RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Extraction
2.3. HPLC Analysis
2.4. Mass Spectroscopy (MS) Instrumentation
2.5. Antioxidant Activity Studies
2.5.1. CUPRAC Assay
2.5.2. FRAP Assay
2.5.3. DPPH Assay
2.6. Antimicrobial Activity
2.6.1. Microorganisms
2.6.2. Microbiological Assay
3. Results and Discussion
3.1. Identification of Phenols
3.2. Quantification of Phenols
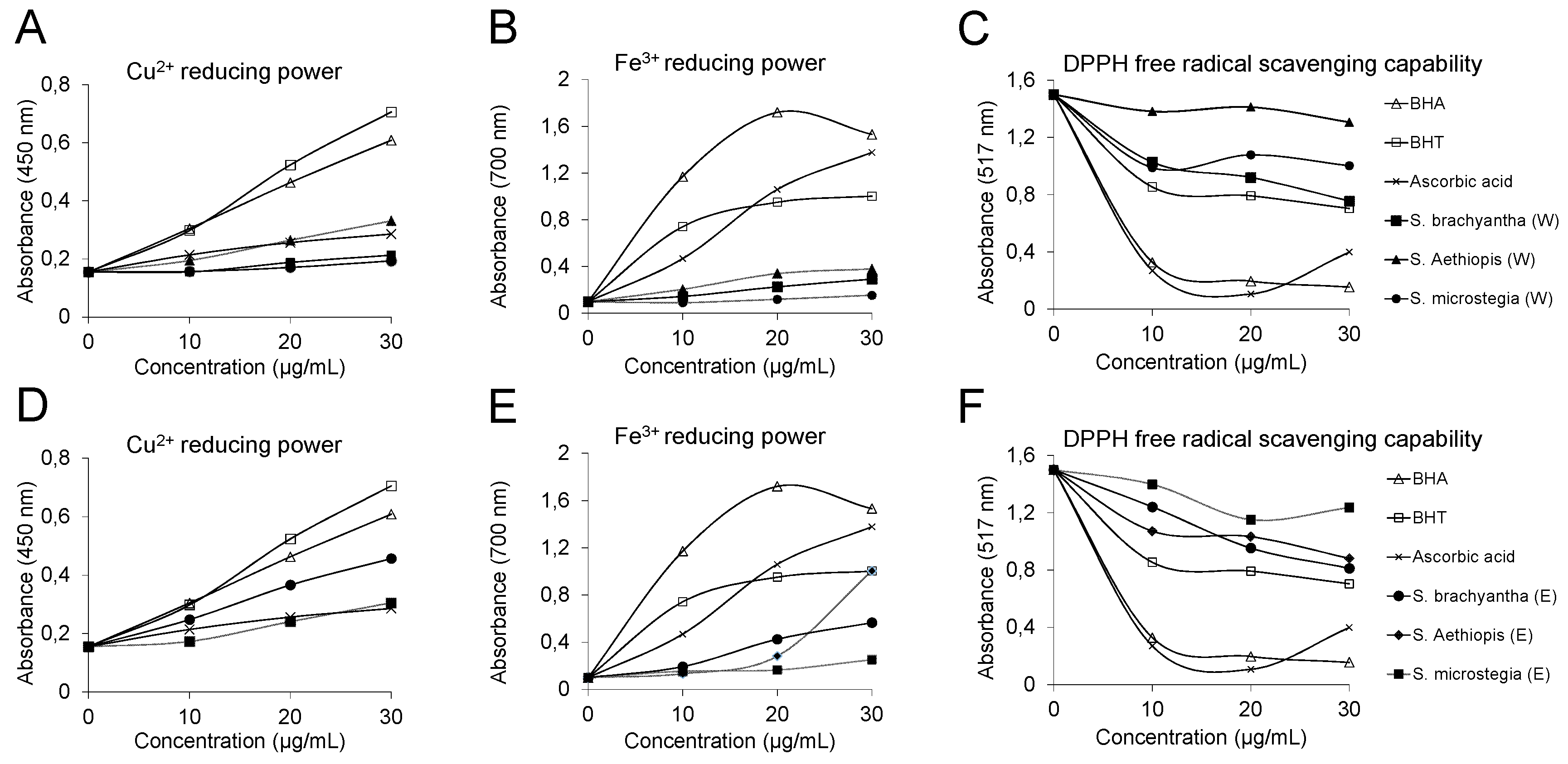
3.3. Antioxidant Activity
3.4. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jia, Z.; Dumont, M.J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents—An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Gülçin, İ.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar] [PubMed]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bursal, E.; Köksal, E.; Gülçin, İ.; Bilsel, G.; Gören, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Fındık, E.; Ceylan, M.; Elmastas, M. Isoeugenol-based novel potent antioxidants: Synthesis and reactivity. Eur. J. Med. Chem. 2011, 46, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Oztaşkın, N.; Çetinkaya, Y.; Taslimi, P.; Göksu, S.; Gülçin, I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015, 60, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Göçer, H.; Gülçin, İ. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.S.; Sarikurkcu, C.; Cengiz, M.; Kocak, S.; Uren, M.C.; Tepe, B. Salvia cadmica: Phenolic composition and biological activity. Ind. Crops Prod. 2016, 85, 204–212. [Google Scholar] [CrossRef]
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-Ela, M.; El-Lakany, A. Anti-inflammatory and antioxidant activities of Salvia fruticosa: An HPLC determination of phenolic contents. Evid. Based Comp. Alter. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y. Antifungal activity of Salvia miltiorrhiza against Candida albicans is associated with the alteration of membrane permeability and (1,3)-β-d-glucan synthase activity. J. Microbiol. Biotechnol. 2016, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wu, H.H.; Chen, T.H.; Leung, W.; Liang, Y.C. 15,16-dihydrotanshinone I from the functional food Salvia miltiorrhiza exhibits anticancer activity in human HL-60 leukemia cells: In vitro and in vivo studies. Int. J. Mol. Sci. 2015, 16, 19387–19400. [Google Scholar] [CrossRef] [PubMed]
