A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products
Abstract
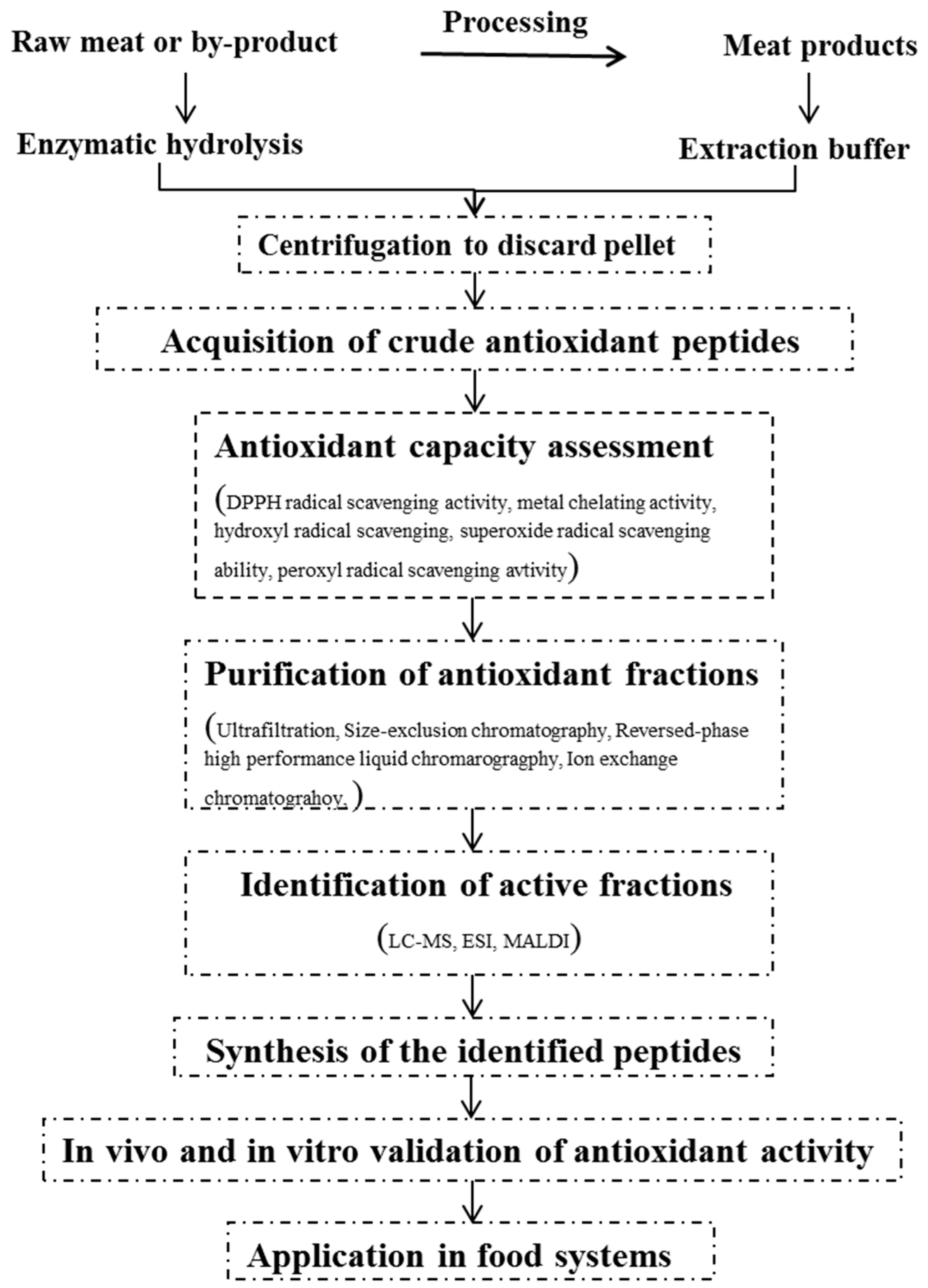
:1. Introduction
2. General Procedures for the Production of Antioxidant Peptides
2.1. Preparation of Antioxidant Fractions
2.1.1. Solvent Extraction
2.1.2. Enzymatic Hydrolysis
2.2. Approaches for Measuring Antioxidant Capacity
2.2.1. Chemical Reactions
2.2.2. Cell Model
2.2.3. Animal Model
2.3. Purification and Identification of Antioxidant Peptides
3. Characteristics of Antioxidant Peptides
3.1. Molecular Weight
3.2. Constitution and Sequence of Amino Acid
4. Implication
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SOD | superoxide dismutase |
| BHA | butyl hydroxy anisd |
| BHT | butylated hydroxytoluene |
| DH | degree of hydrolysis |
| TBARS | thiobarbituric acid-reactive substance |
| ORAC | oxygen radical absorbance capacity |
| TRAP | total radical trapping antioxidant parameter |
| TEAC | trolox equivalent antioxidant capacity |
| FRAP | ferric ion reducing power |
| O2•− | superoxide anion |
| •OH | hydroxyl |
| HO2• | hydroperoxyl |
| ROO• | peroxyl |
| ESR | electron spin resonance |
| PV | peroxide value |
| RP-HPLC | reversed-phase high performance liquid chromatography |
References
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.K.; Simmons, L.W. Reactive oxygen species as universal constraints in life-history evolution. Proc. Biol. Sci. 2009, 276, 1737–1145. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ros: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Freeman, B.A. Reactive oxygen species in human health and disease. Nutrition 2001, 17, 161–165. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dorval, J.; Hontela, A. Role of glutathione redox cycle and catalase in defense against oxidative stress induced by endosulfan in adrenocortical cells of rainbow trout (oncorhynchus mykiss). Toxicol. Appl. Pharmacol. 2003, 192, 191–200. [Google Scholar] [CrossRef]
- Kern, J.C.; Kehrer, J.P. Free radicals and apoptosis: Relationships with glutathione, thioredoxin, and the bcl family of proteins. Front. Biosci. 2005, 10, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Antioxidants in food and food antioxidants. Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Winata, A.; Lorenz, K. Antioxidant potential of 5-n-pentadecylresorcinol. J. Food Process. Preserv. 1996, 20, 417–429. [Google Scholar] [CrossRef]
- Ito, N.; Hirose, M.; Fukushima, S.; Tsuda, H.; Shirai, T.; Tatematsu, M. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem. Toxicol. 1986, 24, 1071–1082. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, K.S.; Choi, S.Y.; Kwon, H.Y.; Won, M.H.; Kang, T.-C. Carnosine and related dipeptides protect human ceruloplasmin against peroxyl radical-mediated modification. Mol. Cells 2002, 13, 498–502. [Google Scholar] [PubMed]
- Toldrá, F.; Aristoy, M.-C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J. Muscle proteins and muscle structure. Food Proteins 1982, 245–260. [Google Scholar]
