The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines
Abstract
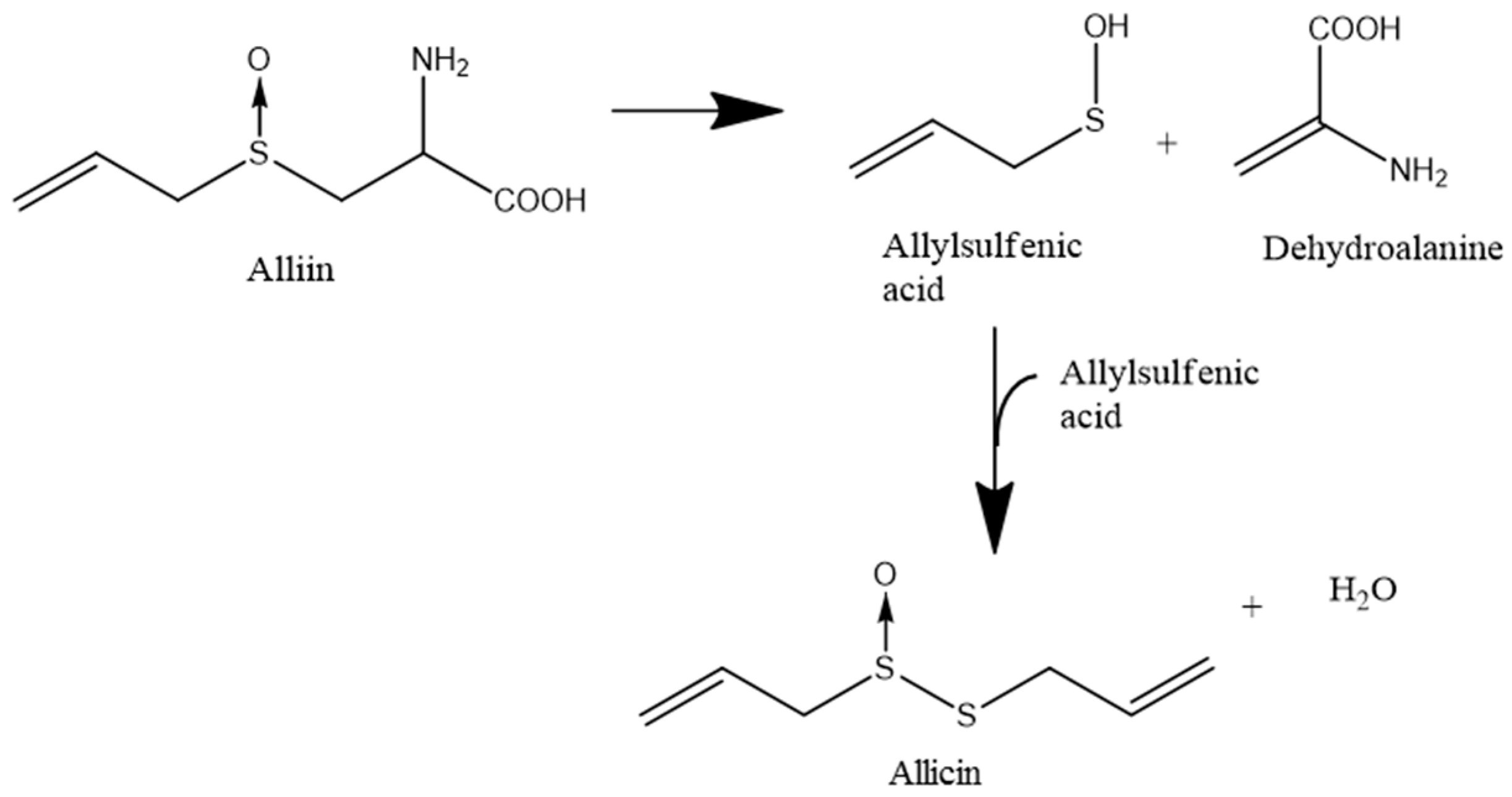
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cultivation
2.2. Methylthiazoltetrazolium (MTT)-Assay
2.3. Cell Proliferation
2.4. Glutathione Determination Using Monochlorobimane
2.5. Detection of Reactive Oxygen Species
2.6. Estimation of Apoptotic Cells Using YO-PRO-1 Iodide
2.7. Preparation of Garlic Juice and Quantification of Allicin by HPLC (High Performance Liquid Chromatography)
2.8. Allicin Synthesis
3. Results
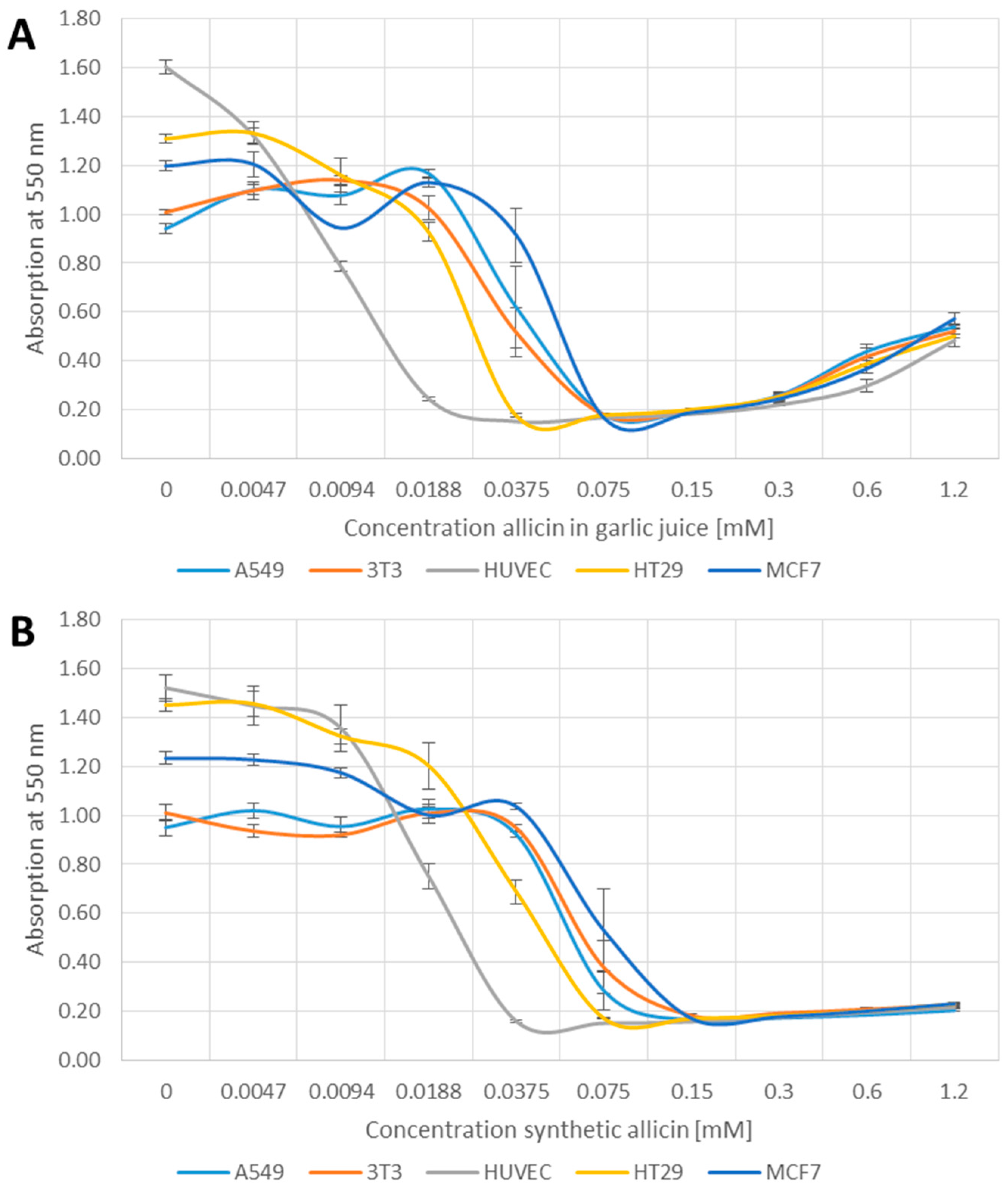
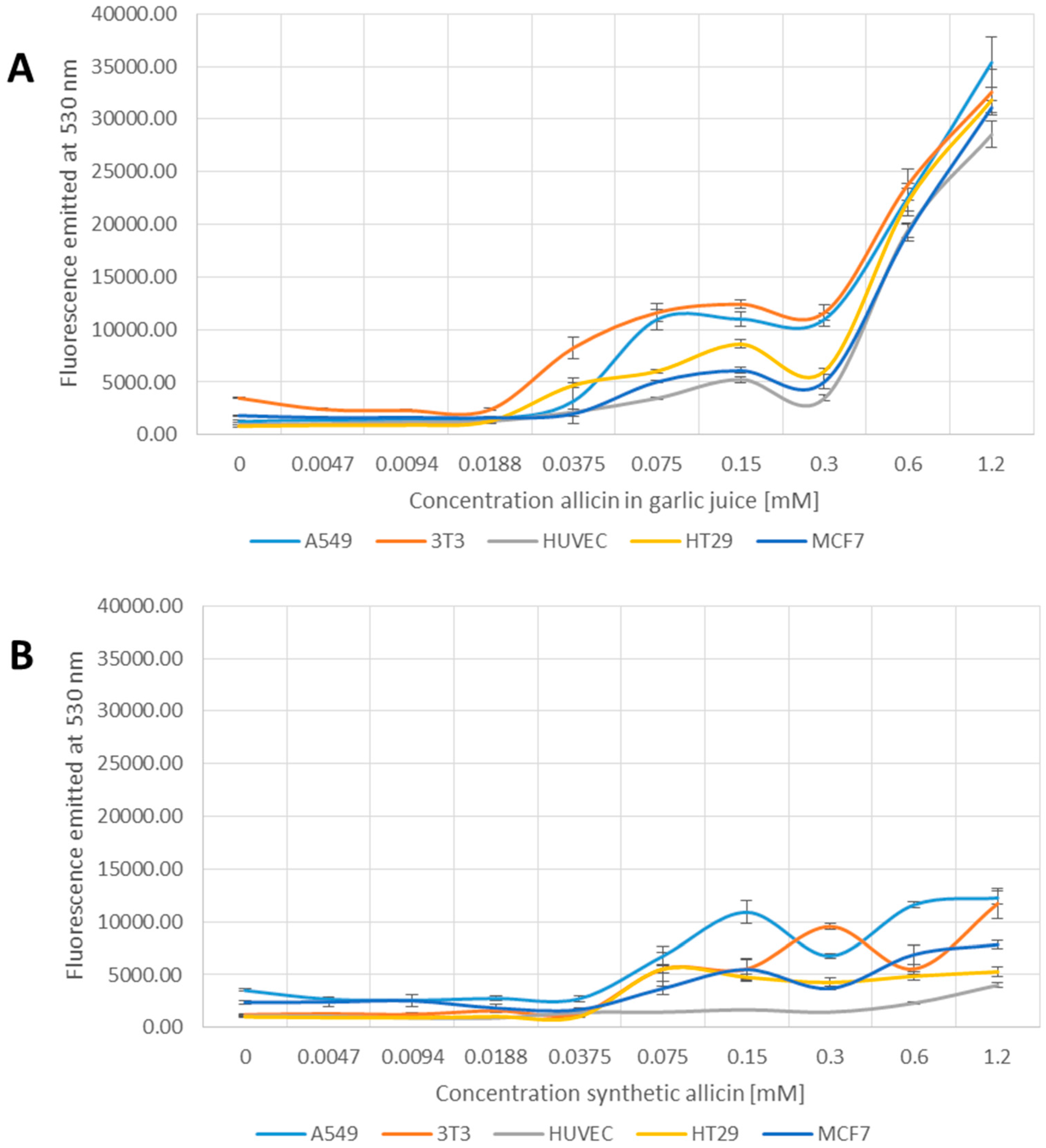
3.1. Effects of Allicin on Cell Viability
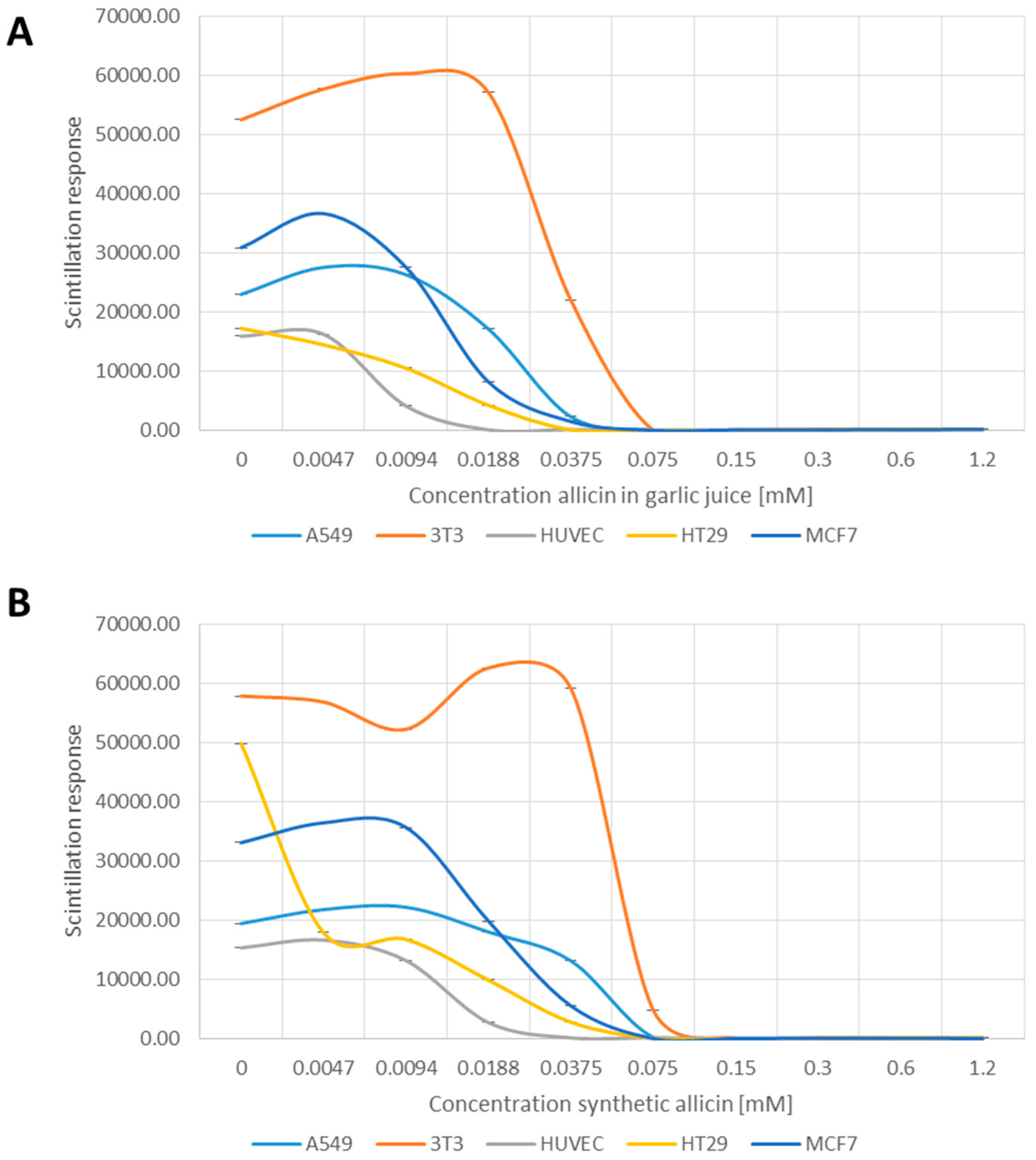
3.2. Allicin Inhibits Cell Proliferation
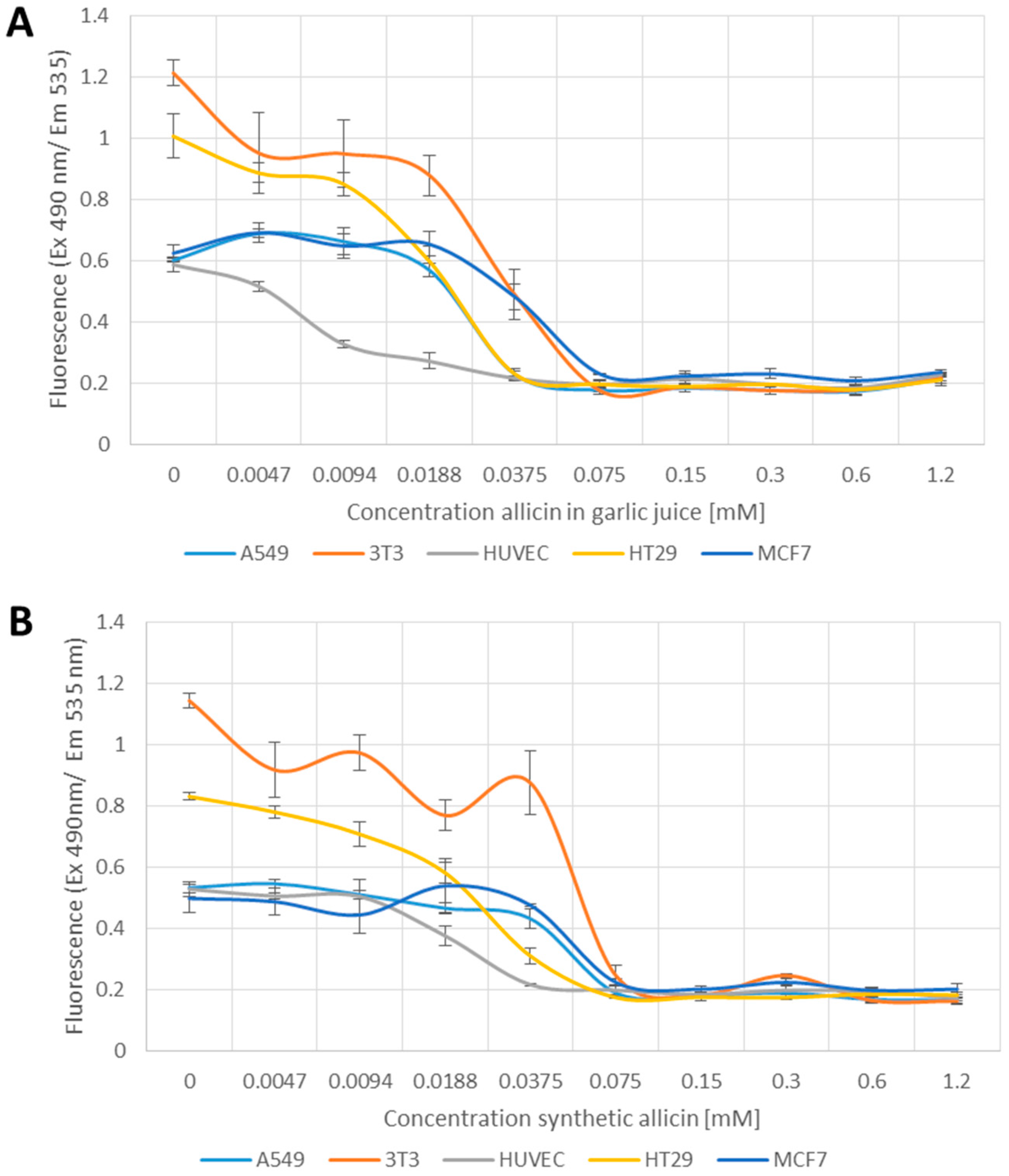
3.3. The Glutathione Pool Is Affected upon Treatment with Garlic Juice and Synthetic Allicin
3.4. Allicin-Induced Accumulation of Reactive Species
3.5. Induction of Apoptosis by Allicin
4. Discussion
4.1. Synthetic Allicin vs. Allicin in Garlic Juice
4.2. Effects of Allicin on Cell Viability and Proliferation
4.3. Effect of Allicin on the Glutathione Pool
4.4. Effect of Allicin on Inducing Apoptosis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Block, E. Garlic and Other Alliums—The Lore and The Science; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Kleijnen, J.; Knipschild, P.; Ter Riet, G. Garlic, onions and cardiovascular risk factors. A review of the evidence from human experiments with emphasis on commercially available preparations. Br. J. Clin. Pharmacol. 