MivacunaLA (MyshotLA): A Community-Partnered Mobile Phone Intervention to Improve COVID-19 Vaccination Behaviors among Low-Income, Spanish-Speaking, and Immigrant Latino Parents or Caregivers
Abstract
1. Introduction
2. Materials and Methods
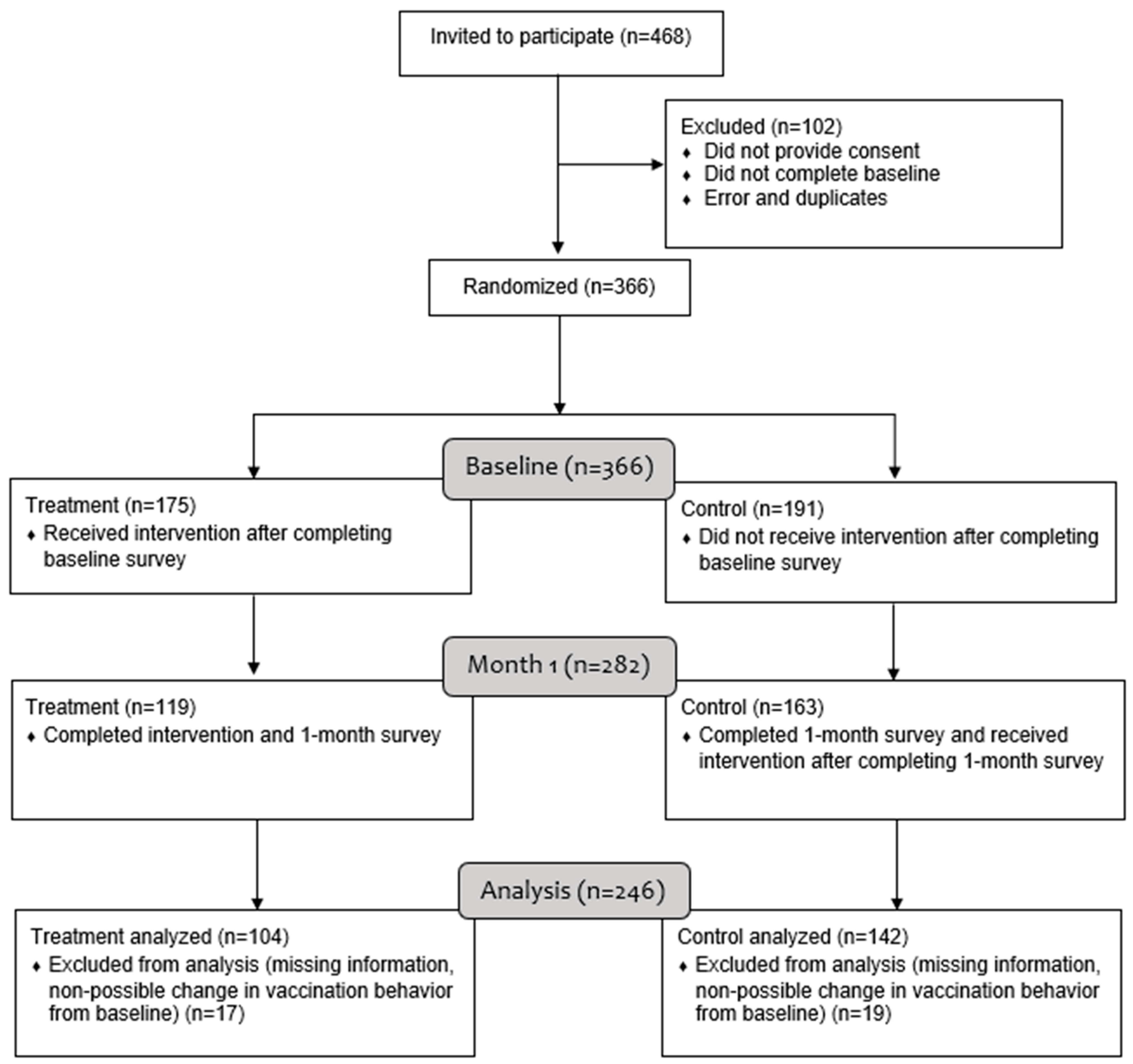
2.1. Study Design, Recruitment and Procedures
2.2. Study Intervention
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Primary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Simon, P.; Ho, A.; Shah, M.D.; Shetgiri, R. Trends in Mortality From COVID-19 and Other Leading Causes of Death Among Latino vs White Individuals in Los Angeles County, 2011–2020. JAMA 2021, 326, 973–974. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html (accessed on 12 January 2022).