- Escudero, E.; Aristoy, M.-C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive effect and antioxidant activity of peptide fractions extracted from Spanish dry-cured ham. Meat Sci. 2012, 91, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Escudero, E.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Proteomic identification of antioxidant peptides from 400 to 2500 da generated in Spanish dry-cured ham contained in a size-exclusion chromatography fraction. Food Res. Int. 2014, 56, 68–76. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Zhang, W.-G.; Zhou, G.-H.; Xu, X.-L.; Kang, Z.-L.; Yin, Y. Isolation and identification of antioxidant peptides from jinhua ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.-J.; Hu, Y.-Y.; Hu, H.-Y.; Ge, Q.-F.; Zhou, G.-H.; Zhang, W.-G. Purification and identification of antioxidative peptides from dry-cured xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, W.; Yang, Y.; Ma, C.; Ahn, D.; Li, X.; Lei, J.; Du, M. Changes of hormone sensitive lipase (HSL) and adipose tissue triglyceride lipase (ATGL) and free fatty acids in subcutaneous adipose tissue throughout the ripening process of dry-cured ham. Food Chem. 2010, 121, 191–195. [Google Scholar] [CrossRef]
- Sacchetti, G.; di Mattia, C.; Pittia, P.; Martino, G. Application of a radical scavenging activity test to measure the total antioxidant activity of poultry meat. Meat Sci. 2008, 80, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Yaya, H.; Lujuan, X.; Guanghong, Z.; Wangang, Z. Effect of extraction methods on the antioxidant activity of crude peptides from Jinhua ham. Sci. Technol. Food Ind. 2015, 36, 115–118. [Google Scholar]
- Matoba, T. How does the radical-scavenging activity of soy protein food change during heating. Daizu Tanpakushitsu Kenkyu 2002, 5, 47–50. [Google Scholar]
- Lee, S.-J.; Kim, Y.-S.; Hwang, J.-W.; Kim, E.-K.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J.; Kim, J.M.; Park, P.-J. Purification and characterization of a novel antioxidative peptide from duck skin by-products that protects liver against oxidative damage. Food Res. Int. 2012, 49, 285–295. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. 2012, 50, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardini, R.; Mullen, A.M.; Bolton, D.; Kerry, J.; O’Neill, E.; Hayes, M. Assessment of the angiotensin-i-converting enzyme (ACE-I) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterisation of associated bioactive peptidic fractions. Meat Sci. 2012, 90, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Lee, S.-J.; Jeon, B.-T.; Moon, S.-H.; Kim, B.; Park, T.-K.; Han, J.-S.; Park, P.-J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009, 114, 1365–1370. [Google Scholar] [CrossRef]
- Arihara, K. Strategies for designing novel functional meat products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, R.; He, L.; Yang, N. Antioxidant activity of hydrolysates derived from porcine plasma. J. Sci. Food Agric. 2009, 89, 1897–1903. [Google Scholar] [CrossRef]
- Park, S.Y.; Chin, K.B. Antioxidant activities of pepsin hydrolysates of water-and salt-soluble protein extracted from pork hams. Int. J. Food Sci. Technol. 2011, 46, 229–235. [Google Scholar] [CrossRef]
