A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Olive Leaf Extracts
- Ethanol/H2O (80:20 v/v %)
- Methanol/H2O (80:20 v/v %)
- Diethyl ether/H2O (80:20 v/v %)
- Hexanol/H2O (80:20 v/v %)
2.2. Determination of Total Phenolic Compounds
2.3. Ferric Thiocyanate
- % Antioxidant activity = 100 − (A/B) × 100
- A: Absorbance of thesample
- B: Absorbance of thecontrol
2.4. Ferric Reducing Antioxidant Power
- % Ferric reducing antioxidant power = 100 − (As/Ac) × 100
- As = absorbance of the sample
- Ac = absorbance of the control
2.5. Free Radical Scavenging Activity
- % Inhibition of DPPH activity= [Ac − As/Ac ] × 100
- Ac: absorbance of the control
- As: absorbance of the sample
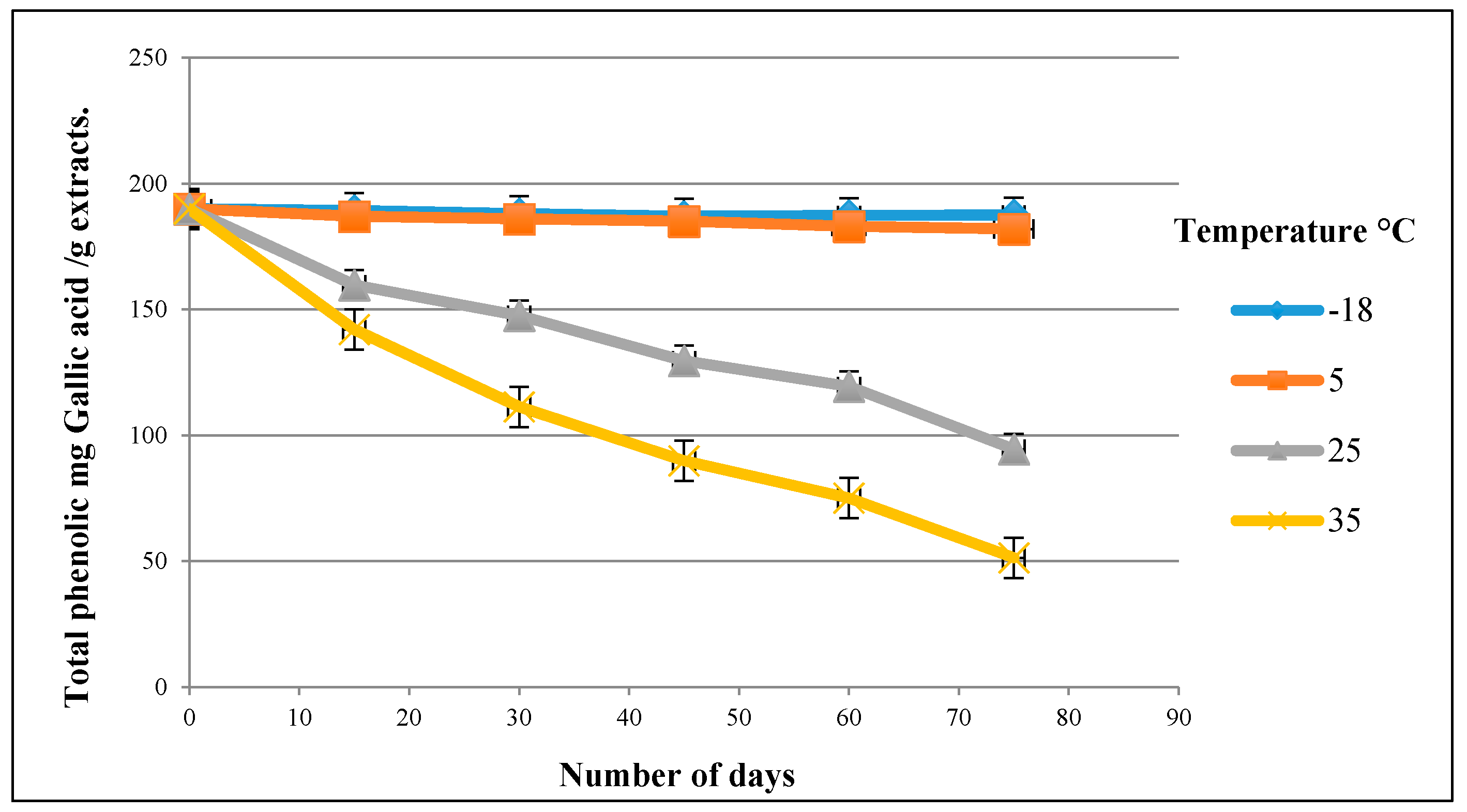
2.6. Studying the Stability of the Olive Leaf Extract
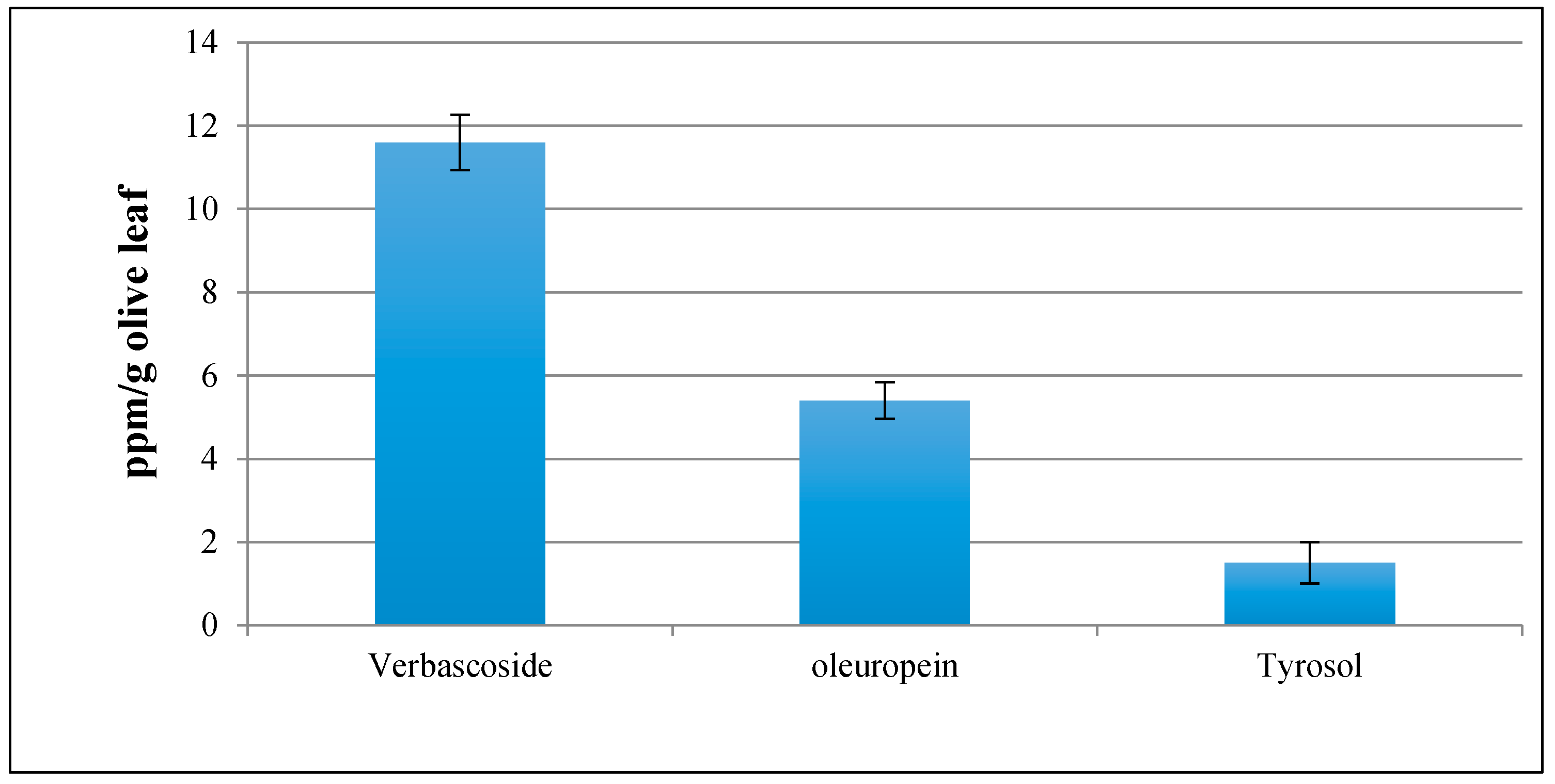
2.7. Determination of the Olive Leaf Extract Profile
2.8. Application of the Olive Leaf Extract on the Sheep Meat Slides
2.8.1. Preparation of Sheep Meat Samples
2.8.2. pH Determination of the Sheep Meat Slides
2.9. Determination of Oxidative Stability of the Sheep Meat Slides
2.9.1. Thiobarbituric Acid (TBA) Assay
2.9.2. Antimicrobial Activity of the Olive Leaf Extract on Sheep Meat Slides
