Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat
Abstract
:1. Introduction
2. Material and Methods
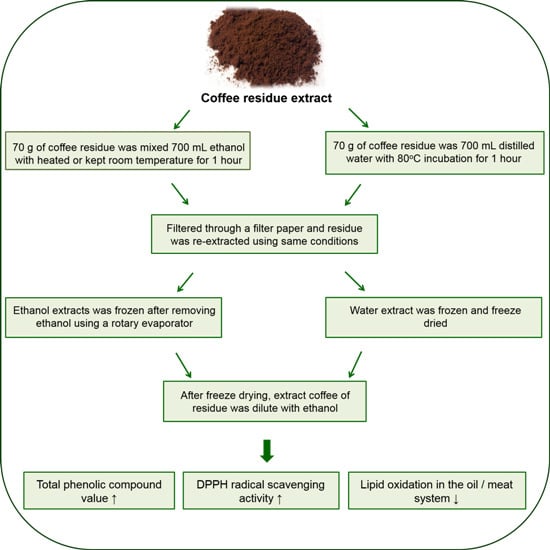
2.1. Extraction of Brewed Coffee Residues
2.2. Determination of Total Phenolic Compounds in the Extract
2.3. DPPH Radical Scavenging Activity
2.4. Antioxidant Activity of ECR
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Compound Values of ECR
3.2. DPPH Radical Scavenging Activity
3.3. Antioxidant Activity of ECR in the Oil Emulsion System
3.4. Antioxidant Activity of ECR in the Meat Systems
3.4.1. Raw-Meat Homogenates TBARS Method
3.4.2. Cooked-Meat Model System Using TBARS Method
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HEE | ethanol extraction with heating |
| CEE | ethanol extraction at room temperature |
| WE | hot water extraction |
References
- Reische, D.W.; Lillard, D.A.; Eitenmiller, R.R. Food Lipid Chemistry, Nutrition and Biotechnology; Akoh, C.C., Min, D.B., Eds.; CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Shaarani, S.M.; Pablos, M.P.A. Study of the properties of bearberry leaf extracts as a natural antioxidant in model foods. Antioxidants 2016, 5, 2–11. [Google Scholar]
- Tan, S.R.; Stathopoulos, C.; Parks, S.; Roach, P. An optimized aqueous extracts of phenolic compounds from Bitter Melon with high antioxidant capacity. Antioxidants 2014, 3, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Pangloli, P.; Melton, S.L.; Collins, J.L.; Penfield, M.P.; Saxton, A.M. Flavor and storage stability of potato chips fried in cottonseed and sunflower oils and palm olein/sunflower oil blends. J. Food Sci. 2002, 67, 97–103. [Google Scholar] [CrossRef]
- Yen, W.J.; Wang, B.S.; Chang, L.W.; Duh, P.D. Antioxidant properties of roasted coffee residues. J. Agric. Food Chem. 2005, 53, 2658–2663. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamied, A.A.; Nassar, A.G.; E1-Badary, N. Investigation on antioxidant and antibacterial activities of some natural extracts. World J. Food Sci. 2009, 4, 1–7. [Google Scholar]
- Bravo, J.; Monente, C.J.; Juániz, I.; Pna, P.D.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. J. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, R.; Napolitano, A.; Ritieni, A.; Rogliano, V. Characterization of a new potential functional ingredient: Coffee sliverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jimenez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–955. [Google Scholar] [CrossRef]
- Ramalakshmi, K.; Rao, L.J.M.; TaKano-Ishikawa, Y.; Goto, M. Bioactivity of low-grade coffee and spent coffee in different in vitro model systems. J. Food Chem. 2009, 115, 79–58. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.M.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; Pena, M.P.; Cid, C. Assessment of total (free and bound) phenolic compounds in spent coffee extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Quantitation of volatiles and nonvolatiles acids in an extract from coffee beverages: Correlation with antioxidant activity. J. Agric. Food Chem. 2006, 54, 6054–6058. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamamre, A.; Foerster, S.; Hartmann, F.; Kroger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. J. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.G.; Simoes, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee ground (Coffea Arabica L.) by subcritical water extraction. Ind. Crops. Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Pavlović, M.D.; Buntic´, A.V.; Šiler-Marinkovic´, S.S.; Dimitrijevic´-Brankovi, S.I. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, R.; Liu, C.Z. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep. Purif. Technol. 2008, 62, 480–483. [Google Scholar] [CrossRef]