- Los Angeles Department of Public Health. Los Angeles County Daily COVID-19 Data. Available online: http://publichealth.lacounty.gov/media/Coronavirus/data/index.htm# (accessed on 5 January 2022).
- Caraballo, C.; Massey, D.; Mahajan, S.; Lu, Y.; Annapureddy, A.R.; Roy, B.; Riley, C.; Murugiah, K.; Valero-Elizondo, J.; Onuma, O.; et al. Racial and Ethnic Disparities in Access to Health Care Among Adults in the United States: A 20-Year National Health Interview Survey Analysis, 1999–2018. medRxiv 2020. [Google Scholar] [CrossRef]
- Martinez, M.E.; Nodora, J.N.; Carvajal-Carmona, L.G. The dual pandemic of COVID-19 and systemic inequities in US Latino communities. Cancer 2021, 127, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, C.E.; Guilamo-Ramos, V.; Mena, L.; Hall, E.; Honermann, B.; Crowley, J.S.; Baral, S.; Prado, G.J.; Marzan-Rodriguez, M.; Beyrer, C.; et al. Risk for COVID-19 infection and death among Latinos in the United States: Examining heterogeneity in transmission dynamics. Ann. Epidemiol. 2020, 52, 46–53.e2. [Google Scholar] [CrossRef]
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S.; et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths: A Systematic Review. Ann. Intern. Med. 2021, 174, 362–373. [Google Scholar] [CrossRef]
- Galdamez, M.; Kesteven, C.; Melaas, A. A Vulnerable State: Hispanic Essential Workers in California; Milken Institute: Santa Monica, CA, USA, 2020. [Google Scholar]
- Gonzalez, C.J.; Aristega Almeida, B.; Corpuz, G.S.; Mora, H.A.; Aladesuru, O.; Shapiro, M.F.; Sterling, M.R. Challenges with social distancing during the COVID-19 pandemic among Hispanics in New York City: A qualitative study. BMC Public Health 2021, 21, 1946. [Google Scholar] [CrossRef]
- GPSN. Educational Recovery Now. Available online: https://gpsnla.org/wp-content/uploads/2021/03/EdRecoveryNow_Final_3-29-21.pdf (accessed on 24 June 2022).
- Los Angeles County Department of Public Health. COVID-19 Vaccinations in LA County. Available online: http://publichealth.lacounty.gov/media/Coronavirus/vaccine/vaccine-dashboard.htm# (accessed on 5 December 2021).
- Fontenot, H.B.; White, B.P.; Rosenberger, J.G.; Lacasse, H.; Rutirasiri, C.; Mayer, K.H.; Zimet, G. Mobile App Strategy to Facilitate Human Papillomavirus Vaccination Among Young Men Who Have Sex With Men: Pilot Intervention Study. J. Med. Internet Res. 2020, 22, e22878. [Google Scholar] [CrossRef]
- Stockwell, M.S.; Kharbanda, E.O.; Martinez, R.A.; Lara, M.; Vawdrey, D.; Natarajan, K.; Rickert, V.I. Text4Health: Impact of text message reminder-recalls for pediatric and adolescent immunizations. Am. J. Public Health 2012, 102, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Albertin, C.S.; Casillas, A.; Valderrama, R.; Duru, O.K.; Ong, M.K.; Vangala, S.; Tseng, C.H.; Humiston, S.G.; Evans, S.; et al. Effect of Personalized Messages Sent by a Health System’s Patient Portal on Influenza Vaccination Rates: A Randomized Clinical Trial. J. Gen. Intern. Med. 2021, 37, 615–623. [Google Scholar] [CrossRef]
- Stockwell, M.S.; Kharbanda, E.O.; Martinez, R.A.; Vargas, C.Y.; Vawdrey, D.K.; Camargo, S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: A randomized controlled trial. JAMA 2012, 307, 1702–1708. [Google Scholar] [CrossRef]
- Chou, W.; Burgdorf, C.; Gaysynsky, A.; Hunter, C. COVID-19 Vaccination Communication: Applying Behavioral and Social Science to Address Vaccine Hesitancy and Foster Vaccine Confidence; National Institute of Health: Bethesda, MD, USA, 2020.