1989, 28, 535–544. [Google Scholar] [PubMed]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Warshafsky, S.; Kamer, R.S.; Sivak, S.L. Effect of garlic on total serum cholesterol. A Meta-Anal. Ann. Intern. Med. 1993, 119, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum L. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar]
- Cavallito, C.J.; Buck, J.S.; Suter, C.M. Allicin, the antibacterial principle of Allium sativum. II. Determination of the chemical structure. J. Am. Chem. Soc. 1944, 66, 1952–1954. [Google Scholar] [CrossRef]
- Jacob, C.; Anwar, A. The chemistry behind redox regulation with a focus on sulphur redox systems. Physiol. Plant. 2008, 133, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Paolo, J.A.D.; Carruthers, C. The Effect of Allicin from Garlic on Tumor Growth. Cancer Res. 1960, 20, 431–434. [Google Scholar]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-Allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.D. Enzyme Inhibition by Allicin, the Active Principle of Garlic. Biochem. J. 1956, 63, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Oommen, S.; Anto, R.J.; Srinivas, G.; Karunagaran, D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur. J. Pharmacol. 2004, 485, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, S.J.; Kwon, H.C.; Lee, K.R.; Rhee, D.K.; Pyo, S. Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA. Cancer Lett. 2005, 224, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Goncharov, I.; Rabinkov, A.; Mirelman, D.; Geiger, B.; Bershadsky, A.D. Allicin inhibits cell polarization, migration and division via its direct effect on microtubules. Cell Motil. Cytoskelet. 2007, 64, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Wilchek, M.; Sharp, A.; Nakagawa, Y.; Naoi, M.; Nozawa, Y.; Akao, Y. Allicin inhibits cell growth and induces apoptosis through the mitochondrial pathway in HL60 and U937 cells. J. Nutr. Biochem. 2008, 19, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.L.; Zhang, W.W.; Li, X.Y.; Yun, D.Z.; Zhang, Y. The Study of Apoptosis Induced by Allicin in HT-9/HL-60 and its Transfection Cell. Adv. Mater. Res. 2012, 340, 409–415. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Slusarenko, A.J. The Cellular “Thiolstat” as an Emerging Target of Some Plant Secondary Metabolites. In Recent Advances in Redox Active Plant and Microbial Products: From Basic Chemistry to Widespread Applications in Medicine and Agriculture; Jacob, C., Slusarenko, A.J., Kirsch, G., Winyard, P., Eds.; Springer: Berlin, Germany, 2014; pp. 235–262. [Google Scholar]
- Melino, S.; Sabelli, R.; Paci, M. Allyl sulfur compounds and cellular detoxification system: Effects and perspectives in cancer therapy. Amino Acids 2011, 41, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bat-Chen, W.; Tal, G.; Irena, P.; Zvi, L.; Betty, S. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2009, 62, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.; Ingold, K.U.; Pratt, D.A. Garlic: Source of the Ultimate Antioxidants—Sulfenic Acids. Angew. Chem. Int. Ed. 2009, 48, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, J.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Arditti, F.D.; Rabinkov, A.; Miron, T.; Reisner, Y.; Berrebi, A.; Wilchek, M.; Mirelman, D. Apoptotic killing of B-chronic lymphocytic leukemia tumor cells by allicin generated in situ using a rituximab-alliinase conjugate. Mol. Cancer Ther. 2005, 4, 325–332. [Google Scholar] [PubMed]
- Kelkel, M.; Cerella, C.; Mack, F.; Schneider, T.; Jacob, C.; Schumacher, M.; Dicato, M.; Diederich, M. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis 2012, 33, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Jacob, C.; Anwar, A.; Burkholz, T.; Ba, L.A.; Cerella, C.; Diederich, M.; Brandt, W.; Wessjohann, L.; Montenarh, M. Diallylpolysulfides induce growth arrest and apoptosis. Int. J. Oncol. 2010, 36, 743–749. [Google Scholar] [PubMed]
- Lawson, L.D.; Wood, S.G.; Hughes, B.G. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Med. 1991, 57, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Wang, Z.Y.J.; Hughes, B.G. Identification and HPLC Quantitation of Sulphides and Dialk(en)yl Thiosulfinates in Commercial Garlic Products. Planta Med. 1991, 57, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Wang, Z.Y.J.; Hughes, B.G. γ-Glutamyl-S-alkylcysteines