- Sivropoulou, A.; Nikolaou, C.; Papanikolaou, E.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial, cytotoxic, and antiviral activities of Salvia fructicosa essential oil. J. Agric. Food Chem. 1997, 45, 3197–3201. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Bollen, C.V.; Perry, E.K.; Ballard, C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran. J. Pharmaceut. Res. 2013, 12, 801–810. [Google Scholar]
- Gülçin, İ.; Tel, A.Z.; Kirecci, E. Antioxidant, antimicrobial, antifungal and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng. Int. J. Food Propert. 2008, 11, 450–471. [Google Scholar] [CrossRef]
- Gülçin, İ.; Kirecci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.). Turk. J. Biol. 2010, 34, 175–188. [Google Scholar]
- Wu, X.W.; Wu, Z.P.; Wang, L.X.; Zhang, H.B.; Chen, J.W.; Zhang, W.; Gu, L.Q.; Huang, Z.S.; An, L.K. Synthesis, antimicrobial activity and possible mechanism of action of 9-bromo-substituted indolizinoquinoline-5,12-dione derivatives. Eur. J. Med. Chem. 2011, 46, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, A.; Contzen, M.; Hartelt, K.; Kleiser, A.; Maassen, S.; Rau, J.; Kraushaar, B.; Layer, F.; Strommenger, B. Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. Int. J. Food Microbiol. 2014, 187, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Isobe, J.; Shima, T.; Kanatani, J.I.; Kimata, K.; Shimizu, M.; Kobayashi, N.; Tanaka, T.; Iyoda, S.; Ohnishi, M.; Sata, T.V.; et al. Serodiagnosis using microagglutination assay during the food-poisoning outbreak in Japan caused by consumption of raw beef contaminated with enterohemorrhagic Escherichia coli O111 and O157. J. Clin. Microbiol. 2014, 52, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Küfrevioğlu, Ö.İ.; Oktay, M.; Büyükokuroğlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.; Leonhardt, I.; Hünniger, K.; Kurzai, O. Host response to Candida albicans bloodstream infection and sepsis. Virulence 2015, 6, 316–326. [Google Scholar] [PubMed]