2.10. Statistical Data Analysis
3. Results and Discussion
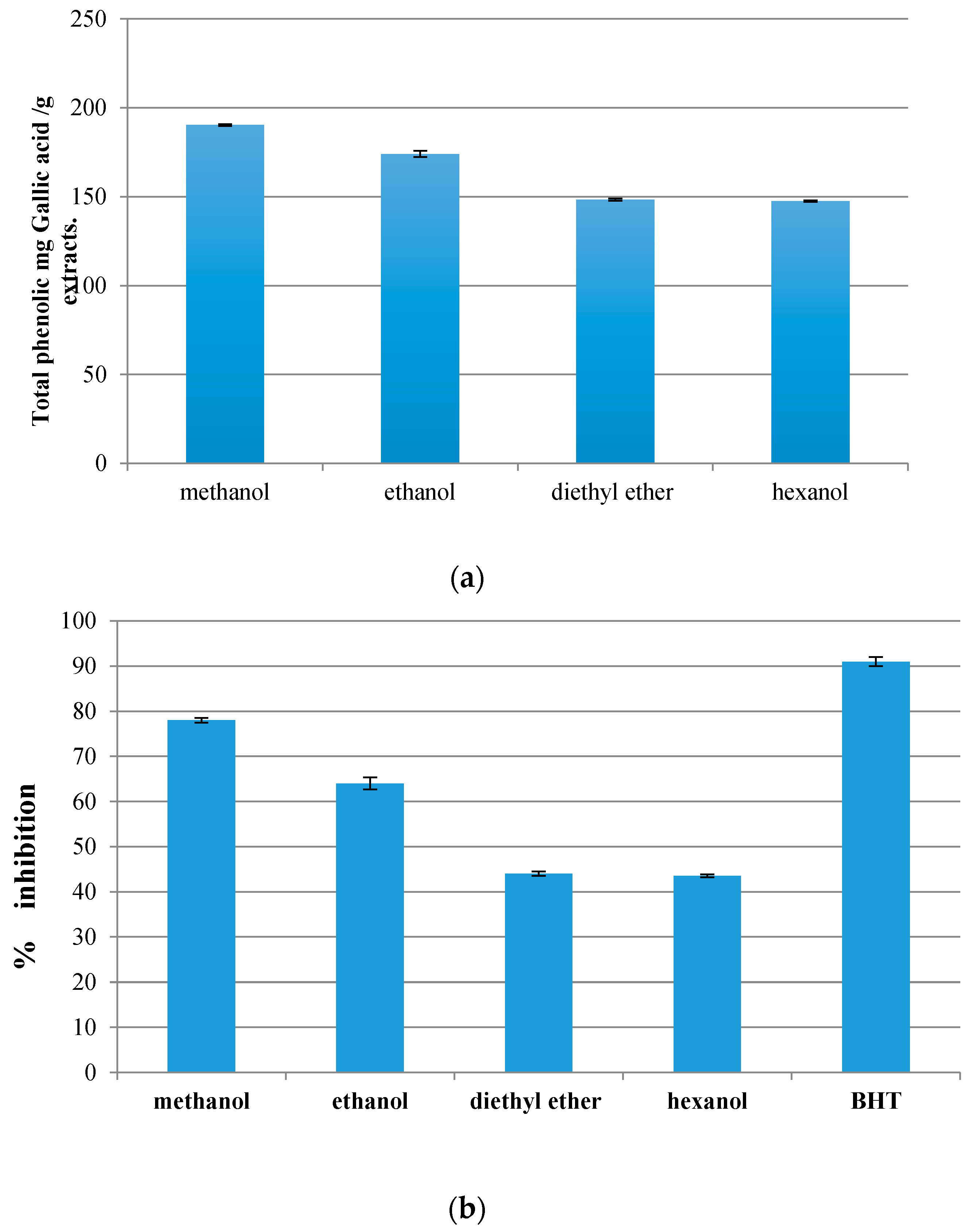
3.1. Total Phenolic Contents
3.2. Antioxidant Properties
3.2.1. Ferric Thiocyanate Method
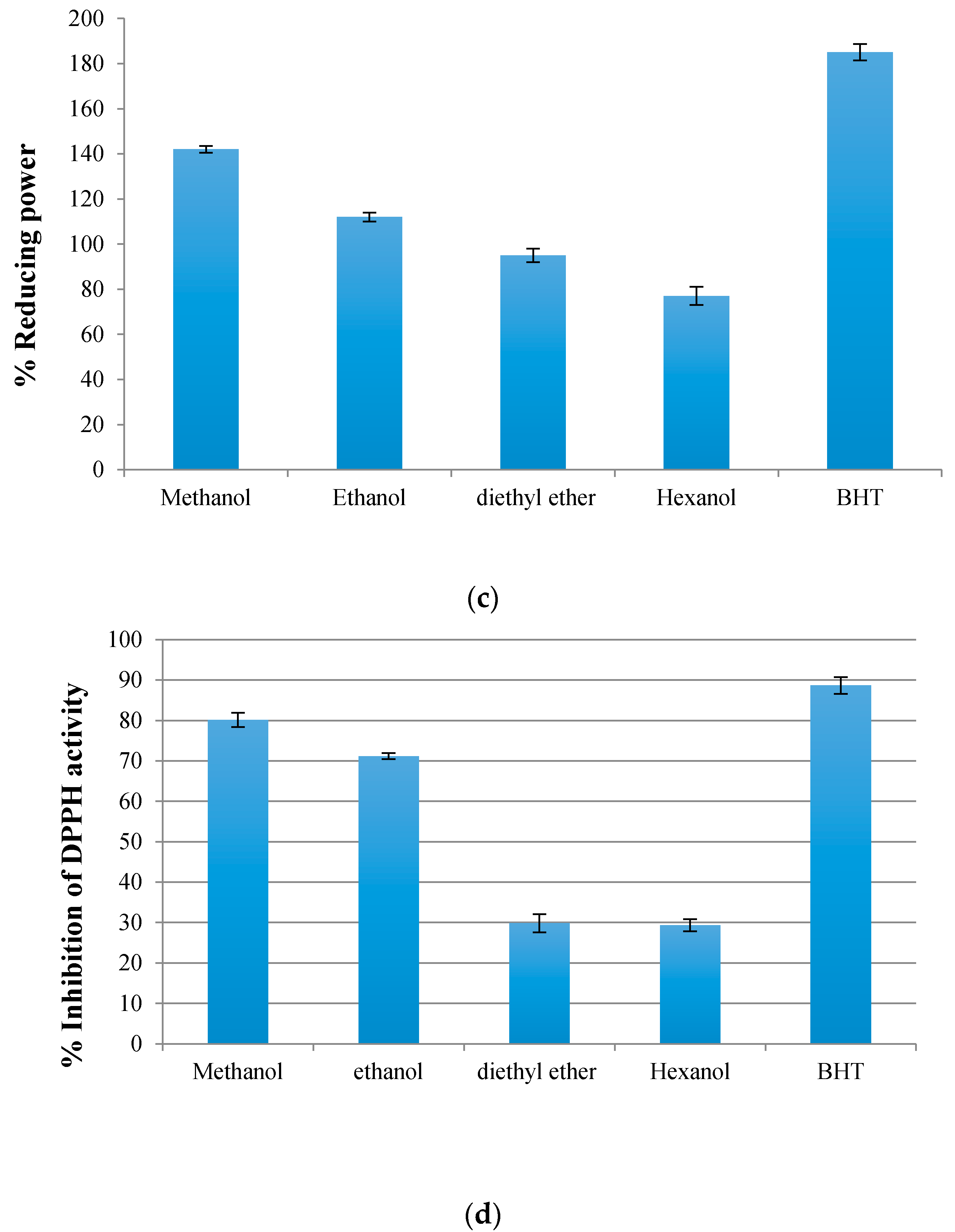
3.2.2. Total Reducing Power
3.2.3. DPPH Radical Scavenging Assay
3.3. Comparison between Total Phenolics Compounds’ and Antioxidant Activities’ Methods Performance
3.4. Stability of the Olive Leaf Extract
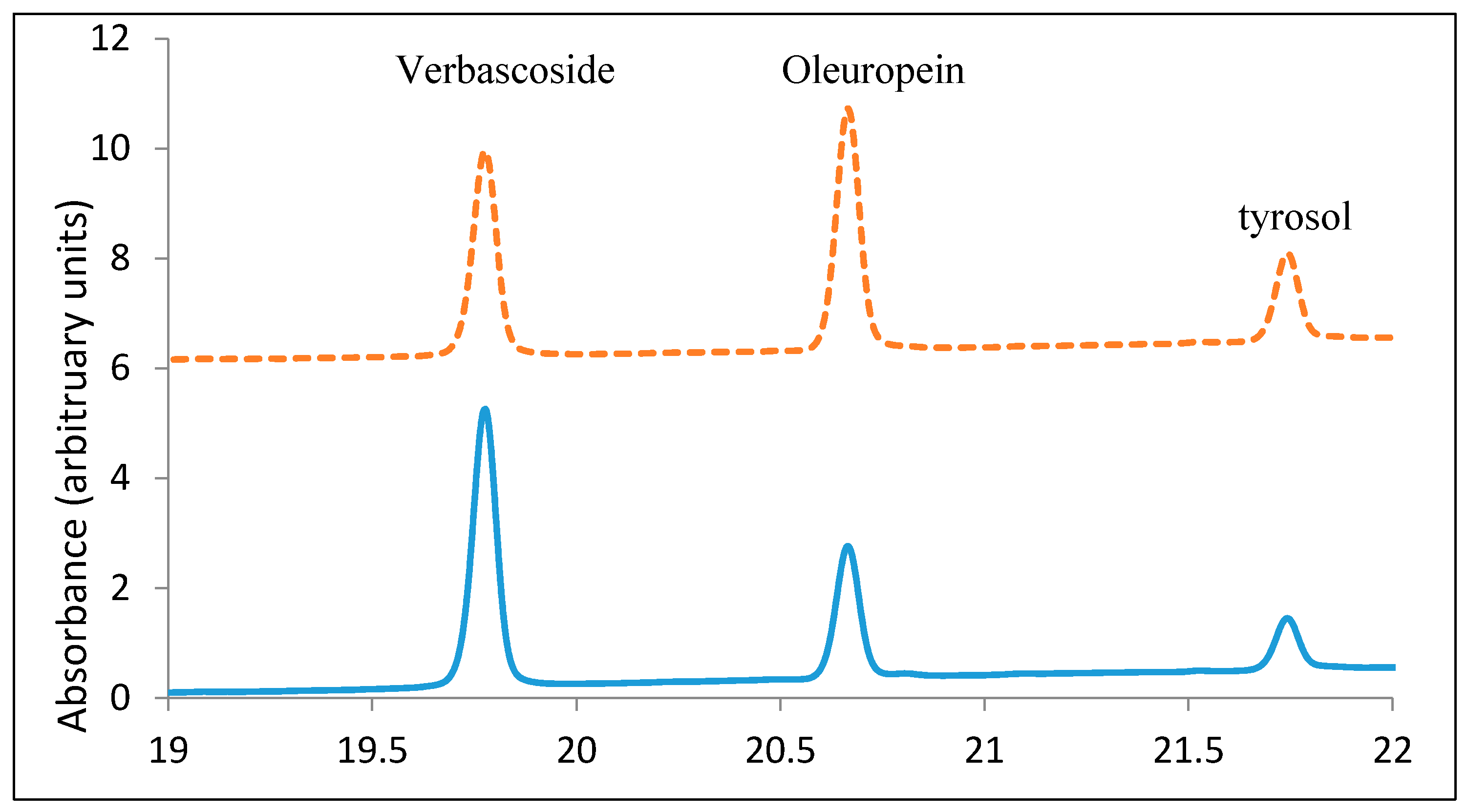
3.5. Olive Leaf Extract Profile
3.6. Application of the Olive Leaf Extract on Sheep Meat Slides
pH Determination of the Sheep Meat Slides
3.7. Determination of Oxidative Stability of Sheep Meat Slides
Thiobarbituric Acid (TBA) Assay
3.8. Antimicrobial Activity of the Olive Leaf Extract on Total Count Bacteria of the Sheep Meat Slides
3.9. Antimicrobial Activity of the Olive Leaf Extract on Total Coliform Bacteria of the Sheep Meat Slides
4. Conclusions
Conflicts of Interest
References
- Taha, F. The development of olive cultivation. In General Company for Forestry and Horticulture; Ministry of Agriculture: Karradah AL-Sharkiya Baghdad, Iraq, 2007. [Google Scholar]
