Comparative Analysis of Vaccine-Induced Neutralizing Antibodies against the Alpha, Beta, Delta, and Omicron Variants of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Neutralization Test
2.3. Nucleocapsid Protein (NCP) ELISA
2.4. Isolation and Sequencing of Virus Variants
2.5. Statistical Analysis
3. Results
3.1. Mutational Changes in Virus Variants
3.2. NCP-ELISA
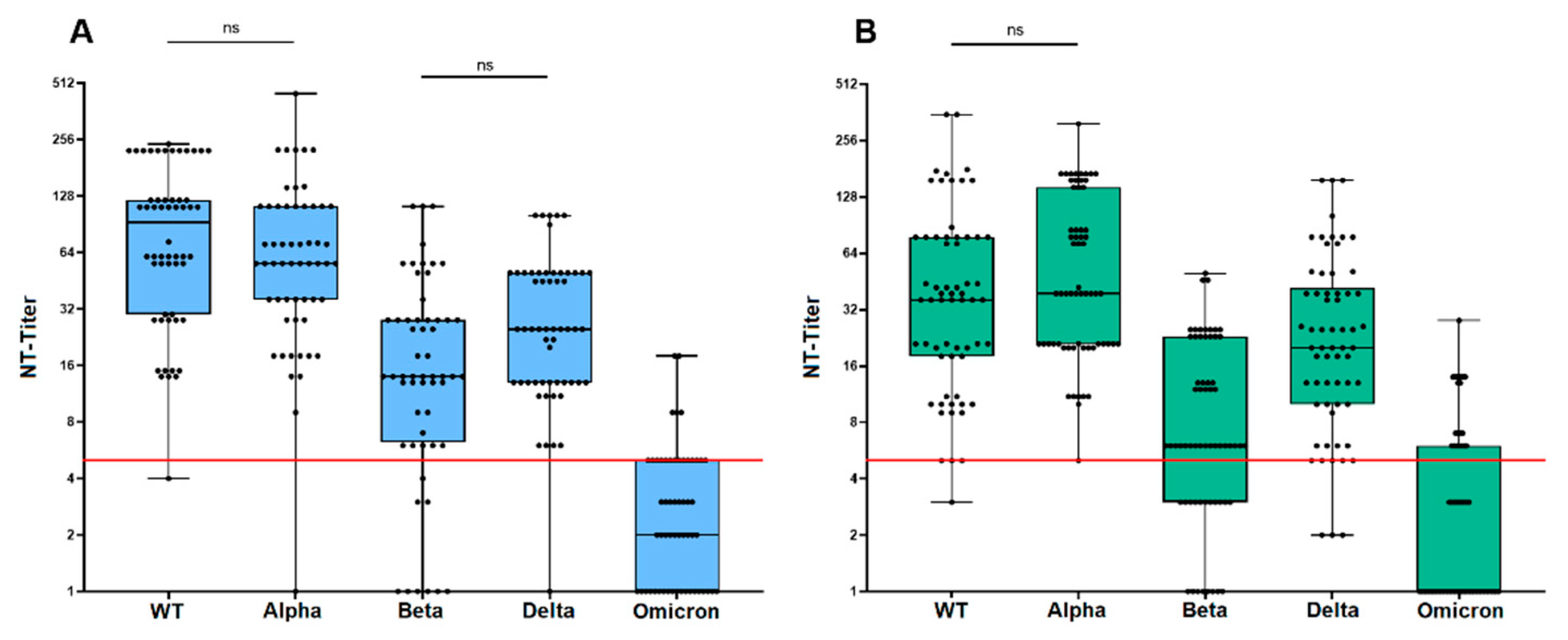
3.3. Neutralization Sensitivity of Virus Variants after Prime/Boost Vaccination
3.4. Neutralization Sensitivity of Virus Variants after Third Vaccination
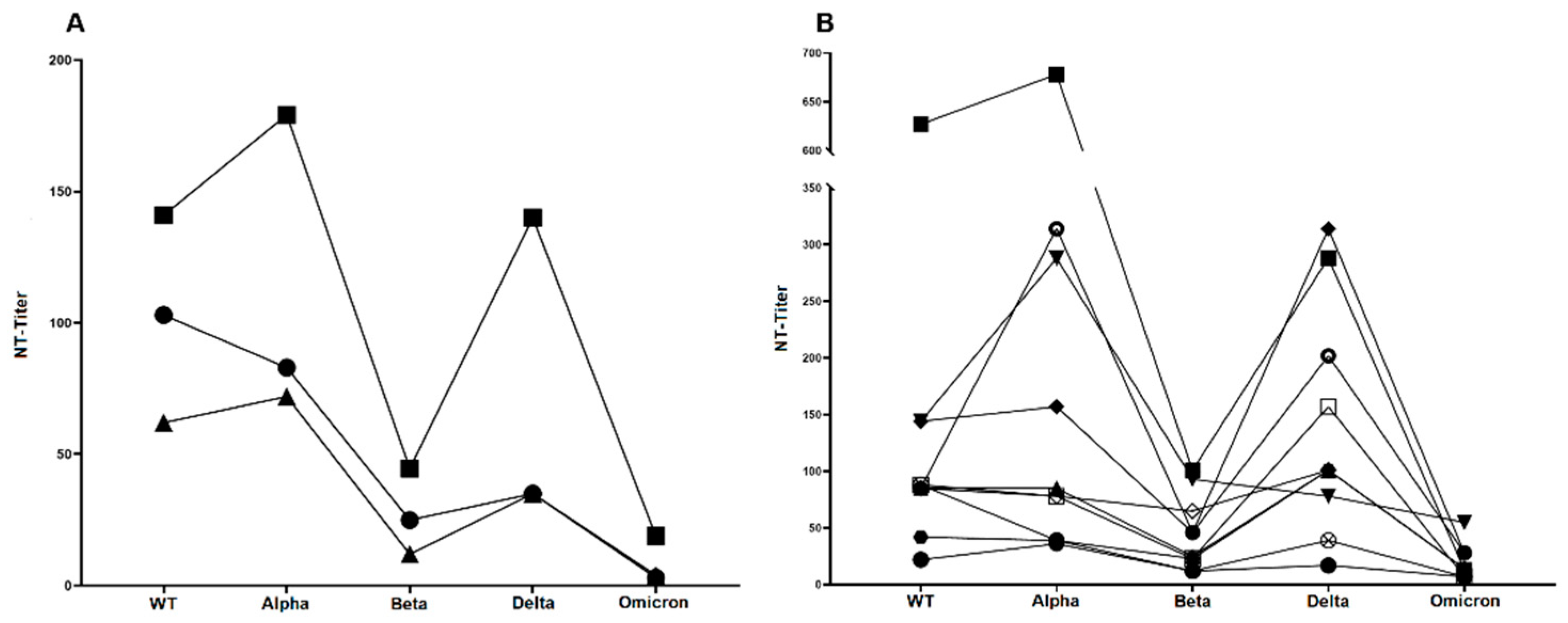
3.5. Neutralization Sensitivity of Virus Variants after Breakthrough Infection
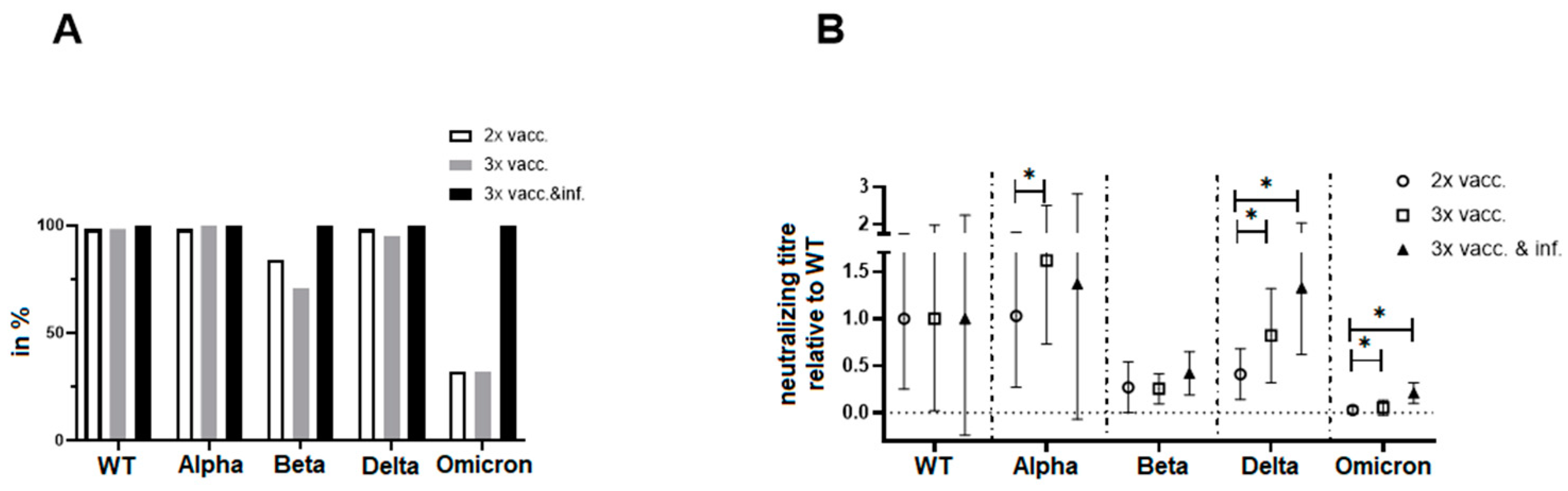
3.6. Direct Comparison of Neutralization Capacities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency (EMA). Authorised COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 19 August 2022).
- European Centre for Disease Prevention and Control (ECDC). Vaccines Authorised for Use in the European Union. Available online: https://vaccination-info.europa.eu/en/covid-19-0 (accessed on 19 August 2022).