- Gülçin, İ.; Oktay, M.; Kireçci, E.; Küfrevioğlu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Hon, P.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, Scotland, 1965; Volume 10. [Google Scholar]
- Köksal, E.; Gülçin, İ. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk. J. Agric. For. 2008, 32, 65–78. [Google Scholar]
- Polat Köse, L.; Gulcin, İ.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Ertaş, A.; Boğa, M.; Yılmaz, M.A.; Yeşil, Y.; Haşim, N.; Kaya, M.Ş.; Temel, H.; Kolak, U. Chemical compositions by using LC-MS/MS and GC-MS and biological activities of Sedum sediforme (Jacq.) Pau. J. Agric. Food Chem. 2014, 62, 4601–4609. [Google Scholar] [CrossRef] [PubMed]
- Kalın, P.; Gülçin, İ.; Gören, A.C. Antioxidant activity and polyphenol content of Vaccinium macrocarpon. Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Aksu, K.; Topal, F.; Gülçin, I.; Tümer, F.; Göksu, S. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch. Pharm. 2015, 348, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Polat Köse, P.; Gulcin, İ.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J. Enzyme Inhib. Med. Chem. 2016, 31, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Sehitoglu, M.H.; Han, H.; Kalin, P.; Gülçin, İ.; Ozkan, A.; Aboul-Enein, H.Y. Pistachio (Pistacia vera L.) Gum: A potent inhibitor of reactive oxygen species. J. Enzyme Inhib. Med. Chem. 2015, 30, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, D.U.; Eun, J.B.; Moon, S.H. Antioxidant effect of extracts from the coffee residue in raw and cooked meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Çakmakçı, S.; Topdaş, E.F.; Kalın, P.; Han, H.; Şekerci, P.; Polat Kose, L.; Gülçin, İ. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int. J. Food Sci. Technol. 2015, 50, 472–481. [Google Scholar] [CrossRef]
