Drivers of the Intention to Receive a COVID-19 Booster Vaccine: Insights from the UK and Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Design
2.2. Procedure
2.3. Survey
2.3.1. Primary Outcome: Booster Vaccine Intention
2.3.2. Predictor Variables: Both Surveys
COVID-19 Virus and Vaccine Perceptions
Months since Last COVID-19 Vaccine
Previous Vaccine Side Effects
General Familiarity with COVID-19 Vaccine Side Effects
Previous Vaccine Type
Age
2.3.3. Predictor Variables: UK Survey
Experience with the COVID-19 Virus
2.3.4. Predictor Variables: Australian Survey
Experience with the Influenza Vaccine
Status as Immunocompromised
2.4. Statistical Analysis and Sample Size
3. Results
3.1. Descriptive Statistics
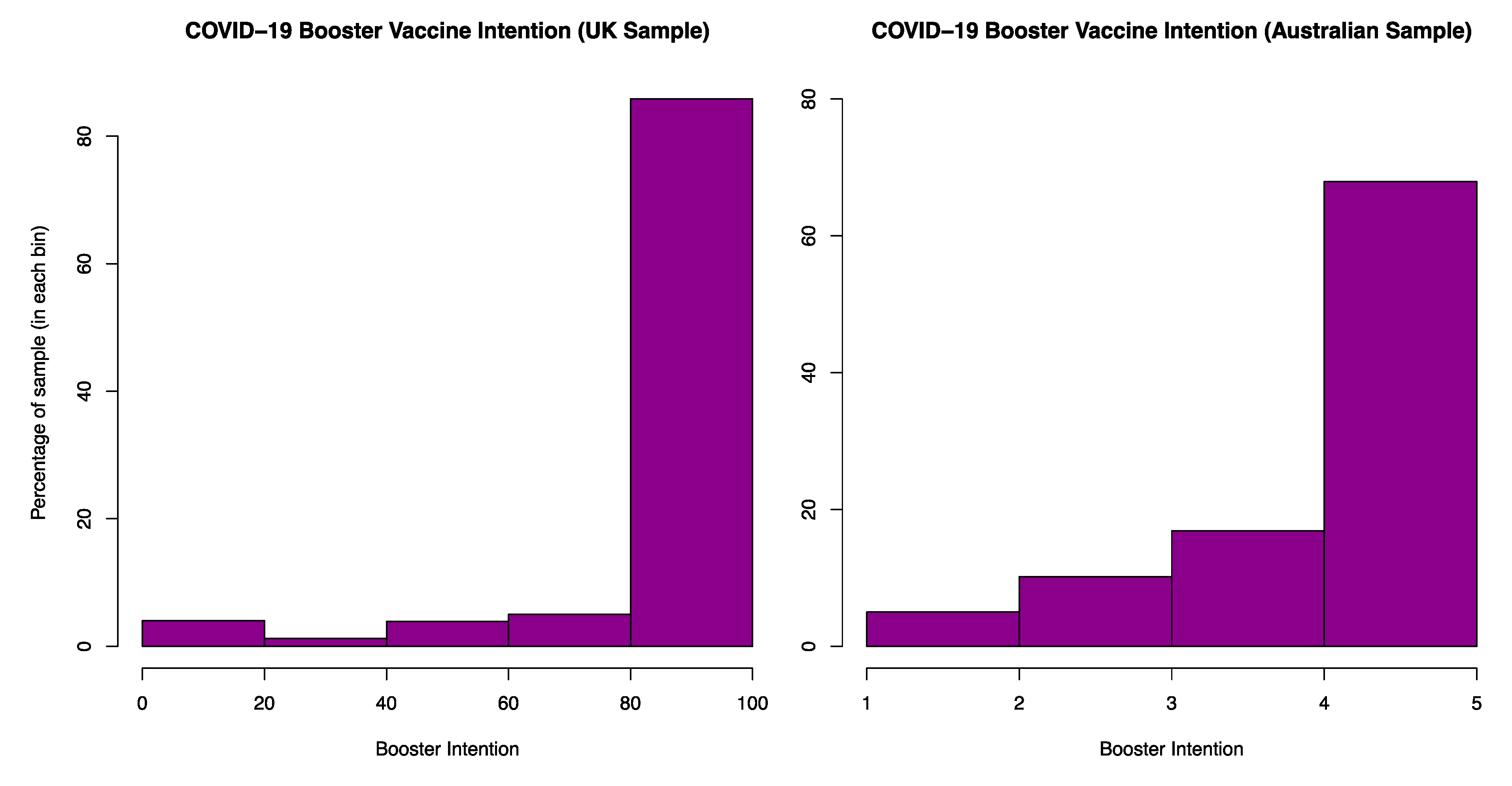
3.2. Vaccine Intention: UK and Australia
3.3. Latent Variables: Psychological Perceptions of the COVID-19 Virus and Vaccine
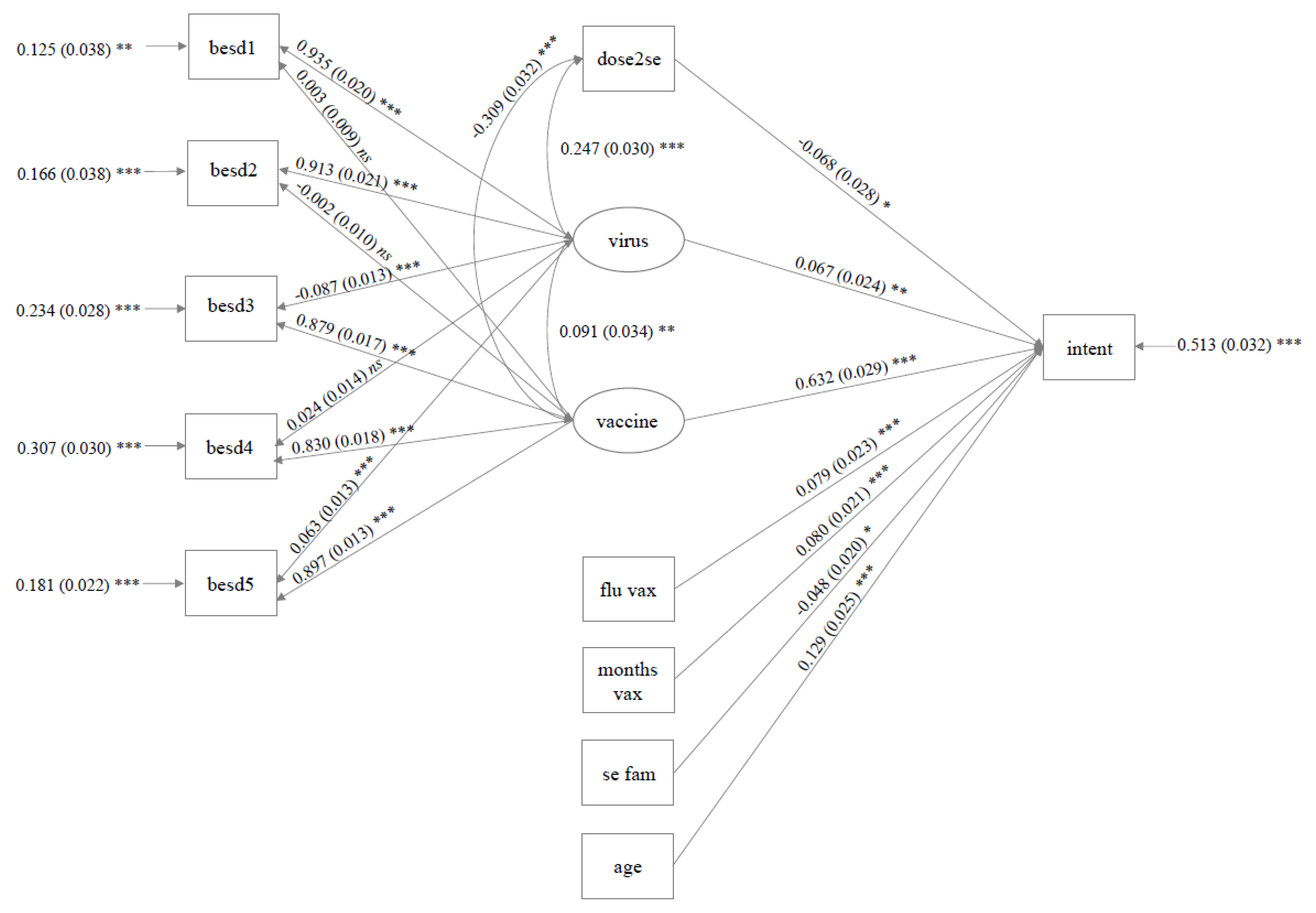
3.4. Primary Analysis
3.4.1. ESEM UK Sample
3.4.2. ESEM Australian Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Universal Coronavirus Vaccines—An Urgent Need. N. Engl. J. Med. 2022, 386, 297–299. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Abu-Raddad, L.J. Waning effectiveness of COVID-19 vaccines. Lancet 2022, 399, 771–773. [Google Scholar] [CrossRef]
- Duarte, N.; Yanes-Lane, M.; Arora, R.K.; Bobrovitz, N.; Liu, M.; Bego, M.G.; Langlois, M.A. Adapting Serosurveys for the SARS-CoV-2 Vaccine Era. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2022; Volume 9, p. ofab632. [Google Scholar]