- Wang, L.-S.; Huang, J.-C.; Chen, Y.-L.; Huang, M.; Zhou, G.-H. Identification and characterization of antioxidant peptides from enzymatic hydrolysates of duck meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhang, H.; Zhang, M.; YAO, W.T.; MAO, X.Y.; REN, F.Z. Antioxidant activity of hydrolysates and peptide fractions of porcine plasma albumin and globulin. J. Food Biochem. 2008, 32, 693–707. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, K.-C.; Chiang, S.-H. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, K.H.; Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Lim, B.O.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J.; Ahn, C.-B. Biological activity from the gelatin hydrolysates of duck skin by-products. Process Biochem. 2012, 47, 1150–1154. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, Y.-T.; Byun, H.-G.; Park, P.-J.; Ito, H. Purification and characterization of antioxidative peptides from bovine skin. BMB Rep. 2001, 34, 219–224. [Google Scholar]
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [PubMed]
- Lee, S.-J.; Kim, E.-K.; Hwang, J.-W.; Oh, H.-J.; Cheong, S.-H.; Moon, S.-H.; Jeon, B.-T.; Lee, S.M.; Park, P.-J. Purification and characterisation of an antioxidative peptide from enzymatic hydrolysates of duck processing by-products. Food Chem. 2010, 123, 216–220. [Google Scholar] [CrossRef]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Dirienzo, D.; Matthews, J. Recent developments in gastrointestinal absorption and tissue utilization of peptides: A review. J. Dairy Sci. 1993, 76, 351–361. [Google Scholar] [CrossRef]
- Davalos, A.; Miguel, M.; Bartolome, B.; Lopez-Fandino, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Protect. 2004, 67, 1939–1944. [Google Scholar]
- Yu, L.L.; Cheng, Z. Application of electron spin resonance (ESR) spectrometry in nutraceutical and food research. Mol. Nutr. Food Res. 2008, 52, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Deak, S.; Csaky, T. Factors regulating the exchange of nutrients and drugs between lymph and blood in the small intestine. Microcirc. Endothel. Lymphat. 1984, 1, 569–588. [Google Scholar]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. A review of the methodology for assessing in vivo antioxidant capacity. J. Sci. Food Agric. 2006, 86, 2057–2066. [Google Scholar] [CrossRef]
- Pan, M.; Jiang, T.S.; Pan, J.L. Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess Technol. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.; Ramarao, B. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Brown, P.; Luu, T.; Gu, Y.; Qian, D.; Duan, J.; Wang, Z.; Guo, J.; Tang, Y.; Guo, S.; Ye, H. Liquid chromatography. J. Sep. Sci. 2010, 33, 999–1174. [Google Scholar] [PubMed]
- Escudero, E.; Sentandreu, M.A.; Toldrá, F. Characterization of peptides released by in vitro digestion of pork meat. J. Agric. Food Chem. 2010, 58, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.; FitzGerald, R. Biofunctional peptides from milk proteins: Mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003, 9, 1289–1296. [Google Scholar] [PubMed]
- Tang, C.-H.; Peng, J.; Zhen, D.-W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- Bougatef, A.; Balti, R.; Haddar, A.; Jellouli, K.; Souissi, N.; Nasri, M. Protein hydrolysates from bluefin tuna (Thunnus thynnus) heads as influenced by the extent of enzymatic hydrolysis. Biotechnol. Bioprocess Eng. 2012, 17, 841–852. [Google Scholar] [CrossRef]