- German Federal Ministry of Health. Current Vaccination Status. Available online: https://impfdashboard.de/en/ (accessed on 19 August 2022).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Overview of EU/EEA Country Recommendations on COVID-19 Vaccination with Vaxzevria, and a Scoping Review of Evidence to Guide Decision-Making; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021. [Google Scholar]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Perez-Olmeda, M.; Castano, L.; Bertran, M.J.; Garcia-Perez, J.; Campins, M.; Portoles, A.; Gonzalez-Perez, M.; Garcia Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Normark, J.; Vikstrom, L.; Gwon, Y.D.; Persson, I.L.; Edin, A.; Bjorsell, T.; Dernstedt, A.; Christ, W.; Tevell, S.; Evander, M.; et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 385, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.B.; Massardier-Pilonchery, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Belik, M.; Jalkanen, P.; Lundberg, R.; Reinholm, A.; Laine, L.; Vaisanen, E.; Skon, M.; Tahtinen, P.A.; Ivaska, L.; Pakkanen, S.H.; et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat. Commun. 2022, 13, 2476. [Google Scholar] [CrossRef]
- Bordi, L.; Sberna, G.; Piscioneri, C.N.; Cocchiara, R.A.; Miani, A.; Grammatico, P.; Mariani, B.; Parisi, G. Longitudinal dynamics of SARS-CoV-2 anti-receptor binding domain IgG antibodies in a wide population of health care workers after BNT162b2 vaccination. Int. J. Infect. Dis. 2022, 122, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Hadj Hassine, I. COVID-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef]
- van den Brink, E.N.; Ter Meulen, J.; Cox, F.; Jongeneelen, M.A.; Thijsse, A.; Throsby, M.; Marissen, W.E.; Rood, P.M.; Bakker, A.B.; Gelderblom, H.R.; et al. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1635–1644. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Fort, H. A very simple model to account for the rapid rise of the alpha variant of SARS-CoV-2 in several countries and the world. Virus Res. 2021, 304, 198531. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021, 26, 2100509. [Google Scholar] [CrossRef] [PubMed]
- Hodcroft, E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. Per Country. Available online: https://covariants.org/per-country (accessed on 19 August 2022).