- Silverman-Lloyd, L.G.; Dominguez Cortez, J.; Godage, S.K.; Valenzuela Araujo, D.; Rivera, T.; Polk, S.; DeCamp, L.R. Immigrant Latino parents demonstrated high interactivity with pediatric primary care text messaging intervention. mHealth 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Thomas, K.; Shah, M.D.; Vizueta, N.; Cui, Y.; Vangala, S.; Fox, C.; Kapteyn, A. The role of trust in the likelihood of receiving a COVID-19 vaccine: Results from a national survey. Prev. Med. 2021, 153, 106727. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.L.; Casillas, A.; Castellon-Lopez, Y.; Mansfield, L.N.; Morris, D.; Barron, J.; Ntekume, E.; Landovitz, R.; Vassar, S.D.; Norris, K.C.; et al. COVID-19 Vaccine Decision-making Factors in Racial and Ethnic Minority Communities in Los Angeles, California. JAMA Netw. Open 2021, 4, e2127582. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, N.A.; Shirazipour, C.H.; Herrera, E.; Figueiredo, J.C.; Salvy, S.J. Exploring Latino Promotores/a de Salud (Community Health Workers) knowledge, attitudes, and perceptions of COVID-19 vaccines. SSM 2021, 2, 100033. [Google Scholar] [CrossRef]
- Gargano, L.M.; Herbert, N.L.; E Painter, J.; Sales, J.M.; Morfaw, C.; Rask, K.; Murray, D.; DiClemente, R.J.; Hughes, J.M. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum. Vaccin. Immunother. 2013, 9, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Cialdini, R.B.; Goldstein, N.J. Social influence: Compliance and conformity. Annu. Rev. Psychol. 2004, 55, 591–621. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.; Kerkhoff, A.D.; Naso, J.; Contreras, M.G.; Castellanos Diaz, E.; Rojas, S.; Peng, J.; Rubio, L.; Jones, D.; Jacobo, J.; et al. A multi-component, community-based strategy to facilitate COVID-19 vaccine uptake among Latinx populations: From theory to practice. PLoS ONE 2021, 16, e0257111. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Vargas, N.; de la Torre, C.; Magana Alvarez, M.; Clark, J.L. Engaging Latino Families About COVID-19 Vaccines: A Qualitative Study Conducted in Oregon, USA. Health Educ. Behav. 2021, 48, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, L.; Santilli, A.; Morone, J.; Ainooson, J.; Roy, B.; Njoku, A.; Mendiola-Iparraguirre, A.; O’Connor Duffany, K.; Macklin, B.; Higginbottom, J.; et al. COVID-19 Vaccine Acceptance and Access Among Black and Latinx Communities. JAMA Netw. Open 2021, 4, e2128575. [Google Scholar] [CrossRef]
- Webb Hooper, M.; Nápoles, A.M.; Pérez-Stable, E.J. No Populations Left Behind: Vaccine Hesitancy and Equitable Diffusion of Effective COVID-19 Vaccines. J. Gen. Intern. Med. 2021, 36, 2130–2133. [Google Scholar] [CrossRef]
- Castellon-Lopez, Y.M.; Carson, S.L.; Mansfield, L.; Garrison, N.A.; Barron, J.; Morris, D.; Ntekume, E.; Vassar, S.D.; Norris, K.C.; Brown, A.F.; et al. “The System Doesn’t Let Us in”—A Call for Inclusive COVID-19 Vaccine Outreach Rooted in Los Angeles Latinos’ Experience of Pandemic Hardships and Inequities. Int. J. Environ. Res. Public Health 2022, 19, 5785. [Google Scholar] [CrossRef]
- McAboy, K. UCLA-Led Coalition Works with Minority Communities on COVID-19 and Vaccine Education. Available online: https://www.foxla.com/news/ucla-led-coalition-works-with-minority-communities-on-covid-19-and-vaccine-education (accessed on 8 March 2021).
- CDC en Español. ¿Son Seguras Para Nosotros Las Vacunas Contra el COVID-19? Available online: https://www.facebook.com/watch/?v=1356313164724043 (accessed on 6 June 2021).
