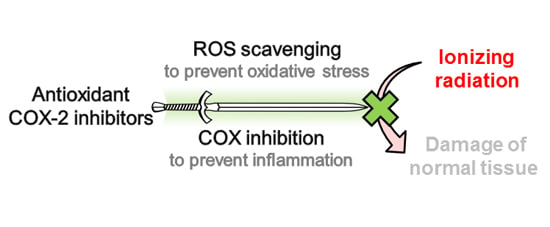
Development of Antioxidant COX-2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review
Abstract
:1. Introductory Remarks
2. Biochemical Background
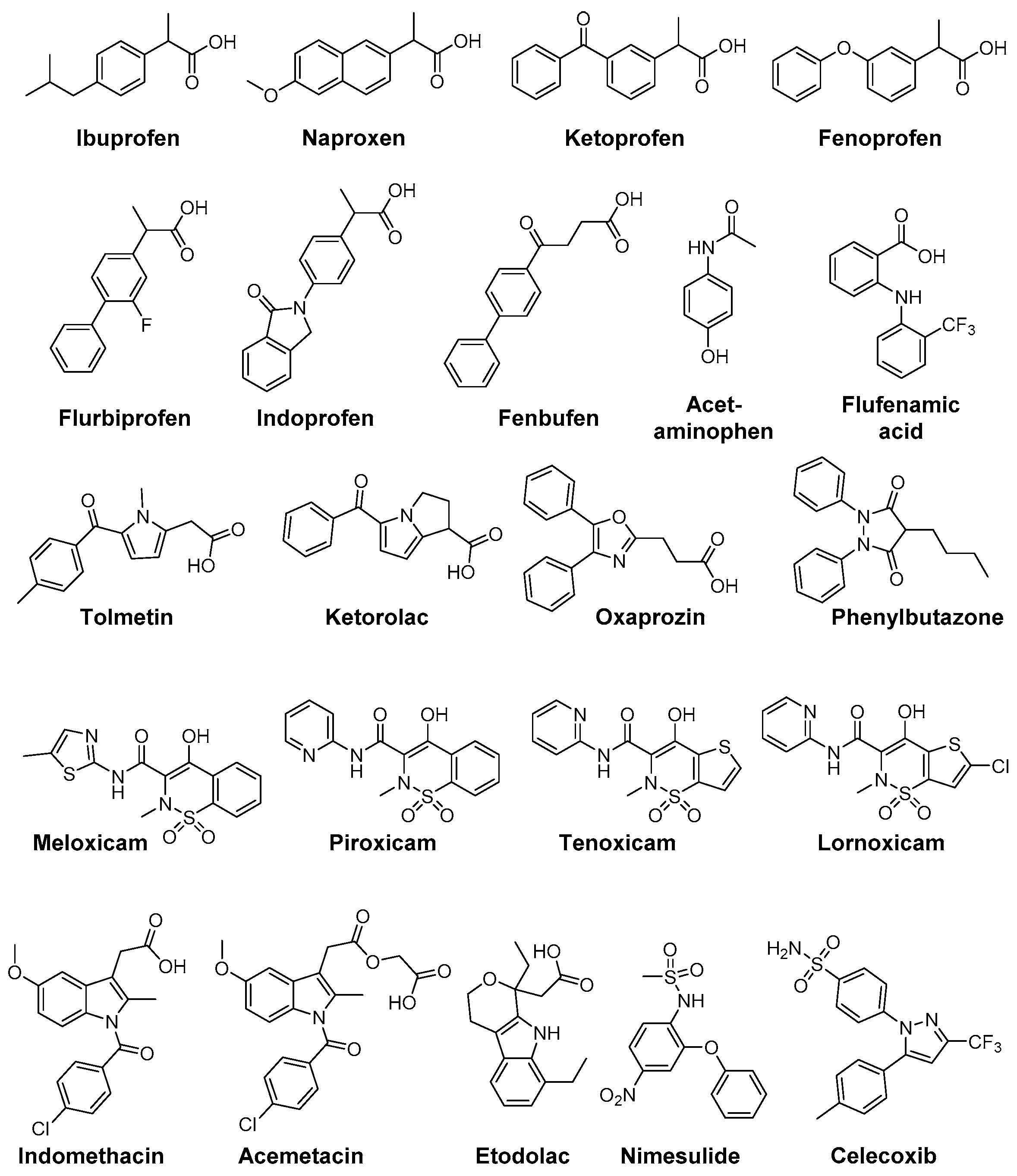
3. Cyclooxygenase-2 and COX-2 Inhibitors
4. COX-2 