- De Leonardis, A.; Acetini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol rich extract from olive leaves (Olea europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Medina, I.; Gllardo, J.M.; Gonzalez, M.J.; Lois, S.; Hedges, N. Effect of Molecular Structure of Phenolic Families as Hydroxycinnamic Acid and Catechins on their Antioxidant Effectiveness in Minced Fish Muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Ishioroshi, M.; Samejima, K. Antioxidant and antimicrobial effects of garlic in chicken sausage. Lebenson Wiss Technol. 2004, 37, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arraez-Roman, D.; Zarrouk, M.; Valverde, J.; Segura-Carretero, A.; Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; et al. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243. [Google Scholar]
- Gharavi, N.; Haggarty, S.; El-Kadi, A. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr. Drug. Metab. 2007, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Loliger, J.; Lambelet, P.; Aeschbach, R.; Prior, E.M. Natural antioxidants: from radical mechanisms to food stabilization. In Food Lipids and Health; McDonald, R.E., Min, D.B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 68–77. [Google Scholar]
- Pokorny, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food Practical Applications; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Gkanatsiou, K.; Kiritsakis, A.; Kiritsakis, K. The effect of saffron extracts (Crocus Sativus L.). In Preventing the Oxidation of Oils, Vol. 2; Hellenic Association of Food Technologists and Alexander Technological Educational Institute of Thessaloniki: Thessaloniki, Greece, 2007; pp. 595–604. [Google Scholar]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, M.S.Y. Effect of Olive Leaf Extracts on the Growth and Metabolism of Two Probiotic Bacteria of Intestinal Origin. Pak. J. Nutr. 2010, 9, 787–793. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Carotenoids, and the Antioxidant Properties of a Wide Selection of Fijian Fruit, Vegetables and Other Readily Available Foods. Food Chem. 2007, 101, 1727–1741. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Yateem, H.; Afaneh, I.; Al-Rimawi, F. Optimum conditions for oleuropein extraction from olive leaves. Int. J. Appl. 2014, 4, 153–157. [Google Scholar]
- Aytul, K.K. Antimicrobial and Antioxidant Activities of Olive Leaf Extract and Its Food Applications. MSc. Thesis, Engineering and Sciences of Izmir Institute of Technology, Izmir, Turkey, 12 July 2010. [Google Scholar]