- Wald, A. Booster Vaccination to Reduce SARS-CoV-2 Transmission and Infection. JAMA 2022, 327, 327–328. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab. Open 2022, 14, 100180. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Primary COVID-19 vaccine cycle and booster doses efficacy: Analysis of Italian nationwide vaccination campaign. Eur. J. Public Health 2022, 32, 328–330. [Google Scholar] [CrossRef]
- Harrison, E.A.; Wu, J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020, 35, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, S.B.; Salmon, D.A.; Orenstein, W.A.; de Hart, M.P.; Halsey, N. Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases. N. Engl. J. Med. 2009, 360, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Chapman, G.B.; Rothman, A.J.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science into Action. Psychol. Sci. Public Interes. 2017, 18, 149–207. [Google Scholar] [CrossRef] [Green Version]
- Nindrea, R.D.; Usman, E.; Katar, Y.; Sari, N.P. Acceptance of COVID-19 vaccination and correlated variables among global populations: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 2021, 12, 100899. [Google Scholar] [CrossRef] [PubMed]
- Fajar, J.K.; Sallam, M.; Soegiarto, G.; Sugiri, Y.J.; Anshory, M.; Wulandari, L.; Kosasih, S.A.P.; Ilmawan, M.; Kusnaeni, K.; Fikri, M.; et al. Global Prevalence and Potential Influencing Factors of COVID-19 Vaccination Hesitancy: A Meta-Analysis. Vaccines 