- Earnest, R.; Uddin, R.; Matluk, N.; Renzette, N.; Turbett, S.E.; Siddle, K.J.; Loreth, C.; Adams, G.; Tomkins-Tinch, C.H.; Petrone, M.E.; et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. Cell Rep. Med. 2022, 3, 100583. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 18 August 2022).
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J. Med. Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Hodcroft, E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. Per Variant. Available online: https://covariants.org/per-variant (accessed on 19 August 2022).
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Haselmann, V.; Ozcurumez, M.K.; Klawonn, F.; Ast, V.; Gerhards, C.; Eichner, R.; Costina, V.; Dobler, G.; Geilenkeuser, W.J.; Wolfel, R.; et al. Results of the first pilot external quality assessment (EQA) scheme for anti-SARS-CoV2-antibody testing. Clin. Chem. Lab. Med. 2020, 58, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Khatamzas, E.; Muenchhoff, M.; Rehn, A.; Graf, A.; Hellmuth, J.; Hollaus, A.; Mohr, A.-W.; Gaitzsch, E.; Weiglein, T.; Georgi, E.; et al. CD8 T cells and antibodies drive SARS-CoV-2 evolution in chronic infection. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, L.J. trackViewer: A Bioconductor package for interactive and integrative visualization of multi-omics data. Nat. Methods 2019, 16, 453–454. [Google Scholar] [CrossRef]
- Muller, K.; Girl, P.; Giebl, A.; Gruetzner, S.; Antwerpen, M.; Khatamzas, E.; Wolfel, R.; von Buttlar, H. Sensitivity of two SARS-CoV-2 variants with spike protein mutations to neutralising antibodies. Virus Genes 2021, 57, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Uzoeto, H.O.; Ajima, J.N.; Arazu, A.V.; Ibiang, G.O.; Cosmas, S.; Durojaye, O.A. Immunity evasion: Consequence of the N501Y mutation of the SARS-CoV-2 spike glycoprotein. J. Genet. Eng. Biotechnol. 2022, 20, 10. [Google Scholar] [CrossRef]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361.e2346. [Google Scholar] [CrossRef]
- Jangra, S.; Ye, C.; Rathnasinghe, R.; Stadlbauer, D.; Personalized Virology Initiative Study Group; Krammer, F.; Simon, V.; Martinez-Sobrido, L.; Garcia-Sastre, A.; Schotsaert, M. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2021, 2, e283–e284. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, B.; Fernandes, M.H.V.; Frazier, L.; Tang, T.; Daniel, S.; Diel, D.G.; Jaimes, J.A.; Whittaker, G.R. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B.1.1.7 (Alpha) spike. iScience 2022, 25, 103589. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Mallik, B. Omicron (B.1.1.529)—A new heavily mutated variant: Mapped location and probable properties of its mutations with an emphasis on S-glycoprotein. Int. J. Biol. Macromol. 2022, 219, 980–997. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Kimura, I.; Shirakawa, K.; Takaori-Kondo, A.; Nakada, T.-a.; Kaneda, A.; Nakagawa, S.; Sato, K. Neutralization of the SARS-CoV-2 Mu Variant by Convalescent and Vaccine Serum. N. Engl. J. Med. 2021, 385, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; De Marco, A.; Ferreira, I.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2021, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. eBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef]