- Tekeli, Y.; Karpuz, E.; Danahaliloglu, H.; Bucak, S.; Guzel, Y.; Erdmann, H. Phenolic composition, Antioxidant capacity of Salvia verticcilata and effect on multidrug resistant bacteria by flow-cytometry. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Krimat, S.; Dob, T.; Toumi, M.; Kesouri, A.; Noasri, A. Assessment of phytochemicals, antioxidant, antimicrobial and cytotoxic properties of Salvia chudaei Batt. et Trab. endemic medicinal plant from Algeria. J. Mat. Environ. Sci. 2015, 6, 70–78. [Google Scholar]
- Ertas, A.; Boga, M.; Yilmaz, M.A.; Yesil, Y.; Tel, G.; Temel, H.; Hasimi, N.; Gazioglu, I.; Ozturk, M.; Ugurlu, P. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crop. Prod. 2015, 67, 336–345. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Göçer, H.; Akıncıoğlu, A.; Öztaşkın, N.; Göksu, S.; Gülçin, İ. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch Pharm. 2013, 346, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Tusevski, O.; Kostovska, A.; Iloska, A.; Trajkovska, L.; Simic, S.G. Phenolic production and antioxidant properties of some Macedonian medicinal plants. Cent. Eur. J. Biol. 2014, 9, 888–900. [Google Scholar] [CrossRef]
- Bravo, L.; Goya, L.; Lecumberri, E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007, 40, 393–405. [Google Scholar] [CrossRef]
- Gülçin, İ.; Topal, F.; Çakmakçı, R.; Gören, A.C.; Bilsel, M.; Erdoğan, U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef] [PubMed]
- Tel, G.; Öztürk, M.; Duru, M.E.; Harmandar, M.; Topçu, G. Chemical composition of the essential oil and hexane extract of Salvia chionantha and their antioxidant and anticholinesterase activities. Food Chem. Toxicol. 2010, 48, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Chaabouni, SE. Antioxidant properties of water-soluble gum from flaxseed hulls. Antioxidants 2016, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F.; Barcellos, T.; Moura, S.; Calloni, C.; Branco, C.S.; Salvador, M.; Roesch-Ely, M.; Henriques, J.A. Pharmacological perspectives from brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. An. Acad. Bras. Cienc. 2016, 88, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Sabbobeh, R.; Hejaz, H.; Jahajha, A.; Al-Akhras, S.; Al-Jaas, H.; Abu-Lafi, S. Antioxidant an antimicrobial activities of the leaf extract of Salvia palaestina. J. Appl. Pharm. Sci. 2016, 6, 76–82. