030019