- Huang, C.Q.; Vishwanath, S.; Carnell, G.W.; Chan, A.C.Y.; Heeney, J.L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 2023, 8, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Woodland, D.L. Jump-starting the immune system: Prime-boosting comes of age. Trends Immunol. 2004, 25, 98–104. [Google Scholar] [CrossRef]
- Pang, H.; Liu, Y.; Han, X.; Xu, Y.; Jiang, F.; Wu, D.; Kong, X.; Bartlam, M.; Rao, Z. Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: Implications for the design of an effective protein-based vaccine. J. Gen. Virol. 2004, 85, 3109–3113. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mohlenberg, M.; Wang, P.; Zhou, H. Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron. Cytokine Growth Factor Rev. 2023, 70, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Schmidt, F.; Schaefer-Babajew, D.; Wang, Z.; JC, C.L.; Flyak, A.I.; DeLaitsch, A.T.; Huey-Tubman, K.E.; et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021, 54, 1853–1868.e7. [Google Scholar] [CrossRef] [PubMed]
- Chandler, T.L.; Yang, A.; Otero, C.E.; Permar, S.R.; Caddy, S.L. Protective mechanisms of nonneutralizing antiviral antibodies. PLoS Pathog. 2023, 19, e1011670. [Google Scholar] [CrossRef]
- Yaugel-Novoa, M.; Bourlet, T.; Paul, S. Role of the humoral immune response during COVID-19: Guilty or not guilty? Mucosal Immunol. 2022, 15, 1170–1180. [Google Scholar] [CrossRef]

| Key Spike Protein Mutations | Location | Described Effect | WT | Alpha | Beta | Delta | Omicron | Reference |
|---|---|---|---|---|---|---|---|---|
| D614G | S1 domain (C-terminal) | Enhanced infectivity and fitness | x | x | x | x | x | [29] |
| N501Y | Receptor-binding motif | Enhanced infectivity and transmission | x | x | x | [30] | ||
| E484K | Receptor-binding motif | Enhanced infectivity, reduced antibody neutralization, and immune escape | x | x (E484A) | [32,33] | |||
| K417N | Receptor-binding domain | Enhanced infectivity, reduced antibody neutralization, and immune escape | x | x | [32] | |||
| P681R | Furin cleavage site | Enhanced spike protein cleavage and increased fitness | x (P681H) | x | x (P681H) | [34] | ||
| T478K | Receptor-binding domain | Enhanced infectivity and reduced antibody neutralization | x | [35] | ||||
| L452R | Receptor-binding domain | Enhanced infectivity and reduced antibody neutralization | x | [35] | ||||
| S477N | Receptor-binding domain | Increased affinity of RBD for ACE2 and immune escape | x | [37] | ||||
| Q493R | Receptor-binding domain | Improved binding between RBD and ACE2 | x | [37] | ||||
| G496S | Receptor-binding motif | Reduced antibody-RBD interaction | x | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girl, P.; von Buttlar, H.; Mantel, E.; Antwerpen, M.H.; Wölfel, R.; Müller, K. Comparative Analysis of Vaccine-Induced Neutralizing Antibodies against the Alpha, Beta, Delta, and Omicron Variants of SARS-CoV-2. Vaccines 2024, 12, 515. https://doi.org/10.3390/vaccines12050515
Girl P, von Buttlar H, Mantel E, Antwerpen MH, Wölfel R, Müller K. Comparative Analysis of Vaccine-Induced Neutralizing Antibodies against the Alpha, Beta, Delta, and Omicron Variants of SARS-CoV-2. Vaccines. 2024; 12(5):515. https://doi.org/10.3390/vaccines12050515
Chicago/Turabian StyleGirl, Philipp, Heiner von Buttlar, Enrico Mantel, Markus H. Antwerpen, Roman Wölfel, and Katharina Müller. 2024. "Comparative Analysis of Vaccine-Induced Neutralizing Antibodies against the Alpha, Beta, Delta, and Omicron Variants of SARS-CoV-2" Vaccines 12, no. 5: 515. https://doi.org/10.3390/vaccines12050515