- Bekhit, A.E.D.; Geesink, G.H.; Ilian, M.A.; Morton, J.D.; Bickerstaffe, R. The Effects of Natural Antioxidants on Oxidative Process and Metmyoglobin Reducing Activity in Sheep meat Patties. Food Chem. 2003, 81, 175–187. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of Minimum Inhibition Concentrations. J. Antimic. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Ilango, K.; Chitra, V.; Kanimozhi, P.; Balaji, G. Antidiabetic, Antioxidant and Antibacterial Activities of Leaf extracts of Adhatoda zeylanica. Medic (Acanthaceae). J. Pharm. Sci. Res. 2009, 1, 67–73. [Google Scholar]
- Aliyu, A.B.; Ibrahim, M.A.; Musa, A.M.; Ibrahim, H.; Abdulkadir, I.E.; Oyewale, A.O. Evaluation of antioxidant activity of leave extract of Bauhinia rufescens Lam. (Caesalpiniaceae). J. Med. Plants Res. 2009, 3, 563–567. [Google Scholar]
- Hamad, I. Antioxidant activity and potential Hepato- protective effect of Saudi olive leaf extract. In Proceedings of the 2nd International Conference on Advances in Environment, Agriculture & Medical Sciences, Antalya, Turkey, 11–12 June 2015. [Google Scholar]