in garlic and other Allium spp.: Precursors of age-dependent trans-1-propenyl thiosulfinates. J. Nat. Prod. 1991, 54, 436–444. [Google Scholar]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Servettaz, A.; Goulvestre, C.; Kavian, N.; Nicco, C.; Guilpain, P.; Chereau, C.; Vuiblet, V.; Guillevin, L.; Mouthon, L.; Weill, B.; et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J. Immunol. 2009, 182, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Marut, W.; Jamier, V.; Kavian, N.; Servettaz, A.; Winyard, P.G.; Eggleton, P.; Anwar, A.; Nicco, C.; Jacob, C.; Chéreau, C.; et al. The natural organosulfur compound dipropyltetrasulfide prevents HOCl-induced systemic sclerosis in the mouse. Arthritis Res. Ther. 2013, 15, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krest, I.; Keusgen, M. Biosensoric flow-through method for the determination of cysteine sulfoxides. Anal. Chim. Acta 2002, 469, 155–164. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Hemmis, B.; Noll, U.; Wagner, R.; Lühring, H.; Slusarenko, A.J. The defense substance allicin from garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor, Chara corallina and artificial lipid bilayers. Biochim. Biophys. Acta 2015, 1850, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic. Biol. Med. 2010, 49, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491–499. [Google Scholar]