2022, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Patwary, M.M.; Alam, A.; Bardhan, M.; Disha, A.S.; Haque, Z.; Billah, S.M.; Kabir, P.; Browning, M.H.E.M.; Rahman, M.; Parsa, A.D.; et al. COVID-19 Vaccine Acceptance among Low- and Lower-Middle-Income Countries: A Rapid Systematic Review and Meta-Analysis. Vaccines 2022, 10, 427. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Jin, H.; Lin, L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev. Med. 2021, 150, 106694. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Katsiroumpa, A.; Siskou, O.; Konstantakopoulou, O.; Katsoulas, T.; Mariolis-Sapsakos, T.; Kaitelidou, D. First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors. Vaccines 2022, 10, 1097. [Google Scholar] [CrossRef]
- Barnes, K.; Colagiuri, B. Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines. Vaccines 2022, 10, 962. [Google Scholar] [CrossRef]
- Barnes, K.; Faasse, K.; Colagiuri, B. The impact of side effect framing on COVID-19 booster vaccine intentions in an Australian sample. medRxiv 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.05.09.22274840v1 (accessed on 1 October 2022).
- World Health Organisation. Data for Action: Achieving High Uptake of COVID-19 Vaccines: Gathering and Using Data on the Behavioural and Social Drivers of Vaccination: A Guidebook for Immunization Programmes and Implementing Partners: Interim Guidance. Available online: https://apps.who.int/iris/handle/10665/340645 (accessed on 1 April 2021).