- Rezaeizadeh, A.; Zuki, A.B.Z.; Abdollahi, M.; Goh, Y.M.; Noordin, M.M.; Hamid, M.; Azmi, T.I. Determination of antioxidant activity in methanolic and chloroformic extracts of Momordica charantia. Afr. J. Biotechnol. 2011, 10, 4932–4940. [Google Scholar]
- Altemimi, A.; Choudhary, R.; Watson, D.G.; Lightfoot, D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015, 24, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gutierrez, A. The occurrence and bioactivity of polyphenols in Tunisian olive products and byproducts: A review. J. Food Sci. 2012, 77, R83–R92. [Google Scholar]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Effect of processing conditions, prestorage treatment, and storage conditions on the phenol content and antioxidant activity of olive mill waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.P.; Sahoo, J. Synergistic effect of l-ascorbic acid and α-tocopherol acetate on the quality of ground chevon during refrigerated storage. J. Food Sci. Technol. 2001, 38, 220–226. [Google Scholar]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Rahman, M.M.; Akhter, S.; Jamal, M.A.H.M.; Pandeya, D.R.; Haque, M.A.; Alam, M.F.; Rahman, A. Control of coliform bacteria detected from diarrhea associated patients by extracts of Moringa oleifera. Nepal. Med. Coll. J. 2010, 12, 12–19. [Google Scholar] [PubMed]