Inhibitors as Radiosensitizers and Radioprotectors
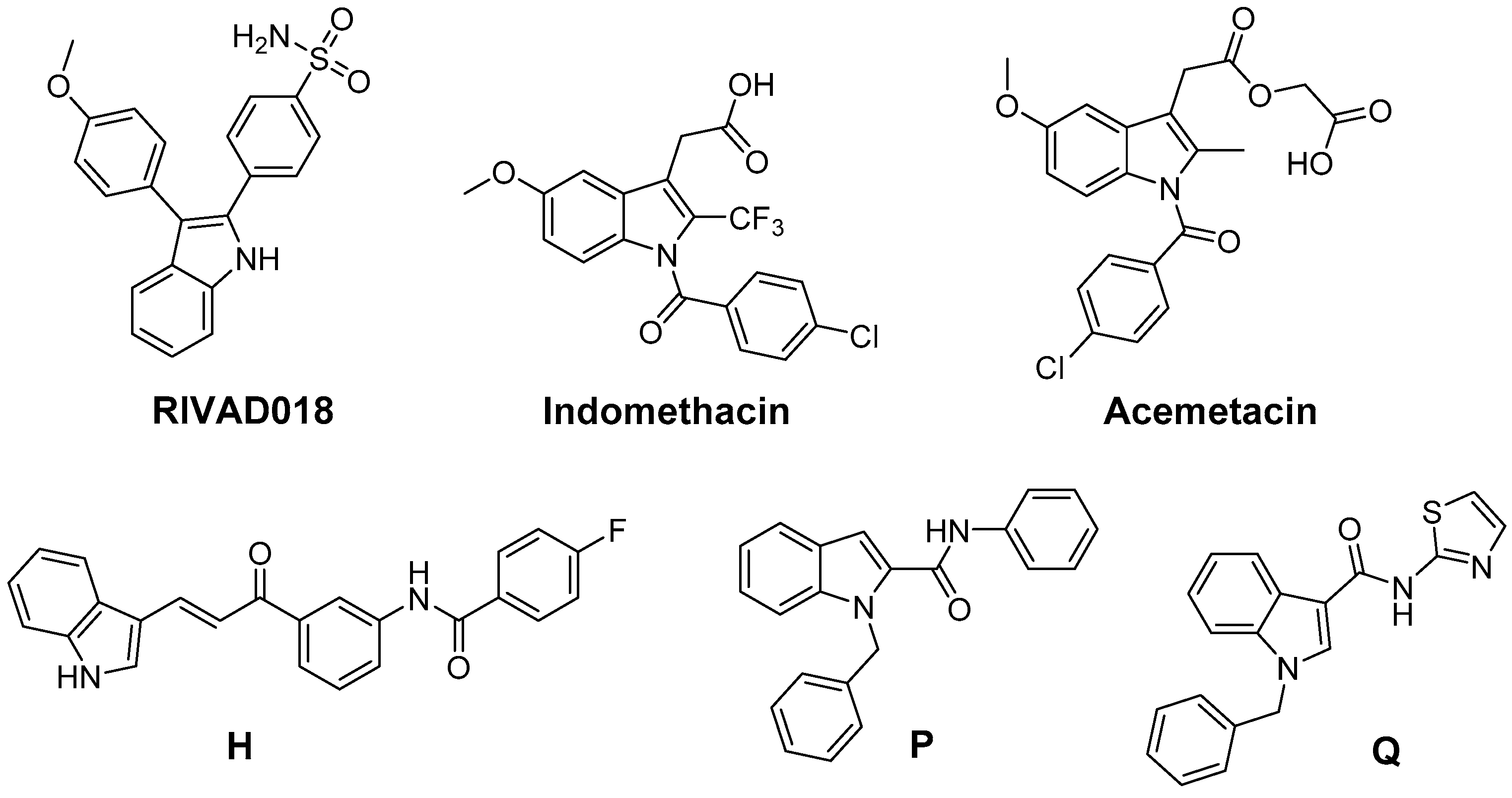