- Arie, K.; Marco, A.; Dan, B.; de Bruin, W.B.; Jill, D.; Tania, G.; Ying, L.; Erik, M.; Francisco, P.-A.; Simone, S.; et al. Tracking the Effect of the COVID-19 Pandemic on the Lives of American Households. Surv. Res. Methods 2020, 14, 179–186. [Google Scholar] [CrossRef]
- Stockwell, M.S.; Hofstetter, A.M.; DuRivage, N.; Barrett, A.; Fernandez, N.; Vargas, C.Y.; Camargo, S. Text message reminders for second dose of influenza vaccine: A randomized controlled trial. Pediatrics 2015, 135, e83–e91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Koopmeiners, J.S.; McHugh, J.; Raveis, V.H.; Ahluwalia, J.S. mHealth Pilot Study: Text Messaging Intervention to Promote HPV Vaccination. Am. J. Health Behav. 2016, 40, 67–76. [Google Scholar] [CrossRef]
- Lustria, M.L.; Noar, S.M.; Cortese, J.; Van Stee, S.K.; Glueckauf, R.L.; Lee, J. A meta-analysis of web-delivered tailored health behavior change interventions. J. Health Commun. 2013, 18, 1039–1069. [Google Scholar] [CrossRef]
| Characteristic | Overall (n = 246) | Control (n = 142) | Treatment (n = 104) | p-Value 2 |
|---|---|---|---|---|
| n (% 1) | n (%) | n (%) | ||
| Language | ||||
| English | 53 (21.5) | 36 (25.4) | 17 (16.3) | 0.09 |
| Spanish | 193 (78.5) | 106 (74.6) | 87 (83.7) | |
| Parent COVID-19 Vaccination Status | ||||
| Vaccinated | 176 (71.5) | 96 (67.6) | 80 (76.9) | 0.28 |
| Not Vaccinated | 65 (26.4) | 43 (30.3) | 22 (21.2) | |
| Unsure | 5 (2.0) | 3 (2.1) | 2 (1.9) | |
| Ethnicity | ||||
| Not Hispanic/Latino/Spanish Origin | 6 (2.4) | 4 (2.8) | 2 (1.9) | 0.85 |
| Mexican/Mexican American/Chicano | 189 (76.8) | 110 (77.5) | 79 (76.0) | |
| Other Hispanic/Latino/Spanish Origin 3 | 49 (19.9) | 27 (19.0) | 22 (21.2) | |
| Missing | 2 (0.8) | 1 (0.7) | 1 (1.0) | |
| Born in the U.S. | ||||
| Yes | 51 (20.7) | 39 (27.5) | 12 (11.5) | 0.01 |
| No | 176 (71.5) | 92 (64.8) | 84 (80.8) | |
| Prefer Not to Respond | 19 (7.7) | 11 (7.7) | 8 (7.7) | |
| Highest Education Attained | ||||
| Some High School or Less | 113 (45.9) | 59 (41.5) | 54 (51.9) | 0.21 |
| High School Graduate/GED | 72 (29.3) | 47 (33.1) | 25 (24.0) | |
| Some College or More | 61 (24.8) | 36 (25.4) | 25 (24.0) | |
| Employment Status | ||||
| Employed | 89 (36.2) | 57 (40.1) | 32 (30.8) | 0.24 |
| Unemployed | 37 (15.0) | 22 (15.5) | 15 (14.4) | |
| Other 4 | 111 (45.1) | 57 (40.1) | 54 (51.9) | |
| Do Not Know/Prefer Not to Respond | 8 (3.3) | 6 (4.2) | 2 (1.9) | |
| Missing | 1 (0.4) | 0 (0.0) | 1 (1.0) | |
| Household Income | ||||
| <USD 25,000 | 180 (73.2) | 104 (73.2) | 76 (73.1) | 0.87 |
| USD 25,000–USD 49,000 | 41 (16.7) | 24 (16.9) | 17 (16.3) | |
| >USD 50,000 | 23 (9.3) | 12 (8.5) | 11 (10.6) | |
| Missing | 2 (0.8) | 2 (1.4) | --- | |
| Health Insurance Status | ||||
| Insured 5 | 158 (64.2) | 97 (68.3) | 61 (58.7) | 0.23 |
| Not Insured | 56 (22.8) | 27 (19.0) | 29 (27.9) | |
| Do Not Know/Prefer Not to Respond | 32 (13.0) | 18 (12.7) | 14 (13.5) | |
| Marital Status | ||||
| Currently Married | 123 (50.0) | 62 (43.7) | 61 (58.7) | 0.13 |
| Cohabitation (Common Law Marriage) | 46 (18.7) | 30 (21.1) | 16 (15.4) | |
| Widowed/Divorced/Separated | 28 (11.4) | 17 (12.0) | 11 (10.6) | |
| Never Married | 49 (19.9) | 33 (23.2) | 16 (15.4) | |