| Treatment | Number of Days * | |||
|---|---|---|---|---|
| 2 | 4 | 8 | 12 | |
| Control | 6.53 ± 0.02 a | 6.55 ± 0.02 a | 6.66 ± 0.03 a | 6.75 ± 0.03 a |
| 0.5 % olive leaf | 6.52 ± 0.03 a | 6.54 ± 0.02 a | 6.62 ± 0.03 a | 6.74 ± 0.02 a |
| 1.5 % olive leaf | 6.52 ± 0.02 a | 6.52 ± 0.02 c | 6.49 ± 0.03 b | 6.73 ± 0.04 a |
| 2.5 % olive leaf | 6.39 ± 0.03 b | 6.36 ± 0.02 b | 6.32 ± 0.02 c | 6.29 ± 0.03 b |
| Treatment | Number of Days * | |||
|---|---|---|---|---|
| 2 | 4 | 8 | 12 | |
| Control | 2.24 ± 0.15 a | 3.76 ± 0.23 a | 4.68 ± 0.18 a | 5.75 ± 0.10 a |
| 0.5% olive leaf | 2.27 ± 0.06 a | 3.73 ± 0.20 a | 4.69 ± 0.06 a | 5.76 ± 0.02 a |
| 1.5% olive leaf | 2.23 ± 0.06 a | 3.72 ± 0.03 a | 4.09 ± 0.11 b | 4.92 ± 0.04 b |
| 2.5% olive leaf | 1.27± 0.15 b | 1.33 ± 0.06 b | 1.69 ± 0.12 c | 1.92 ± 0.12 c |
| BHT 0.02% | 0.99 ± 0.12 c | 1.12 ± 0.16 c | 1.22 ± 0.21 d | 1.44 ± 0.17 d |
| Treatment | Number of Days * | |||
|---|---|---|---|---|
| 3 | 6 | 9 | 12 | |
| Control | 5.37 ± 0.15 a | 6.37 ± 0.21 a | 8.95 ± 0.13 a | 10.06 ± 0.16 a |
| 0.5% olive leaf | 5.33 ± 0.36 a | 6.33 ± 0.25 a | 8.99 ± 0.31 a | 10.02 ± 0.27 a |