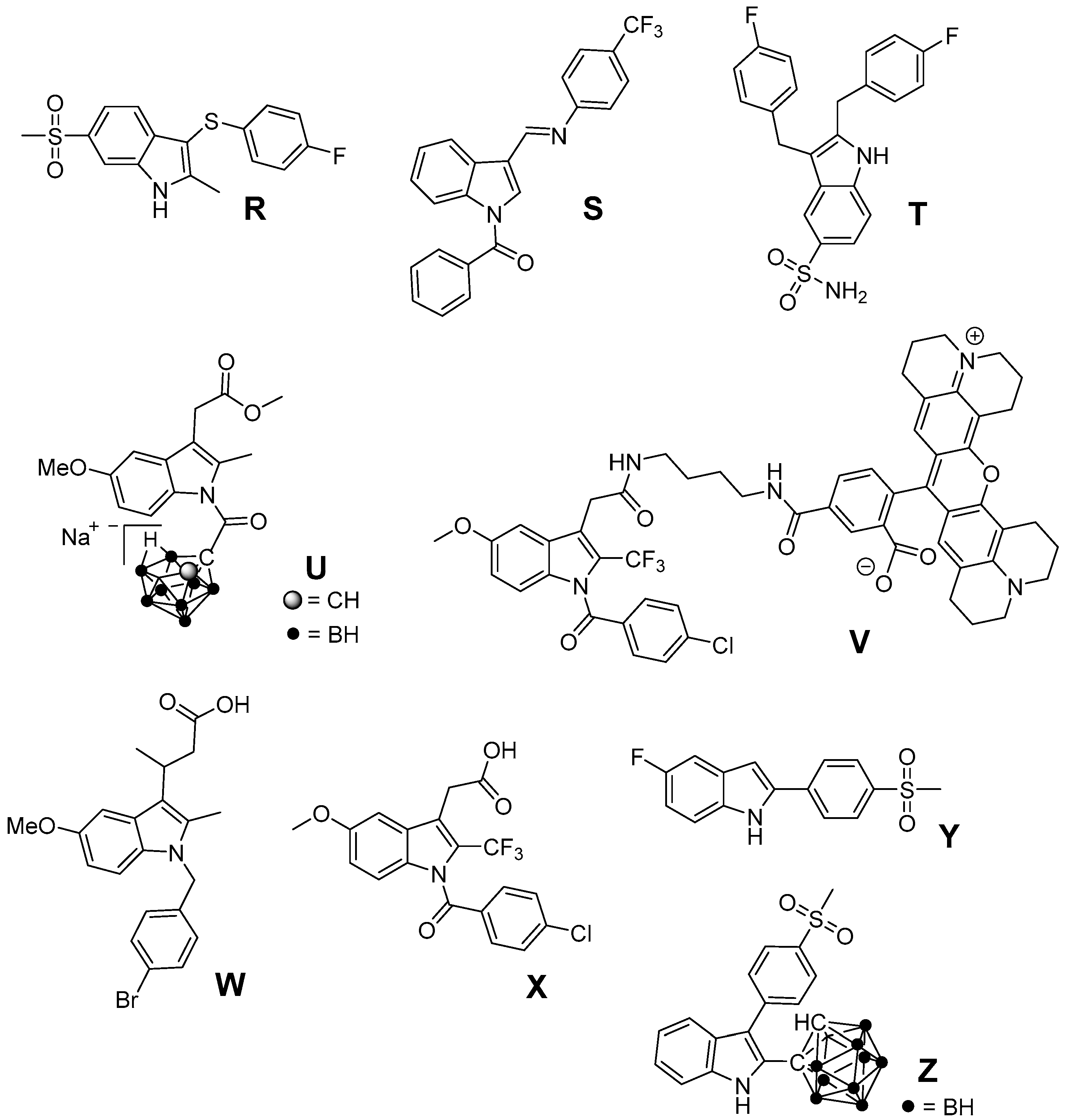
5. 2,3-Diaryl-Substituted Indoles as Promising Radioprotectors