| Type of Household | ||||
| Married With Children | 143 (58.1) | 74 (52.1) | 69 (66.3) | 0.18 |
| Single/Married Without Children | 13 (5.3) | 10 (7.0) | 3 (2.9) | |
| Single With Children | 34 (13.8) | 21 (14.8) | 13 (12.5) | |
| Other | 24 (9.8) | 17 (12.0) | 7 (6.7) | |
| Do Not Know/Prefer Not to Respond | 32 (13.0) | 20 (14.1) | 12 (11.5) | |
| Any Minors in Household Under 2 | ||||
| Yes | 32 (13.0) | 15 (10.6) | 17 (16.3) | 0.21 |
| No | 214 (87.0) | 127 (89.4) | 87 (83.7) | |
| Any Minors in Household 2–11 Years | ||||
| Yes | 209 (85.0) | 121 (85.2) | 88 (84.6) | 0.51 |
| No | 37 (15.0) | 21 (14.8) | 16 (15.4) | |
| Any Minors in Household 12–17 Years | ||||
| Yes | 133 (54.1) | 71 (50.0) | 62 (59.6) | 0.20 |
| No | 113 (45.9) | 71 (50.0) | 42 (40.4) | |
| Other (continuous variables) | ||||
| Age of Parent (Mean, SD) 6,7 | 39.2 (8.8) | 38.8 (9.5) | 39.7 (7.7) | 0.43 |
| Number of Minors in Household (Mean, SD) | 2.2 (1.0) | 2.2 (0.8) | 2.3 (1.1) | 0.23 |
| Baseline | 1-Month Follow-Up | Change, Δ (95% CI) | p-Value | |
|---|---|---|---|---|
| Intention to Vaccinate Minors 2–11 years old | ||||
| Control | 71.0% | 75.4% | 4.4% (−1.6%, 10.4%) | 0.15 |
| Tx | 71.6% | 88.4% | 16.8% (7.7%, 25.8%) | <0.001 |
| Difference | 12.4% (1.5%, 23.2%) | 0.03 | ||
| Vaccination of Minors 12–17 years old | ||||
| Control | 52.1% | 67.6% | 15.5% (7.1%, 23.9%) | <0.001 |
| Tx | 50.0% | 80.7% | 30.7% (19.2%, 42.1%) | <0.001 |
| Difference | 15.2% (0.9%, 29.4%) | 0.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellon-Lopez, Y.M.; Klomhaus, A.M.; Garcia, C.; Marquez, D.; Avila, H.; Gravette, H.; Lopez-Chang, R.; Ortega, B.; Norris, K.C.; Brown, A.F.; et al. MivacunaLA (MyshotLA): A Community-Partnered Mobile Phone Intervention to Improve COVID-19 Vaccination Behaviors among Low-Income, Spanish-Speaking, and Immigrant Latino Parents or Caregivers. Vaccines 2024, 12, 511. https://doi.org/10.3390/vaccines12050511
Castellon-Lopez YM, Klomhaus AM, Garcia C, Marquez D, Avila H, Gravette H, Lopez-Chang R, Ortega B, Norris KC, Brown AF, et al. MivacunaLA (MyshotLA): A Community-Partnered Mobile Phone Intervention to Improve COVID-19 Vaccination Behaviors among Low-Income, Spanish-Speaking, and Immigrant Latino Parents or Caregivers. Vaccines. 2024; 12(5):511. https://doi.org/10.3390/vaccines12050511
Chicago/Turabian StyleCastellon-Lopez, Yelba M., Alexandra M. Klomhaus, Cruz Garcia, Denise Marquez, Hilda Avila, Hannah Gravette, Ray Lopez-Chang, Brenda Ortega, Keith C. Norris, Arleen F. Brown, and et al. 2024. "MivacunaLA (MyshotLA): A Community-Partnered Mobile Phone Intervention to Improve COVID-19 Vaccination Behaviors among Low-Income, Spanish-Speaking, and Immigrant Latino Parents or Caregivers" Vaccines 12, no. 5: 511. https://doi.org/10.3390/vaccines12050511
APA StyleCastellon-Lopez, Y. M., Klomhaus, A. M., Garcia, C., Marquez, D., Avila, H., Gravette, H., Lopez-Chang, R., Ortega, B., Norris, K. C., Brown, A. F., & Blanco, L. (2024). MivacunaLA (MyshotLA): A Community-Partnered Mobile Phone Intervention to Improve COVID-19 Vaccination Behaviors among Low-Income, Spanish-Speaking, and Immigrant Latino Parents or Caregivers. Vaccines, 12(5), 511. https://doi.org/10.3390/vaccines12050511