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessiere, J.M.; Viano, J. Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Valizadeh, H.; Asghari, B.; Dinparast, L.; Farimani, M.; Bahadori, S. Chemical composition and antimicrobial, cytotoxicity, antioxidant and enzyme inhibitory activities of Salvia spinosa L. J. Funct. Foods 2015, 18, 727–736. [Google Scholar] [CrossRef]
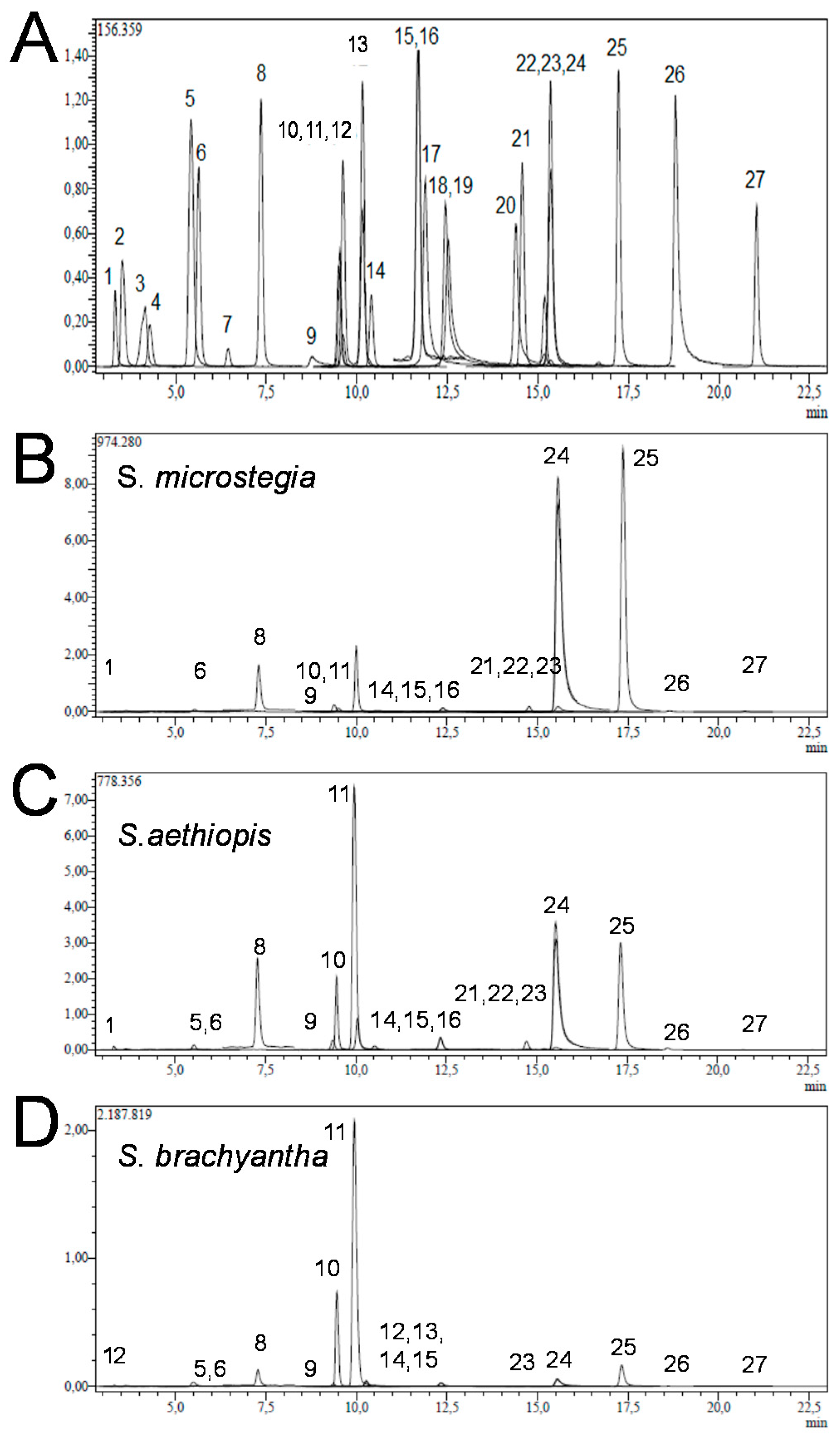
| No | Analyses | RT a | Parent Ion (m/z) b | Ionization Mode | r2 c | RSD (%) d | Linearity Range (µg/L) | LOD / LOQ (µg/L) e | Recovery (%) | U f | S. brachyantha | S. aethiopis | S. microstegia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 3.32 | 190.95 | Neg | 0.9927 | 0.0388 | 250–10,000 | 22.3 / 74.5 | 103.3 | 4.8 | 161.26 | 224.58 | 48.8 |
| 2 | Malic acid | 3.54 | 133.05 | Neg | 0.9975 | 0.1214 | 250–10,000 | 19.2 / 64.1 | 101.4 | 5.3 | 71.67 | 0 | 0 |
| 3 | trans-Aconitic acid | 4.13 | 172.85 | Neg | 0.9933 | 0.3908 | 250–10,000 | 15.6 / 51.9 | 102.8 | 4.9 | ND | ND | ND |
| 4 | Gallic acid | 4.29 | 169.05 | Neg | 0.9901 | 0.4734 | 25–1000 | 4.8 / 15.9 | 102.3 | 5.1 | ND | ND | ND |
| 5 | Chlorogenic acid | 5.43 | 353 | Neg | 0.9932 | 0.1882 | 250–10,000 | 7.3 / 24.3 | 99.7 | 4.9 | 355.05 | 25.42 | 0 |
| 6 | Protocatechuic acid | 5.63 | 152.95 | Neg | 0.9991 | 0.5958 | 100–4000 | 25.8 / 85.9 | 100.2 | 5.1 | 31.39 | 88.24 | 57.04 |
| 7 | Tannic acid | 6.46 | 182.95 | Neg | 0.9955 | 0.9075 | 100–4000 | 10.2 / 34.2 | 97.8 | 5.1 | ND | ND | ND |
| 8 | trans-Caffeic acid | 7.37 | 178.95 | Neg | 0.9942 | 1.0080 | 25–1000 | 4.4 / 14.7 | 98.6 | 5.2 | 151.77 | 289.68 | 176.8 |
| 9 | Vanillin | 8.77 | 151.05 | Neg | 0.9995 | 0.4094 | 250–10,000 | 10.1 / 33.7 | 99.2 | 4.9 | 40.75 | 52.28 | 28.91 |
| 10 | p-Coumaric acid | 9.53 | 162.95 | Neg | 0.9909 | 1.1358 | 100–4000 | 15.2 / 50.8 | 98.4 | 5.1 | 260.97 | 486.72 | 437.15 |
| 11 | Rosmarinic acid | 9.57 | 358.9 | Neg | 0.9992 | 0.5220 | 250–10,000 | 10.4 / 34.8 | 101.7 | 4.9 | 7619.58 | 1904.53 | 173.62 |
| 12 | Hesperidin | 9.69 | 611.1 | Poz | 0.9973 | 0.1363 | 250–10,000 | 21.6 / 71.9 | 100.2 | 4.9 | 354.74 | ND | ND |