| 1.5% olive leaf | 4.2 ± 0.1 b | 5.37 ± 0.15 b | 7.5 ± 0.17 b | 8.84 ± 0.03 b |
| 2.5% olive leaf | 4.13 ± 0.12 b | 3.67 ± 0.15 c | 5.47 ± 0.25 c | 6.23 ± 0.05 c |
| Treatment | Number of Days * | |||
|---|---|---|---|---|
| 3 | 6 | 9 | 12 | |
| Control | 3.13 ± 0.32 a | 5.09 ± 0.21 a | 7.89 ± 0.4 a | 9.11 ± 0.23 a |
| 0.5% olive leaf | 3.12 ± 0.36 a | 5.11 ± 0.41 a | 7.87 ± 0.31 a | 9.1 ± 0.37 a |
| 1.5% olive leaf | 2.32 ± 0.21 b | 3.09 ± 0.13 b | 5.1 ± 0.31 b | 6.87 ± 0.27 b |
| 2.5% olive leaf | 2.3 ± 0.31 b | 3.07 ± 0.15 b | 4.05 ± 0.29 c | 5.2 ± 0.35 c |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altemimi, A.B. A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications. Antioxidants 2017, 6, 34. https://doi.org/10.3390/antiox6020034
Altemimi AB. A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications. Antioxidants. 2017; 6(2):34. https://doi.org/10.3390/antiox6020034
Chicago/Turabian StyleAltemimi, Ammar B. 2017. "A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications" Antioxidants 6, no. 2: 34. https://doi.org/10.3390/antiox6020034
APA StyleAltemimi, A. B. (2017). A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications. Antioxidants, 6(2), 34. https://doi.org/10.3390/antiox6020034