- Li, X.X.; Han, L.j.; Chen, L.j. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Rubas, W.; Grass, G.M. Gastrointestinal lymphatic absorption of peptides and proteins. Adv. Drug Deliv. Rev. 1991, 7, 15–69. [Google Scholar] [CrossRef]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of chain length on absorption of biologically active peptides from the gastrointestinal tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. The significance of peptides in clinical nutrition. Rev. Surg. 1972, 29, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin I–Converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Fitzgerald, R.J.; Meisel, H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br. J. Nutr. 2000, 84, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Yonekura, T.; Nishizawa, N.; Kitts, D.D. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol. Cell. Biochem. 2001, 225, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Itoh, H.; Miyoshi, M.; Chibata, I. Antioxidant properties of branched-chain amino acid derivatives. Chem. Pharm. Bull. 1979, 27, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Murase, H.; Nagao, A.; Terao, J. Antioxidant and emulsifying activity of N-(long-chain-acyl)histidine and N-(long-chain-acyl)carnosine. J. Agric. Food Chem 1993, 41, 1601–1604. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of l-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Chan, K.M.; Decker, E.A.; Feustman, D.C. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Niranjan Rajapakse, A.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Uchida, K.; Kawakishi, S. Sequence-dependent reactivity of histidine-containing peptides with copper (II)/ascorbate. J. Agric. Food Chem. 1992, 40, 13–16. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Tsuge, N.; Eikawa, Y.; Nomura, Y.; Yamamoto, M.; Sugisawa, K. En japonais. Nippon Nôgei Kagakukaishi 1991, 65, 1635–1641. [Google Scholar] [CrossRef]
- Suetsuna, K.; Ukeda, H.; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000, 11, 128–131. [Google Scholar] [CrossRef]
- Patterson, C.E.; Rhoades, R.A. Protective role of sulfhydryl reagents in oxidant lung injury. Exp. Lung Res. 1988, 14, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
| Source | Antioxidant Fractions | Preparation | Parental Protein | Antioxidant Activity Assessment | Mw(Da) | IC50 or Scavenging Activity | Reference |
|---|---|---|---|---|---|---|---|
| Fermented meat sauce | GYP | Centrifugation of fermented mash | - | DPPH radical-scavenging activity Hydroxyl radical scavenging | 406.26 | OH− (97.6% at 100 mM) | [26] |
| Pork hams | Water-soluble and salt-soluble protein hydrolysate | Pepsin in solution pH 2.0 at 37 °C for 2–10 h 1/100 (w/w) | - | Linoleic acid emulsion system Ferric-reducing antioxidant power DPPH radical scavenging activity | <7 kDa | DPPH (50% at 0.1% (w/w)) | [36] |
| Spanish dry-cured ham | Elution zone between 220 and 345 mL | 0.01 N HCl extraction buffer | DPPH radical-scavenging activity Superoxide ion extinguishing ability | 1700 | DPPH (fraction 220–345 mL, 39%–92%) O2− (fraction 240–280 mL with 41.67%, 50.27%) | [18] | |
| Spanish dry-cured ham | SAGNPN, GLAGA (2 most active peptides from 27 identified peptides) | 0.01 N HCl extraction buffer | Integrin alpha-3, Collagen, type VII, alpha 1 | DPPH radical-scavenging activity, Ferric-reducing antioxidant power | 558.24 387.21 | DPPH (SAGNPN, 50% at 1.5 mg/mL), Reducing power (GLAGA, 0.5 units of absorbance, at 1 mg/mL) | [19] |