| 13 | Rutin | 10.18 | 609.1 | Neg | 0.9971 | 0.8146 | 250–10,000 | 17.0 / 56.6 | 102.2 | 5.0 | 399.43 | ND | ND |
| 14 | Hyperoside | 10.43 | 463.1 | Neg | 0.9549 | 0.2135 | 100–4000 | 12.4 / 41.4 | 98.5 | 4.9 | 30.85 | 71.43 | 23.38 |
| 15 | 4-Hydroxybenzoic acid | 11.72 | 136.95 | Neg | 0.9925 | 1.4013 | 25–1000 | 3.0 / 10.0 | 106.2 | 5.2 | 23.37 | 29.17 | 11.45 |
| 16 | Salicylic acid | 11.72 | 136.95 | Neg | 0.9904 | 0.6619 | 25–1000 | 4 / 13.3 | 106.2 | 5.0 | 19.96 | 26.33 | 11.08 |
| 17 | Myricetin | 11.94 | 317 | Neg | 0.9991 | 2.8247 | 100–4000 | 9.9 / 32.9 | 106.0 | 5.9 | ND | ND | ND |
| 18 | Fisetin | 12.61 | 284.95 | Neg | 0.9988 | 2.4262 | 100–4000 | 10.7 / 35.6 | 96.9 | 5.5 | ND | ND | ND |
| 19 | Coumarin | 12.52 | 146.95 | Poz | 0.9924 | 0.4203 | 100–4000 | 9.1 / 30.4 | 104.4 | 4.9 | ND | ND | ND |
| 20 | Quercetin | 14.48 | 300.9 | Neg | 0.9995 | 4.3149 | 25–1000 | 2.0 / 6.8 | 98.9 | 7.1 | ND | ND | ND |
| 21 | Naringenin | 14.66 | 270.95 | Neg | 0.9956 | 2.0200 | 25–1000 | 2.6 / 8.8 | 97.0 | 5.5 | ND | 28.91 | 22.55 |
| 22 | Hesperetin | 15.29 | 300.95 | Neg | 0.9961 | 1.0164 | 25–1000 | 3.3/ 11.0 | 102.4 | 5.3 | ND | 11.42 | 4.05 |
| 23 | Luteolin | 15.43 | 284.95 | Neg | 0.9992 | 3.9487 | 25–1000 | 5.8 / 19.4 | 105.4 | 6.9 | 25.41 | 171.50 | 437.46 |
| 24 | Kaempferol | 15.43 | 284.95 | Neg | 0.9917 | 0.5885 | 25–1000 | 2.0 / 6.6 | 99.1 | 5.2 | 129.92 | 764.82 | 1954.28 |
| 25 | Apigenin | 17.31 | 268.95 | Neg | 0.9954 | 0.6782 | 25–1000 | 0.1 / 0.3 | 98.9 | 5.3 | 222.54 | 416.76 | 1207.27 |
| 26 | Rhamnetin | 18.94 | 314.95 | Neg | 0.9994 | 2.5678 | 25–1000 | 0.2 / 0.7 | 100.8 | 6.1 | 2.50 | 25.19 | 12.89 |
| 27 | Chrysin | 21.18 | 253 | Neg | 0.9965 | 1.5530 | 25–1000 | 0.05 / 0.17 | 102.2 | 5.3 | 0.94 | 0.42 | 2.51 |
| Microorganisms | Salvia brachyantha | Salvia aethiopis | Salvia microstegia | Antibiotic Discs (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | Erythromycin | Ampicillin/Sulbactam | Amikacin | Rifampicin | ||
| Gram-positive | B.subtilis | 10 ± 0.00 | 11 ± 0.47 | 13 ± 0.47 | - | - | - | 11 ± 0.00 | 12 ± 1.69 | 13 ± 0.47 | 20 ± 1.24 | 14 ± 0.47 | 11 ± 1.24 | 21 ± 1.24 |