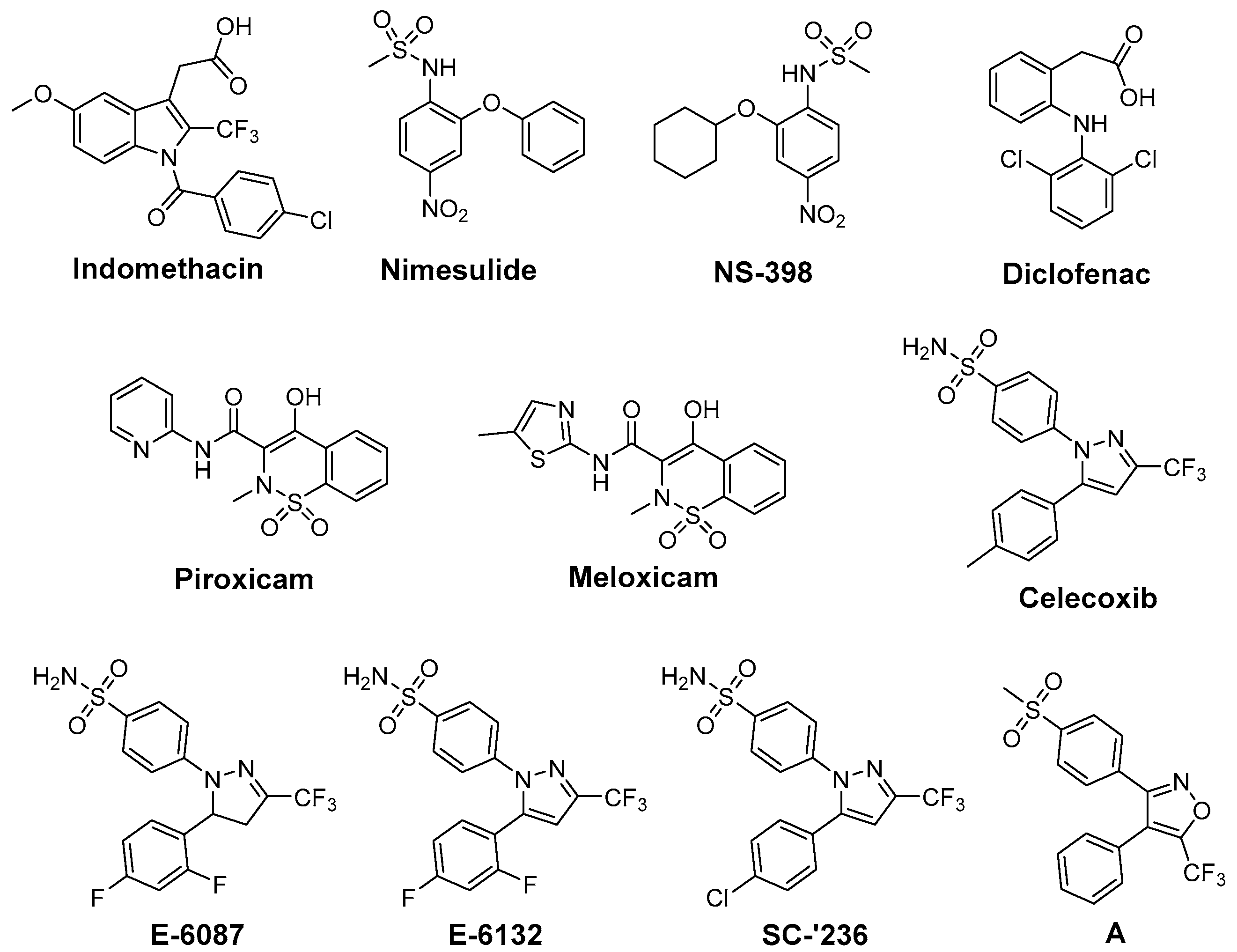
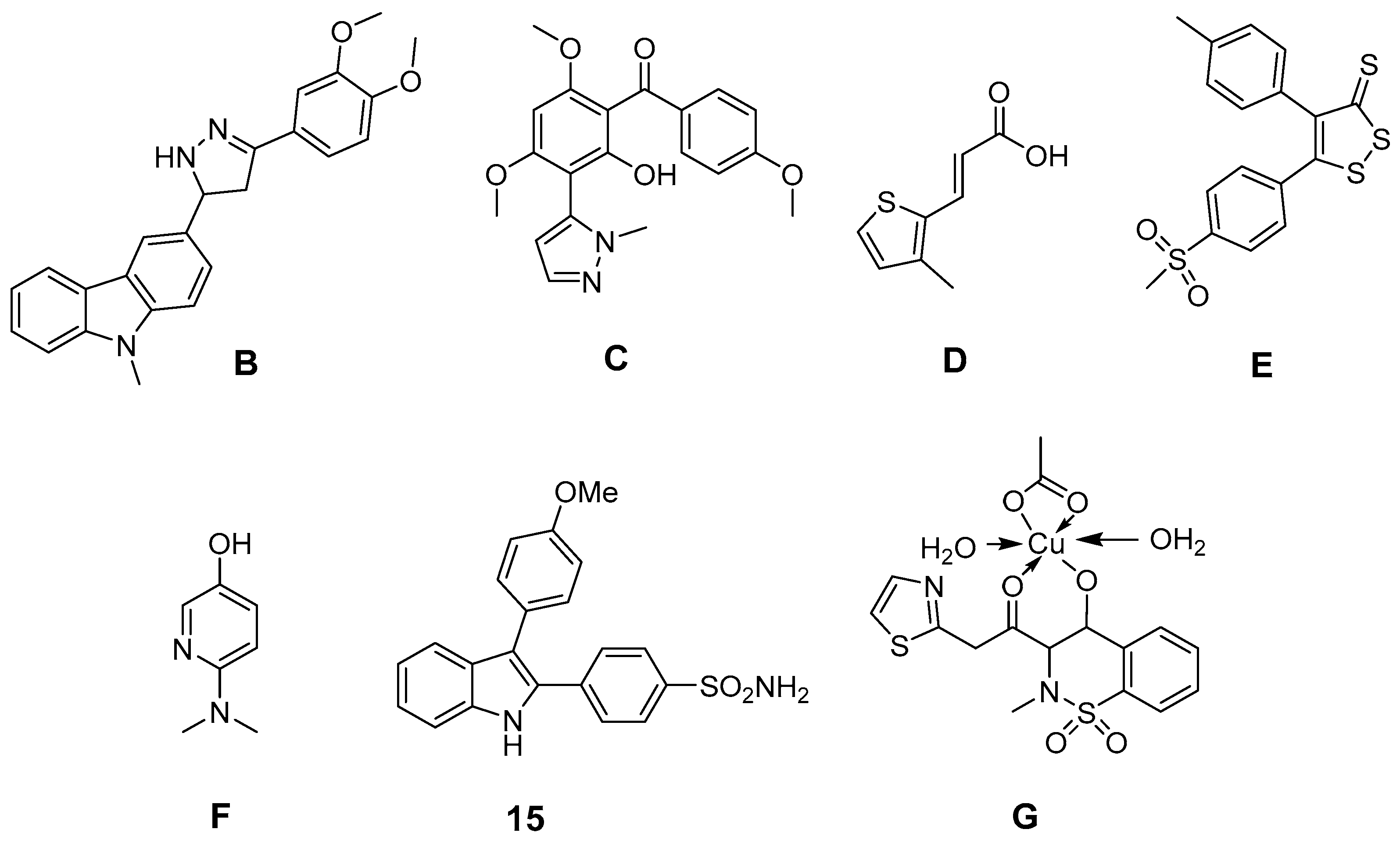