- Singleto, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Goffman, F.D.; Bergman, C.J. Rice kernel phenolic content and its relationship with antiradical efficiency. J. Sci. Food Agric. 2004, 84, 1235–1240. [Google Scholar] [CrossRef]
- Ahn, D.U.; Jo, C. Fluorometric analysis of 2-thiobarbituric acid reactive substances in Turkey. J. Poult. Sci. 1998, 77, 475–480. [Google Scholar]
- Ahn, D.U.; Kim, S.M. Prooxidant effects of ferrous iron, hemoglobin, and ferritin in oil emulsion and cooked-meat homogenates are different from those in raw-meat homogenates. J. Poult. Sci. 1998, 77, 348–355. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Cominho, J.; Oilvella, M.A.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops. Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from Mashua (Tropaeolum tuberoum Ru´ız & Pavon) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effect of extraction solvents on concentration and antioxidant activity of black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. J. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Rehman, Z.-U. Citrus peel extract-a natural source of antioxidant. J. Food Chem. 2006, 99, 450–454. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Bio. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Chen, J.; Chuah, C.; Wibisono, R.; Melton, L.D.; Laing, W.; Ferguson, L.R.; Skinner, M.A. Kiwifruit-based polyphenols and related antioxidants for functional foods: Kiwifruit extract-enhanced gluten-free bread. Int. J. Food Sci. Nut. 2009, 60, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, K.T.; Nah, S.Y.; Chung, M.S.; Cho, S.W.; Paik, H.D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Antioxidant activity of some roasted foods. J. Food Chem. 2001, 72, 223–229. [Google Scholar] [CrossRef]
- Illy, A.; Viani, R. Espresso Coffee-the Chemistry of Quality; Academic Press: London, UK, 1995. [Google Scholar]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sanchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Nunez, M.J. Polyphenols from plant materials: extraction and antioxidant power. Elec. J. Env. Agricult. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Wong, J.W.; Hashimoto, K.; Shibamoto, T. Antioxidant activity of rosemary and sage extracts and vitamin E in a model meat system. J. Agric. Food Chem. 1995, 43, 2707–2712. [Google Scholar] [CrossRef]
- Mc Carthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Assessment of the antioxidant potential of natural food and plant extracts in fresh and previously frozen pork patties. Meat Sci. 2001, 57, 177–184. [Google Scholar] [CrossRef]
- Ahn, J.; Grun, I.U.; Mustapha, A. Antimicrobial and antioxidant activity of natural extracts in vitro and in ground beef. J. Food Prot. 2004, 67, 148–155. [Google Scholar]
- Brannan, R.G. Effect of grape seed extract on physicochemical properties of ground, salted chicken thigh meat during refrigerated storage at different relative humidity levels. Food Chem. 2008, 73, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Shim, S.Y. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extraction refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010, 84, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Farhangfar, A.; Moradi, M.; Razavi Rohani, S.M. Effectiveness of clove essential oil and grape seed extract combination on microbial and lipid oxidation characteristics of raw buffalo patty during storage at abuse refrigeration temperature. J. Food Process. Preserv. 2014, 38, 31–38. [Google Scholar] [CrossRef]