| Spanish dry-cured ham | SNAAC (the most active peptide from 93 identified peptides) | 0.01 N HCl extraction buffer | myosin heavy chain 1,4 | DPPH radical-scavenging activity, Ferric-reducing antioxidant power | 464.17 | DPPH (IC50 = 75.2 µM) Reducing power (IC50 = 205 µM) | [20] |
| Jinhua ham | GKFNV | 0.01 N HCl extraction buffer | - | Hydroxyl radical scavenging activity, DPPH radical scavenging activity, Fe2+ chelating ability | 564.4 | Crude extracted peptides: OH− (54% at 1 mg/mL) DPPH(77.39% at 1 mg/mL) Fe2+ (63.20% at 1 mg/mL) | [21] |
| Xuanwei ham | DLEE | 0.2 mM phosphate buffer (pH 7.2) | - | DPPH radical-scavenging activity Hydroxyl radical scavenging activity Superoxide ion scavenging activity | 505.2 | DPPH(74.45% at 0.5 mg/mL) | [22] |
| Duck breast meat | LQAEVEELRAALE IEDPFDQDDWGAWKK | Protamex, 0.75/100 (w/w) 50 °C for 4 h, pH 6.0 | Myosin heavy chain Alpha-enolase | Hydroxyl radical scavenging activity DPPH radical scavenging activity, Fe2+ chelating ability | 1471.8 and 1851.9 | LQAEVEELRAALE: DPPH (93.36% at 1 mg/mL), Fe2+ (87.13% at 1 mg/mL). IEDPFDQDDWGAWKK: OH− (46.51% at 1 mg/mL) | [37] |
| Duck skin | HTVGCMPG | Pepsin in Glycine-HCl pH 2.0 at 37 °C for 8 h 0.2/1000 (w/w) | - | Hydroxyl radical scavenging activity DPPH radical scavenging activity Alkyl radical scavenging activity Superoxide radical scavenging activity | 941.43 | OH− (IC50 = 32.6 μg/mL) DPPH (IC50 = 22.7 μg/mL) Alkyl (IC50 = 55.1 μg/mL) O2−(IC50 = 49.8 μg/mL) | [29] |
| Duck skin | AVCGAAVAGT | Pepsin in Glycine-HCl pH 2.0 at 37 °C for 8 h 0.2/1000 (w/w) | - | Hydroxyl radical scavenging activity | 1096 | OH− (IC50 = 75 μg/mL ) | [45] |
| Gelatin from duck skin | gelatin hydrolysate of two enzymes combination | Collagenase pH 7.0, 37 °C , 8 h and pepsin, pH 2.0, 37 °C, 8 h with 0.2/1000 (w/w) | - | Hydroxyl radical scavenging activity DPPH radical scavenging activity Alkyl radical scavenging activity | - | DPPH (IC50 = 0.632 mg/mL) OH− (IC50 = 0.222 mg/mL) Alkyl (IC50 = 0.708 mg/mL) | [41] |
| Porcine myofibrillar protein | DSGVT IEAEGE DAQEKLE EELDNALN VPSIDDQEELM | Papain in water pH 7.0, at 37 °C for 24 h with 1/100 (w/w) | Actin Tropomyosin Myosin heavy chain | Measurement of hydroperoxides in a Peroxidation System DPPH radical scavenging activity Metal ion chelating activity | 650.3, 646.4 832.5, 916.9 1275.0 | - | [30] |
| Chicken breast protein | Breast protein hydrolysate | Papain in water for 6.15 h at 51.2 °C, pH 6.5 1.5/1000 (w/w) | - | Reducing power assay DPPH scavenging activity assay ICR mice model | - | Reducing power (0.5 at 2.37 mg/mL) DPPH (IC50 = 1.28 mg/mL) | [31] |
| Bovine brisket sarcoplasmic proteins | Potential peptides of Sarcoplasmic protein hydrolysates: EAWAEDVDLRVN GGWQMEEADDWLR GWQMEEADDWLR RIGEEYIADLDQLRKLL VFEWEAFAR AIMENANVLAR LAIMENANVLAR | Papain in water for 24 h at 37 °C, pH 7.0 with 1:100 (w/v). | Pyruvate kinase, Phosphorylase Phosphoglycerate kinase 1 Fructase-biphosphate aldolase | DPPH scavenging activity assay Fe2+ chelating ability assay Ferric ion reducing antioxidant power (FRAP) | Ranging from 1154.56 to 2045.13 | DPPH(18.68% at 1 mg/mL) in NUFH FRAP (6.85% µg/5 mg peptide) in10-kDa-UFH Fe2+ (82.42% at 5 mg/mL) in 3-kDa-UFH (show the most active fractions) | [32] |
| Porcine blood plasma | HNGN | Alcalase at 55, pH 8.0 for 5 h with 2:100 (g/g) | Thiobarbituric acid-reactive substances (TBARS) DPPH scavenging activity assay Ferric ion reducing antioxidant power (FRAP) Metal chelating activity | 441 | FRAP (236.4 µM at 1 mg/mL) | [38] | |