- Geers, A.L.; Clemens, K.S.; Colagiuri, B.; Jason, E.; Colloca, L.; Webster, R.; Vase, L.; Seig, M.; Faasse, K. Do Side Effects to the Primary COVID-19 Vaccine Reduce Intentions for a COVID-19 Vaccine Booster? Ann. Behav. Med. 2022, 56, 761–768. [Google Scholar] [CrossRef]
- Tan, W.; Colagiuri, B.; Barnes, K. Factors Moderating the Link between Personal Recounts of COVID-19 Vaccine Side Effects Viewed on Social Media and Viewer Postvaccination Experience. Vaccines 2022, 10, 1611. [Google Scholar] [CrossRef]
- Chu, H.; Liu, S. Integrating health behavior theories to predict American’s intention to receive a COVID-19 vaccine. Patient Educ. Couns. 2021, 104, 1878–1886. [Google Scholar] [CrossRef]
- van Zyl, L.E.; ten Klooster, P.M. Exploratory Structural Equation Modeling: Practical Guidelines and Tutorial with a Convenient Online Tool for Mplus. Front. Psychiatry 2022, 12, 795672. [Google Scholar] [CrossRef]
- Morin, A.J.S.; Myers, N.D.; Lee, S. Modern Factor Analytic Techniques. In Handbook of Sport Psychology; Tenenbaum, G., Eklund, R.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1044–1073. [Google Scholar] [CrossRef]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research; The Guilford Press Publications: New York, NY, USA, 2015. [Google Scholar]
- Browne, M.W.; Cudek, R. Alternative Ways of Assessing Model Fit. In Testing Structural Equation Models; Bollen, K., Long, J.S., Eds.; Sage Publications: Thousand Oaks, CA, USA, 1993; pp. 136–162. [Google Scholar]
- Jöreskog, K.G.; Sörbom, D. LISREL 8: Structural Equation Modeling with the SIMPLIS Command Language; Lawrence Erlbaum Associates Publishers: Hillsdale, NJ, USA, 1993. [Google Scholar]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Bentler, P.M.; Bonett, D.G. Significance tests and goodness of fit in the analysis of covariance structures. Psychol. Bull. 1980, 88, 588–606. [Google Scholar] [CrossRef]
- Satorra, A.; Bentler, P.M. Ensuring Positiveness of the Scaled Difference Chi-square Test Statistic. Psychometrika 2010, 75, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehal, K.R.; Steendam, L.M.; Ponce, M.C.; van der Hoeven, M.; Smit, G.S.A. Worldwide Vaccination Willingness for COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Costa, D.; Costa, D.; Keating, J.; Arantes, J. Predicting COVID-19 Vaccination Intention: The Determinants of Vaccine Hesitancy. Vaccines 2021, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Faasse, K.; Newby, J. Public Perceptions of COVID-19 in Australia: Perceived Risk, Knowledge, Health-Protective Behaviors, and Vaccine Intentions. Front. Psychol. 2020, 11, 551004. [Google Scholar] [CrossRef]
- Sherman, S.M.; Smith, L.E.; Sim, J.; Amlôt, R.; Cutts, M.; Dasch, H.; Rubin, G.J.; Sevdalis, N. COVID-19 vaccination intention in the UK: Results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum. Vaccines Immunother. 2020, 17, 1612–1621. [Google Scholar] [CrossRef]
- Mertens, G.; Lodder, P.; Smeets, T.; Duijndam, S. Fear of COVID-19 predicts vaccination willingness 14 months later. J. Anxiety Disord. 2022, 88, 102574. [Google Scholar] [CrossRef]
- Hagger, M.S.; Hamilton, K. Predicting COVID-19 booster vaccine intentions. Appl. Psychol. Health Well-Being 2022, 14, 819–841. [Google Scholar] [CrossRef]
- Tan, K.Y.K.; Soh, A.S.E.; Ong, B.W.L.; Chen, M.I.; Griva, K. Determining the Prevalence and Correlates of COVID-19 Booster Vaccine Hesitancy in the Singapore Population Following the Completion of the Primary Vaccination Series. Vaccines 2022, 10, 1088. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2021, 9, 16. [Google Scholar] [CrossRef]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.-L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef] [Green Version]