| Sample | Conc. (ppm) | TPC (mg GAE activity/g ECR) |
|---|---|---|
| HEE 1 | 1000 | 41.97 a ± 2.49 |
| CEE | 35.51 b ± 2.93 | |
| WE | 28.10 c ± 0.76 |
| Sample | Conc. (ppm) | SEM 2 | ||
|---|---|---|---|---|
| 250 | 500 | 1000 | ||
| HEE 1 | 38.16 ax ± 1.33 | 72.15 ay ± 1.37 | 90.39 az ± 0.14 | 7.66 |
| CEE | 37.10 ax ± 1.28 | 69.39 ay ± 0.67 | 89.05 az ± 0.74 | 7.58 |
| WE | 12.03 bx ± 2.76 | 28.86 bx ± 1.52 | 55.42 by ± 0.75 | 6.34 |
| SEM | 4.28 | 7.00 | 5.74 | |
| Sample | Con. (ppm) | Incubation Time (h) | SEM 2 | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 24 | 30 | 48 | 72 | |||
| Control 1 | 0.066 av | 0.094 aw | 0.182 ax | 0.292 ay | 0.309 ay | 0.365 az | 0.027 | |
| BHA | 50 | 0.031 bv | 0.034 cwv | 0.038 cw | 0.047 cx | 0.056 cy | 0.081 cz | 0.005 |
| HEE | 500 | 0.025 bv | 0.040 cw | 0.051 cx | 0.052 cx | 0.056 cy | 0.082 cz | 0.004 |
| 1000 | 0.027 bv | 0.039 cw | 0.048 cy | 0.049 cy | 0.049 cy | 0.078 cz | 0.003 | |
| CEE | 500 | 0.026 bx | 0.040 cx | 0.031 cx | 0.048 cy | 0.061 cy | 0.089 cz | 0.005 |
| 1000 | 0.027 bv | 0.042 cw | 0.050 cx | 0.050 cx | 0.061 cy | 0.086 cz | 0.004 | |
| WE | 500 | 0.034 bu | 0.075 bv | 0.145 abw | 0.222 bx | 0.284 by | 0.326 bz | 0.025 |
| 1000 | 0.033 bv | 0.096 aw | 0.112 bw | 0.214 bx | 0.277 by | 0.331 bz | 0.026 | |
| SEM | 0.003 | 0.005 | 0.012 | 0.020 | 0.024 | 0.026 | ||
| Sample | Conc. (ppm) | Incubation Time (hours) | SEM 2 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 12 | |||
| Control 1 | 0.190 ax | 0.363 ay | 0.454 az | 0.436 az | 0.469 az | 0.027 | |
| BHA | 50 | 0.150 b | 0.191 e | 0.193 e | 0.191 d | 0.192 e | 0.006 |
| HEE | 500 | 0.202 ay | 0.267 cz | 0.261 dz | 0.267 cz | 0.227 cdy | 0.007 |
| 1000 | 0.202 ayx | 0.228 dz | 0.237 dz | 0.219 dzy | 0.196 dex | 0.004 | |
| CEE | 500 | 0.202 ay | 0.313 bz | 0.304 cz | 0.351 bz | 0.298 bz | 0.014 |
| 1000 | 0.202 ay | 0.272 cz | 0.256 dz | 0.258 cz | 0.215 dey | 0.008 | |
| WE | 500 | 0.202 ax | 0.309 by | 0.377 bz | 0.363 bz | 0.284 by | 0.017 |
| 1000 | 0.202 ax | 0.273 cz | 0.291 cz | 0.286 cz | 0.249 cy | 0.009 | |
| SEM | 0.008 | 0.010 | 0.016 | 0.016 | 0.018 | ||
| Sample | Conc. (ppm) | Storage Time (Days) | SEM 2 | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | |||
| Control 1 | 0.105 ax | 0.393 ay | 0.807 az | 0.790 az | 0.063 | |
| BHA | 140 | 0.022 cy | 0.026 dy | 0.040 dz | 0.039 dz | 0.001 |
| HEE | 1000 | 0.025 cw | 0.076 cx | 0.197 cy | 0.263 cz | 0.020 |
| CEE | 1000 | 0.031 bx | 0.121 by | 0.380 bz | 0.396 bz | 0.034 |
| WE | 1000 | 0.029 bw | 0.128 bx | 0.396 by | 0.494 bz | 0.023 |
| SEM | 0.005 | 0.023 | 0.048 | 0.048 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Ahn, D.U.; Eun, J.B.; Moon, S.H. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2016, 5, 21. https://doi.org/10.3390/antiox5030021
Kim J-H, Ahn DU, Eun JB, Moon SH. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants. 2016; 5(3):21. https://doi.org/10.3390/antiox5030021
Chicago/Turabian StyleKim, Ji-Hee, Dong Uk Ahn, Jong Bang Eun, and Sun Hee Moon. 2016. "Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat" Antioxidants 5, no. 3: 21. https://doi.org/10.3390/antiox5030021