| S. aureus | 9 ± 0.47 | 10 ± 0.81 | 11 ± 0.47 | 9 ± 0.47 | 11 ± 0.81 | 12 ± 0.00 | 9 ± 0.47 | 10 ± 0.47 | 11 ± 0.81 | 21 ± 0.00 | 10 ± 0.81 | 9 ± 0.00 | 18 ± 1.69 | |
| B. megaterium | 9 ± 0.00 | 10 ± 0.00 | 11 ± 0.00 | - | - | - | 9 ± 0.47 | 11 ± 0.00 | 12 ± 1.69 | 25 ± 1.69 | - | 10 ± 0.81 | 16 ± 1.24 | |
| Gram-negative | E. aerogenes | 9 ± 0.81 | 10 ± 0.47 | 12 ± 0.81 | - | 9 ± 0.00 | 10 ± 0.47 | 9 ± 0.00 | 10 ± 0.81 | 11 ± 0.00 | 27 ± 1.24 | 10 ± 0.47 | 9 ± 0.00 | 16 ± 0.47 |
| E. coli | 9 ± 0.47 | 10 ± 0.00 | 12 ± 1.69 | - | 9 ± 0.00 | 10 ± 0.00 | 9 ± 0.00 | 11 ± 0.81 | 12 ± 0.81 | 19 ± 0.00 | 13 ± 1.24 | 13 ± 0.81 | 18 ± 1.24 | |
| P. aeruginosa | 10 ± 0.00 | 12 ± 0.81 | 10 ± 0.47 | - | 9 ± 0.47 | 9 ± 0.47 | 9 ± 0.81 | 10 ± 0.00 | 11 ± 0.47 | 19 ± 1.69 | - | 14 ± 0.00 | 8 ± 0.00 | |
| K. pneumoniae | 9 ± 0.00 | 10 ± 0.47 | 10 ± 0.00 | - | - | - | 9 ± 0.00 | 10 ± 0.47 | 11 ± 0.00 | 19 ± 0.47 | 16 ± 1.69 | 10 ± 0.47 | 19 ± 1.69 | |
| Fungus | Y. lipolytica | - | - | 10 ± 0.00 | - | - | 13 ± 1.69 | - | - | - | - | - | - | - |
| C. albicans | - | 9 ± 0.00 | 10 ± 0.00 | - | - | - | - | - | - | - | - | - | - | |
| S. cerevisiae | - | - | - | - | - | - | - | - | - | - | - | - | - | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Yılmaz, M.A.; Gülçin, İ.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants 2016, 5, 38. https://doi.org/10.3390/antiox5040038
Tohma H, Köksal E, Kılıç Ö, Alan Y, Yılmaz MA, Gülçin İ, Bursal E, Alwasel SH. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants. 2016; 5(4):38. https://doi.org/10.3390/antiox5040038
Chicago/Turabian StyleTohma, Hatice, Ekrem Köksal, Ömer Kılıç, Yusuf Alan, Mustafa Abdullah Yılmaz, İlhami Gülçin, Ercan Bursal, and Saleh H. Alwasel. 2016. "RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species" Antioxidants 5, no. 4: 38. https://doi.org/10.3390/antiox5040038
APA StyleTohma, H., Köksal, E., Kılıç, Ö., Alan, Y., Yılmaz, M. A., Gülçin, İ., Bursal, E., & Alwasel, S. H. (2016). RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants, 5(4), 38. https://doi.org/10.3390/antiox5040038