- Wilder-Smith, A.B.; Qureshi, K. Resurgence of Measles in Europe: A Systematic Review on Parental Attitudes and Beliefs of Measles Vaccine. J. Epidemiol. Glob. Health 2019, 10, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, W.; Stoker, G.; Willis, H.; Valgardsson, V.; Gaskell, J.; Devine, D.; Mills, M.C. Lack of trust and social media echo chambers predict COVID-19 vaccine hesitancy. medRxiv 2021. [Google Scholar] [CrossRef]
- Robertson, E.; Reeve, K.S.; Niedzwiedz, C.L.; Moore, J.; Blake, M.; Green, M.; Katikireddi, S.V.; Benzeval, M.J. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain, Behav. Immun. 2021, 94, 41–50. [Google Scholar] [CrossRef]
- Palm, R.; Bolsen, T.; Kingsland, J.T. The Effect of Frames on COVID-19 Vaccine Resistance. Front. Polit. Sci. 2021, 3, 661257. [Google Scholar] [CrossRef]
- Witus, L.S.; Larson, E. A randomized controlled trial of a video intervention shows evidence of increasing COVID-19 vaccination intention. PLoS ONE 2022, 17, e0267580. [Google Scholar] [CrossRef]
- Argote Tironi, P.; Barham, E.; Zuckerman Daly, S.; Gerez, J.E.; Marshall, J.; Pocasangre, O. Messages that increase COVID-19 vaccine acceptance: Evidence from online experiments in six Latin American countries. PLoS ONE 2021, 16, e0259059. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Loe, B.S.; Yu, L.M.; Freeman, J.; Chadwick, A.; Vaccari, C.; Lambe, S. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): A single-blind, parallel-group, randomised controlled trial. Lancet Public Health 2021, 6, e416–e427. [Google Scholar] [CrossRef]
- Sinclair, S.; Agerström, J. Do Social Norms Influence Young People’s Willingness to Take the COVID-19 Vaccine? Health Commun. 2021. [Google Scholar] [CrossRef]
- Davis, C.J.; Golding, M.; McKay, R. Efficacy information influences intention to take COVID-19 vaccine. Br. J. Health Psychol. 2022, 27, 300–319. [Google Scholar] [CrossRef]
- Ashworth, M.; Thunström, L.; Cherry, T.L.; Newbold, S.C.; Finnoff, D.C. Emphasize personal health benefits to boost COVID-19 vaccination rates. Proc. Natl. Acad. Sci. USA 2021, 118, e2108225118. [Google Scholar] [CrossRef]
- Kachurka, R.; Krawczyk, M.; Rachubik, J. Persuasive Messages Will Not Increase COVID-19 Vaccine Acceptance: Evidence from a Nationwide Online Experiment. Vaccines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Thorpe, A.; Fagerlin, A.; Butler, J.; Stevens, V.; Drews, F.A.; Shoemaker, H.; Scherer, L.D. Communicating about COVID-19 vaccine development and safety. PLoS ONE 2022, 17, e0272426. [Google Scholar] [CrossRef] [PubMed]
- Merkley, E.; Loewen, P.J. Assessment of Communication Strategies for Mitigating COVID-19 Vaccine-Specific Hesitancy in Canada. JAMA Netw. Open 2021, 4, e2126635. [Google Scholar] [CrossRef]
- Motta, M.; Sylvester, S.; Callaghan, T.; Lunz-Trujillo, K. Encouraging COVID-19 Vaccine Uptake Through Effective Health Communication. Front. Polit. Sci. 2021, 3, 1. [Google Scholar] [CrossRef]
- Batteux, E.; Mills, F.; Jones, L.F.; Symons, C.; Weston, D. The Effectiveness of Interventions for Increasing COVID-19 Vaccine Uptake: A Systematic Review. Vaccines 2022, 10, 386. [Google Scholar] [CrossRef]
- Berliner Senderey, A.B.; Ohana, R.; Perchik, S.; Erev, I.; Balicer, R. Encouraging Uptake of the COVID-19 Vaccine Through Behaviorally Informed Interventions: National Real-World Evidence from Israel. SSRN. 2021. Available online: Ssrn.com/abstract=3852345 (accessed on 1 October 2021).