| Venison muscle | MQIFVKTLTG DLSDGEQGVL | Papain in PB at 37 °C for 8 h pH 6.0 with 1/2500 (w/w) | - | Hydroxyl radical scavenging activity DPPH radical scavenging activity Superoxide radical scavenging activity Peroxyl radical scavenging activity | 1137.40 1023.07 (predicted) | DPPH (IC50 = 77 µg/ml) OH− (IC50 = 44 µg/ml) O2− (IC50 = 217 µg/ml) Peroxyl (IC50 = 85 µg/ml) | [33] |
| Chicken breast meat | Hydrophilic fraction | Water extraction | - | ABTS radical cation decoloration assay | - | TEAC (total fractions 2.4 µmol/g) | [24] |
| Porcine muscle | DLYA, SLYA,VW | Papain | Actomyosin | - | - | - | [34] |
| Porcine skin collagen | QGAR | Cocktail mixture of three enzymes: PP, PS, PB at 25 °C pH 7.5 for 24 h with 1/125 (w/w) | Collagen | Linoleic acid oxidation system Ferrous ion chelating assay DPPH radical scavenging assay | 430.2 | DPPH (37.27% at 20 mM) Linoleic acid (38.48% at 20 mM) | [43] |
| Porcine plasma | Hydrolysates | Alcalase at 55 °C pH 7.5 for 24 h with 1/1000 (w/w) | Albumin and globulin | Reducing power DPPH radical scavenging activity Inhibition ability of lipid peroxidation Hydroxyl radical scavenging activity Superoxide radical scavenging activity | - | - | [39] |
| Porcine blood | Pepsin hydrolysate Papain hydrolysate | Pepsin at 37 °C pH 2 for 5 h with 1/25 (w/w) Papain at 37 °C pH 8 for 16 h with 1/20 (w/w) | Plasma proteins | Antioxidant activity in linoleic acid system DPPH radical-scavenging activity Iron(II)-chelating activity | - | DPPH (48.4% and 43.1% at 500 µg/ml for PPE and PPA respectively) | [35] |
| Porcine blood | Porcine hydrolysates | 2% Alcalase for 4 h pH 8.5 and followed by 1% Flavourzyme for 6 h pH 7.5, at 50 °C for 6 h | Hemoglobin | Reducing power Ferrous ion chelating ability DPPH radical-scavenging activity | - | Fe2+ (63.54% at 5.0 mg/mL) | [40] |
| Bovine skin gelatin bovine | GE-Hrp-GP-Hrp-GA-Hrp GP-Hrp-GP-Hrp-GP-Hrp-G GP-Hrp-GP-Hrp-GP-Hrp | Alcalase, pronase E amd collagenase sequentially | Collagen | TBARS | 826 877 820 | - | [42] |
| Enzymatic Hydrolysate | Buffer | pH | Temperature (°C) | Time (h) | Hydroxyl Radical Scavenging Activity (%) |
|---|---|---|---|---|---|
| a-Chymotrypsin | Phosphate | 7.0 | 37 | 8 | 19.45 ± 0.41 |
| Alcalase | Phosphate | 7.0 | 50 | 8 | 26.61 ± 0.56 |
| Flavozyme | Phosphate | 7.0 | 50 | 8 | 29.46 ± 0.39 |
| Neutrase | Phosphate | 7.0 | 50 | 8 | 24.45 ± 0.27 |
| Papain | Phosphate | 6.0 | 37 | 8 | 34.38 ± 0.32 |
| Pepsin | Glycine-HCl | 2.0 | 37 | 8 | 54.29 ± 0.14 |
| Protamax | Phosphate | 7.0 | 50 | 8 | 27.74 ± 0.25 |
| Trypsin | Phosphate | 7.0 | 37 | 8 | 28.33 ± 0.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Xing, L.; Fu, Q.; Zhou, G.-h.; Zhang, W.-g. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. https://doi.org/10.3390/antiox5030032
Liu R, Xing L, Fu Q, Zhou G-h, Zhang W-g. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants. 2016; 5(3):32. https://doi.org/10.3390/antiox5030032
Chicago/Turabian StyleLiu, Rui, Lujuan Xing, Qingquan Fu, Guang-hong Zhou, and Wan-gang Zhang. 2016. "A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products" Antioxidants 5, no. 3: 32. https://doi.org/10.3390/antiox5030032