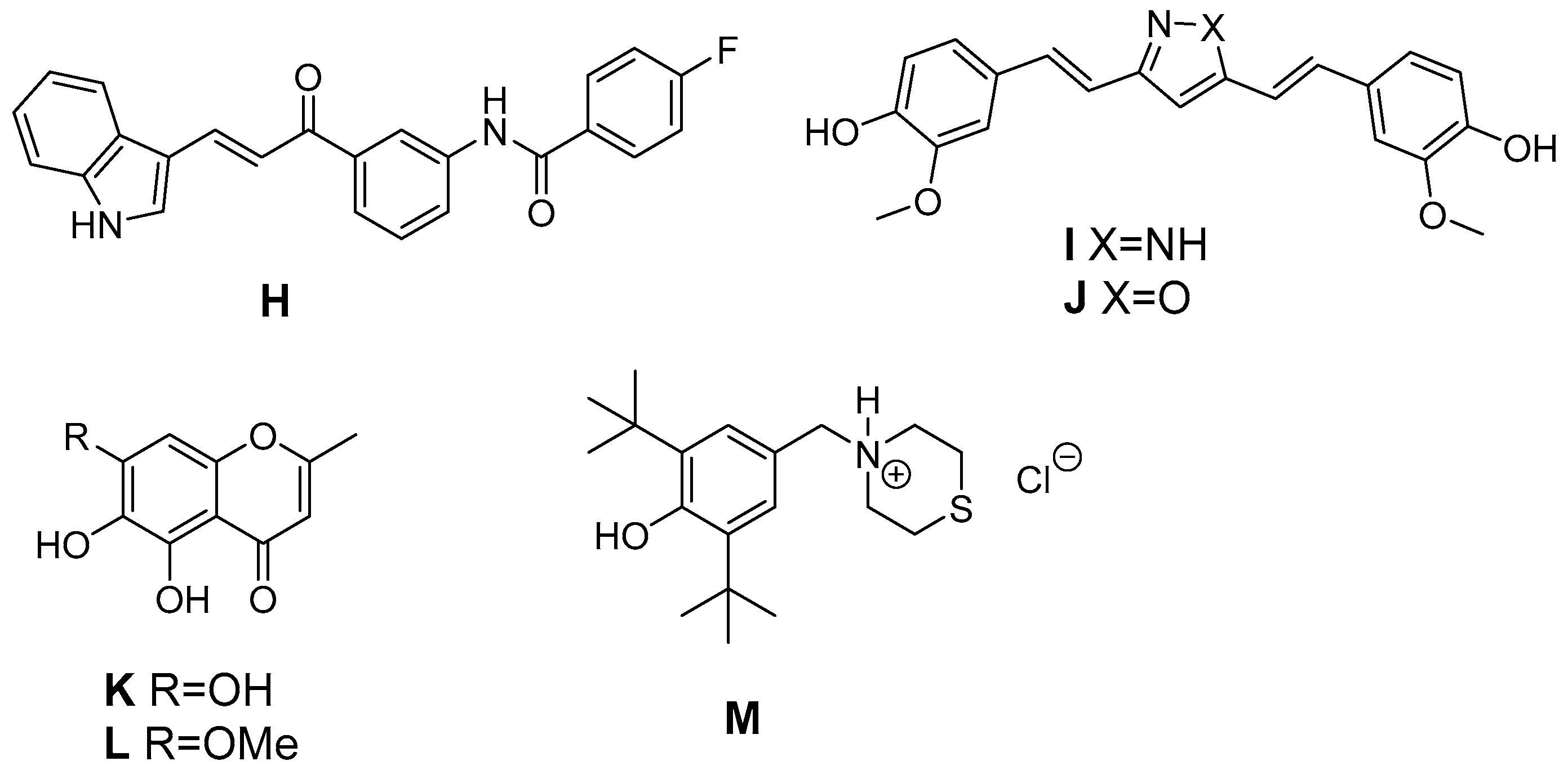
6. Antioxidant COX-2 Inhibitors as an Extensive Library for Promising