- Santos, H.C.; Goren, A.; Chabris, C.F.; Meyer, M.N. Effect of Targeted Behavioral Science Messages on COVID-19 Vaccination Registration Among Employees of a Large Health System: A Randomized Trial. JAMA Netw. Open 2021, 4, e2118702. [Google Scholar] [CrossRef]
- Kerr, J.R.; Freeman, A.L.J.; Marteau, T.M.; van der Linden, S. Effect of Information about COVID-19 Vaccine Effectiveness and Side Effects on Behavioural Intentions: Two Online Experiments. Vaccines 2021, 9, 379. [Google Scholar] [CrossRef]
- Paul, E.; Fancourt, D. Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: An observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg. Health-Eur. 2022, 14, 100317. [Google Scholar] [CrossRef] [PubMed]
- Wirawan, G.B.S.; Harjana, N.P.A.; Nugrahani, N.W.; Januraga, P.P. Health Beliefs and Socioeconomic Determinants of COVID-19 Booster Vaccine Acceptance: An Indonesian Cross-Sectional Study. Vaccines 2022, 10, 724. [Google Scholar] [CrossRef]
- Gonsalves, G.; Yamey, G. Political interference in public health science during covid-19. BMJ 2020, 371, m3878. [Google Scholar] [CrossRef]
- Patterson, L. Encouraging covid vaccine uptake and safe behaviours—An uphill struggle against government complacency. BMJ 2021, 375, n2773. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. Offline: Complacency threatens progress against COVID-19. Lancet 2022, 399, 615. [Google Scholar] [CrossRef]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; El Khoury, A.C. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Schmitzberger, F.F.; Scott, K.W.; Nham, W.; Mathews, K.; Schulson, L.; Fouche, S.; Berri, N.; Shehab, A.; Gupta, A.; Salhi, R.A.; et al. Identifying Strategies to Boost COVID-19 Vaccine Acceptance in the United States. Rand Health Q. 2022, 9, 12. [Google Scholar]
- Nan, X.; Iles, I.A.; Yang, B.; Ma, Z. Public Health Messaging during the COVID-19 Pandemic and Beyond: Lessons from Communication Science. Health Commun. 2022, 37, 1–19. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Fal, A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines 2021, 9, 1286. [Google Scholar] [CrossRef]
- Al-Qerem, W.; Al Bawab, A.Q.; Hammad, A.; Ling, J.; Alasmari, F. Willingness of the Jordanian Population to Receive a COVID-19 Booster Dose: A Cross-Sectional Study. Vaccines 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yuan, Y.; Deng, Z.; Yin, D.; Shen, Q.; Zeng, J.; Xie, Y.; Xu, M.; Yang, M.; Jiang, S.; et al. Acceptance of COVID-19 booster vaccination based on the protection motivation theory: A cross-sectional study in China. J. Med. Virol. 2022, 94, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. Npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Haas, J.W.; Bender, F.L.; Ballou, S.; Kelley, J.M.; Wilhelm, M.; Miller, F.G.; Kaptchuk, T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2143955. [Google Scholar] [CrossRef]
- Colloca, L.; Miller, F.G. The nocebo effect and its relevance for clinical practice. Psychosom. Med. 2011, 73, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geers, A.L.; Clemens, K.S.; Faasse, K.; Colagiuri, B.; Webster, R.; Vase, L.; Sieg, M.; Jason, E.; Colloca, L. Psychosocial Factors Predict COVID-19 Vaccine Side Effects. Psychother. Psychosom. 2021, 91, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rief, W. Fear of Adverse Effects and COVID-19 Vaccine Hesitancy: Recommendations of the Treatment Expectation Expert Group. JAMA Health Forum 2021, 2, e210804. [Google Scholar] [CrossRef]
- Attwell, K.; Lake, J.; Sneddon, J.; Gerrans, P.; Blyth, C.; Lee, J. Converting the maybes: Crucial for a successful COVID-19 vaccination strategy. PLoS ONE 2021, 16, e0245907. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Pei, M.; Li, X.; Li, N. Willingness of the General Public to Receive A COVID-19 Vaccine Booster–China, April–May 2021. China CDC Wkly 2022, 4, 66–70. [Google Scholar] [CrossRef]
- Kong, G.; Lim, N.-A.; Chin, Y.H.; Ng, Y.P.M.; Amin, Z. Effect of COVID-19 Pandemic on Influenza Vaccination Intention: A Meta-Analysis and Systematic Review. Vaccines 2022, 10, 606. [Google Scholar] [CrossRef]
- Alley, S.J.; Stanton, R.; Browne, M.; To, Q.G.; Khalesi, S.; Williams, S.L.; Vandelanotte, C. As the Pandemic Progresses, How Does Willingness to Vaccinate against COVID-19 Evolve? Int. J. Environ. Res. Public Health 2021, 18, 797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mathur, M.; Kumar, N.; Rana, R.K.; Tiwary, R.C.; Raghav, P.R.; Kumar, A.; Kapoor, N.; Mathur, M.; Tanu, T.; et al. Understanding the phases of vaccine hesitancy during the COVID-19 pandemic. Isr. J. Health Policy Res. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Siegler, A.J.; Luisi, N.; Hall, E.W.; Bradley, H.; Sanchez, T.; Lopman, B.A.; Sullivan, P.S. Trajectory of COVID-19 Vaccine Hesitancy Over Time and Association of Initial Vaccine Hesitancy with Subsequent Vaccination. JAMA Netw. Open 2021, 4, e2126882. [Google Scholar] [CrossRef] [PubMed]
- Mazar, A.; Tomaino, G.; Carmon, Z.; Wood, W. Distance to Vaccine Sites is Associated with Lower COVID-19 Vaccine Uptake. PsyArXiv 2022. Available online: https://psyarxiv.com/mux5s/ (accessed on 1 October 2022).