- Eilat, S.; Oestraicher, Y.; Rabinkov, A.; Ohad, D.; Mirelman, D.; Battler, A.; Eldar, M.; Vered, Z. Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron. Artery Dis. 1995, 6, 985–990. [Google Scholar] [PubMed]
- Prasad, K.; Laxdal, V.; Yu, M.; Raney, B. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995, 148, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lynett, P.T.; Butts, K.; Vaidya, V.; Garrett, G.E.; Pratt, D.A. The mechanism of radical-trapping antioxidant activity of plant-derived thiosulfinates. Org. Biomol. Chem. 2011, 9, 3320–3330. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Tanaka, K.; Satoc, E.; Okajima, H. Kinetic and mechanistic studies of allicin as an antioxidant. Org. Biomol. Chem. 2006, 4, 4113–4117. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.P.; Lawson, L.D. Garlic—The Science and Therapeutic Application of Allium sativum L. and Related Species, 2nd ed.; Williams and Wilkins: Baltimore, MD, USA, 1996. [Google Scholar]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar]
- Miron, T.; Mironchik, M.; Mirelman, D.; Wilchek, M.; Rabinkov, A. Inhibition of tumor growth by a novel approach: In situ allicin generation using targeted alliinase delivery. Mol. Cancer Ther. 2003, 2, 1295–1301. [Google Scholar] [PubMed]
- Bernas, T.; Dobrucki, J. Mitochondrial and nonmitochondrial reduction of MTT: Interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 2002, 47, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.P.; Steffen, B.; Röbke, A.; Pentz, R. The effects of garlic preparations against human tumor cell proliferation. Phytomedicine 1999, 6, 7–11. [Google Scholar] [CrossRef]