- Gerend, M.A.; Shepherd, J.E. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann. Behav. Med. 2012, 44, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.A.; Ruiter, R.A.; Chapman, G.; Kok, G. The intention to get vaccinated against influenza and actual vaccination uptake of Dutch healthcare personnel. Vaccine 2014, 32, 6986–6991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juraskova, I.; Bari, R.A.; O’Brien, M.T.; McCaffery, K.J. HPV Vaccine Promotion: Does Referring to Both Cervical Cancer and Genital Warts Affect Intended and Actual Vaccination Behavior? Women’s Health Issues 2011, 21, 71–79. [Google Scholar] [CrossRef]
- Jensen, U.T.; Ayers, S.; Koskan, A.M. Video-based messages to reduce COVID-19 vaccine hesitancy and nudge vaccination intentions. PLoS ONE 2022, 17, e0265736. [Google Scholar] [CrossRef]
- Sheeran, P. Intention—Behavior Relations: A Conceptual and Empirical Review. Eur. Rev. Soc. Psychol. 2002, 12, 1–36. [Google Scholar] [CrossRef]
- Shi, D.; Maydeu-Olivares, A. The Effect of Estimation Methods on SEM Fit Indices. Educ. Psychol. Meas. 2019, 80, 421–445. [Google Scholar] [CrossRef] [PubMed]

| Demographic Information | ||
|---|---|---|
| UK Sample N (% of Sample) | Australian Sample N (% of Sample) | |
| Age bracket (years) | ||
| 18–24 | 47 (3.8) | 126 (10.5) |
| 25–34 | 116 (9.5) | 235 (19.6) |
| 35–44 | 211 (17.3) | 205 (17.1) |
| 45–54 | 246 (20.1) | 202 (16.9) |
| 55–64 | 315 (25.8) | 179 (15.0) |
| 65+ | 287 (23.5) | 250 (20.9) |
| Gender | ||
| Woman | 706 (57.8) | 603 (50.4) |
| Man | 511 (41.8) | 592 (49.5) |
| Non-binary/other | 5 (0.4) | 2 (0.2) |
| Employment | ||
| Employed full-time | 407 (33.3) | 494 (41.3) |
| Employed part-time | 153 (12.5) | 224 (18.7) |
| Self employed | 98 (8.0) | 45 (3.8) |
| Unemployed (looking) | 38 (3.1) | 62 (5.2) |
| Unemployed (not looking)/long-term sick or disabled | 92 (7.5) | - |
| Parent/Carer | 70 (5.7) | 68 (5.7) |
| Student | 24 (2.0) | 43 (3.6) |
| Retired | 321 (26.3) | 256 (21.4) |
| Other | 19 (1.6) | 5 (0.4) |
| Predictor Variables | ||
| M (SD)/N (%) | M (SD)/N (%) | |
| BeSD1 (How concerned are you about getting COVID-19?) | 50.5 (31.3) | 47.7 (30.4) |
| BeSD2 (How concerned are you about your close family and friends getting COVID-19?) | 60.6 (31.0) | 56.3 (31.3) |
| BeSD3 (How much do you trust the COVID-19 vaccines?) | 80.0 (22.6) | 73.9 (24.6) |
| BeSD4 (How important do you think getting a COVID-19 vaccine will be for your health?) | 77.5 (24.6) | 75.7 (24.7) |
| BeSD5 (How much do you think getting a COVID-19 vaccine for yourself will protect other people in your community?) | 86.1 (22.4) | 81.1 (23.0) |
| Months Vax (Months since last COVID-19 vaccine) | 4.8 (1.5) | 3.4 (2.3) |
| Dose1SE (Previous vaccine side effects—dose 1) | 21.3 (27.9) | 23.3 (25.4) |
| Dose2SE (Previous vaccine side effects—dose 2) | 13.7 (21.7) | 21.7 (25.1) |
| Familiarity (familiarity with COVID-19 vaccine side effects) | 48.2 (28.3) | 52.3 (22.8) |
| Age | 52.5 (14.5) | 47.3 (17.2) |