- Miron, T.; Listowsky, I.; Wilchek, M. Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules. Eur. J. Med. Chem. 2010, 45, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Hedley, D.W.; Chow, S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry 1994, 15, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Myhre, O.; Andersen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Amon, R.; Reuven, E.M.; Leviatan Ben-Arye, S.; Padler-Karavani, V. Glycans in immune recognition and response. Carbohydr. Res. 2014, 389, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Wörle-Knirsch, J.M.; Hennrich, F.; Kern, K.; Krug, H.F. Human lung epithelial cells show biphasic oxidative burst after single-walled carbon nanotube contact. Carbon 2007, 45, 2241–2249. [Google Scholar] [CrossRef]
- Meier, B.; Radeke, H.H.; Selle, S.; Raspe, H.H.; Sies, H.; Resch, K.; Habermehl, G.G. Human fibroblasts release reactive oxygen species in response to treatment with synovial fluids from patients suffering from arthritis. Free Radic. Res. Commun. 1990, 8, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hieke, N.; Plum, L.M.; Gruhlke, M.C.H.; Slusarenko, A.J.; Rink, L. Impact of allicin on macrophage activity. Food Chem. 2012, 134, 141–148. [Google Scholar] [CrossRef]
- Su, C.C.; Chen, G.W.; Tan, T.W.; Lin, J.G.; Chung, J.G. Crude extract of garlic induced caspase-3 gene expression leading to apoptosis in human colon cancer cells. In Vivo 2006, 20, 85–90. [Google Scholar] [PubMed]
- Tu, G.; Zhang, Y.; Wei, W.; Li, L.; Zhang, Y.; Yang, J.; Xing, Y. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol. Med. Rep. 2016, 13, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Romin, Y.; Barlas, A.; Petrovic, L.M.; Turkekul, M.; Fan, N.; Xu, K.; Garcia, A.R.; Monette, S.; Klimstra, D.S.; et al. Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases. Cytotechnology 2014, 66, 259–273. [Google Scholar] [CrossRef] [PubMed]

| Test | A549 | 3T3 | HUVEC | HT29 | MCF7 |
|---|---|---|---|---|---|
| MTT (viability) | + | + | +++ | ++ | + |
| 3H-thymidine (proliferation) | ++ | + | +++ | ++ | ++ |
| Monobromobimane (GSH) | ++ | + | +++ | ++ | ++ |
| DCFDA (reactive species) | − | − | − | − | − |
| YO-PRO-1 (apoptosis) | + | +++ | + | + | +++ |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruhlke, M.C.H.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants 2017, 6, 1. https://doi.org/10.3390/antiox6010001
Gruhlke MCH, Nicco C, Batteux F, Slusarenko AJ. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants. 2017; 6(1):1. https://doi.org/10.3390/antiox6010001
Chicago/Turabian StyleGruhlke, Martin C. H., Carole Nicco, Frederic Batteux, and Alan J. Slusarenko. 2017. "The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines" Antioxidants 6, no. 1: 1. https://doi.org/10.3390/antiox6010001