| Vax status (primary course of vaccination: 0 = AstraZeneca/1 = Pfizer) | AZ = 615 (50.3%) | AZ = 503 (42.0%) |
| COVID Self (personal infection with COVID-19: 0 = no/1 = yes) | yes = 148 (12.1%) | - |
| COVID Other (infection among close others: 0 = no/1 = yes) | yes = 565 (46.2%) | - |
| Flu Vax (previous experience with the flu vaccine: 1 = yearly/0 = other) | - | yearly = 545 (45.5%) |
| Immunocompromised (Are you regarded as immunocompromised by your GP: 0 = no/1 = yes) | - | yes = 117 (9.8%) |
| UK Sample | Australian Sample | |||
|---|---|---|---|---|
| EFA: Model Fit | ||||
| One-Factor | Two-Factor | One-Factor | Two-Factor | |
| CFI | 0.57 | 1.0 | 0.50 | 1.0 |
| TLI | 0.14 | 0.99 | 0.01 | 1.0 |
| SRMR | 0.18 | 0.003 | 0.19 | 0.001 |
| RMSEA | 0.38 | 0.03 | 0.40 | <0.001 |
| Predictive Value of the Variable Pruned from the Model | ||||
|---|---|---|---|---|
| Variable | β | S.E. | p | 95% CI |
| Model Stage 1: Age | −0.01 | 0.03 | 0.78 | [−0.07, 0.06] |
| Model Stage 2: COVID other 1 | −0.01 | 0.02 | 0.70 | [−0.05, 0.03] |
| Model Stage 3: COVID self 2 | −0.01 | 0.02 | 0.64 | [−0.06, 0.04] |
| Model Stage 4: Familiarity 3 | −0.10 | 0.02 | 0.61 | [−0.05, 0.03] |
| Model Stage 5: Dose1SE 4 | 0.03 | 0.03 | 0.43 | [−0.04, 0.09] |
| Model Stage 6: Months vax 5 | 0.03 | 0.03 | 0.19 | [−0.02, 0.09] |
| Improvement in Model Fit after Variable Removal | ||||
| Model Comparison | CD 6 | TRd 7 | Δdf | p (for TRd/Δdf) |
| Full Model vs. Stage 1 | 1.10 | 57.13 | 5 | <0.001 |
| Stage 1 vs. Stage 2 | 0.99 | 9.74 | 5 | 0.083 |
| Stage 2 vs. Stage 3 | 1.25 | 26.91 | 5 | <0.001 |
| Stage 3 vs. Stage 4 | 0.98 | 35.75 | 5 | <0.001 |
| Stage 4 vs. Stage 5 | 1.22 | 23.74 | 5 | <0.001 |
| Stage 5 vs. Stage 6 | 1.07 | 60.72 | 5 | <0.001 |
| Predictive Value of the Variable Pruned from the Model | ||||
|---|---|---|---|---|
| Variable | β | S.E. | p | 95% CI |
| Model Stage 1: Vax Status 1 | −0.004 | 0.03 | 0.89 | [−0.06, 0.06] |
| Model Stage 2: Immunocompromised 2 | −0.02 | 0.02 | 0.30 | [−0.06, 0.02] |
| Model Stage 3: Dose1SE 3 | 0.03 | 0.03 | 0.22 | [−0.02, 0.08] |
| Improvement in model fit after variable removal | ||||
| Model Comparison | CD 4 | TRd 5 | Δdf | p (for TRd/Δdf) |
| Full Model vs. Stage 1 | 1.06 | 24.10 | 5 | <0.001 |
| Stage 1 vs. Stage 2 | 1.00 | 20.55 | 5 | 0.001 |
| Stage 2 vs. Stage 3 | 1.09 | 37.31 | 5 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, K.; Colagiuri, B. Drivers of the Intention to Receive a COVID-19 Booster Vaccine: Insights from the UK and Australia. Vaccines 2022, 10, 1730. https://doi.org/10.3390/vaccines10101730
Barnes K, Colagiuri B. Drivers of the Intention to Receive a COVID-19 Booster Vaccine: Insights from the UK and Australia. Vaccines. 2022; 10(10):1730. https://doi.org/10.3390/vaccines10101730
Chicago/Turabian StyleBarnes, Kirsten, and Ben Colagiuri. 2022. "Drivers of the Intention to Receive a COVID-19 Booster Vaccine: Insights from the UK and Australia" Vaccines 10, no. 10: 1730. https://doi.org/10.3390/vaccines10101730