Radioprotectants
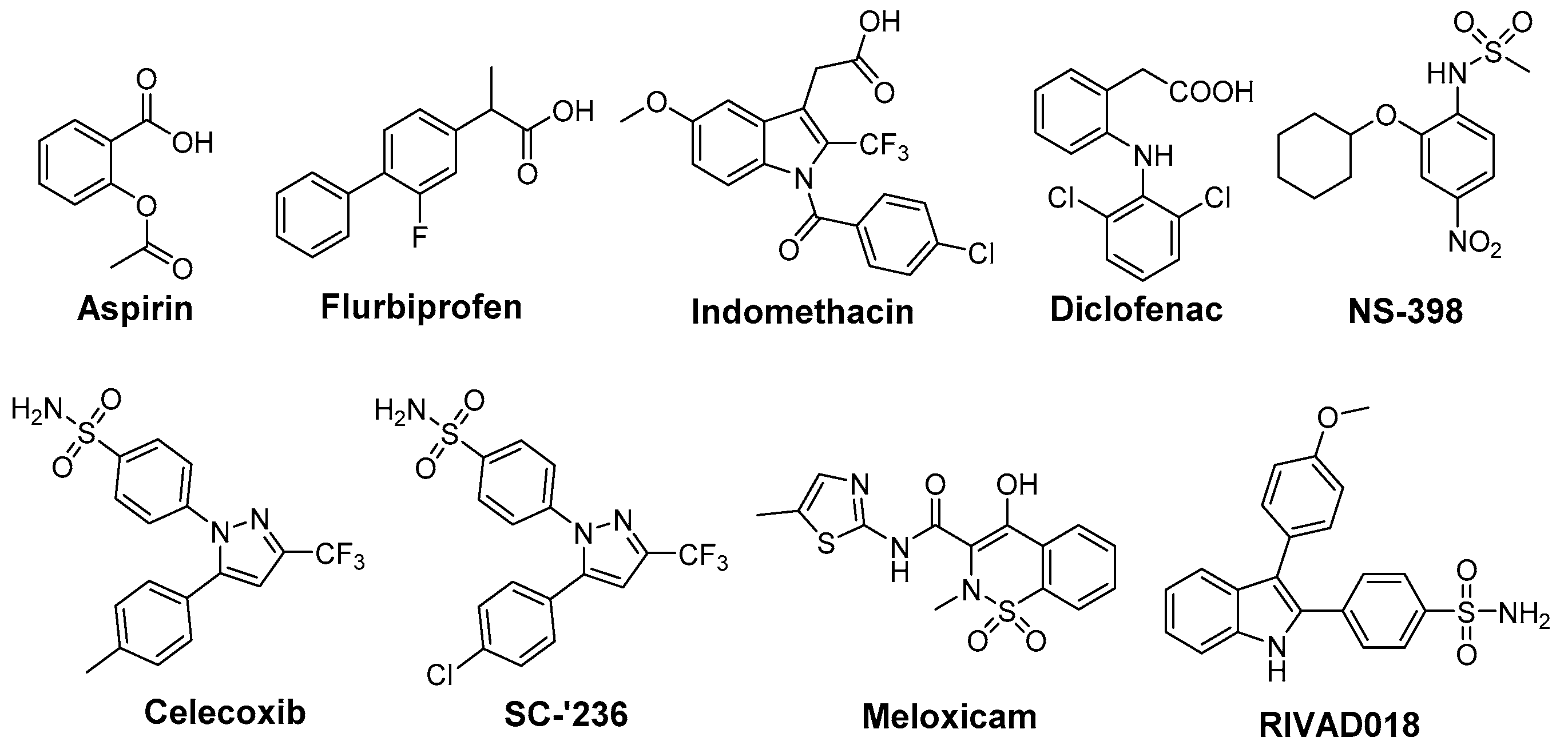
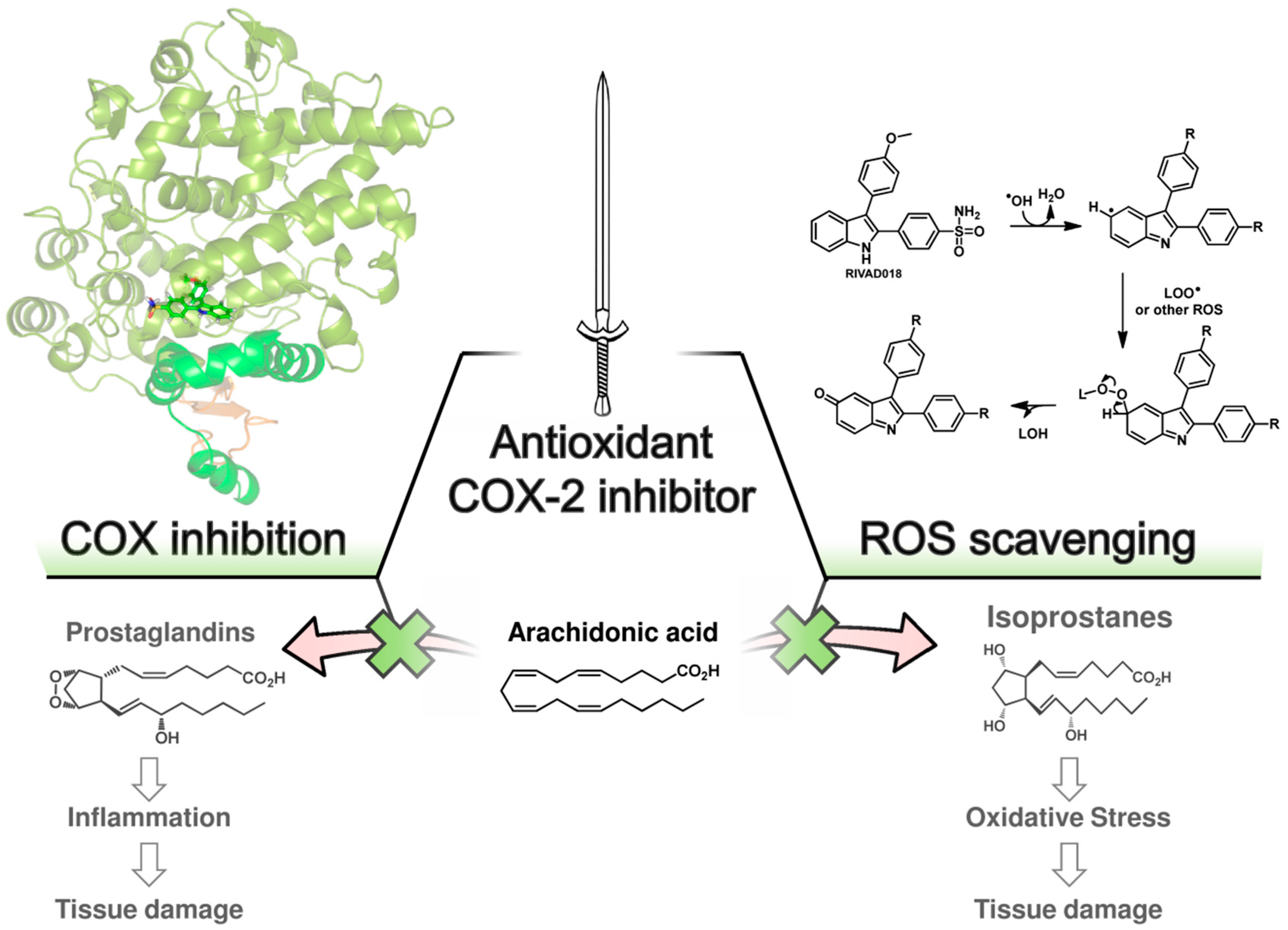
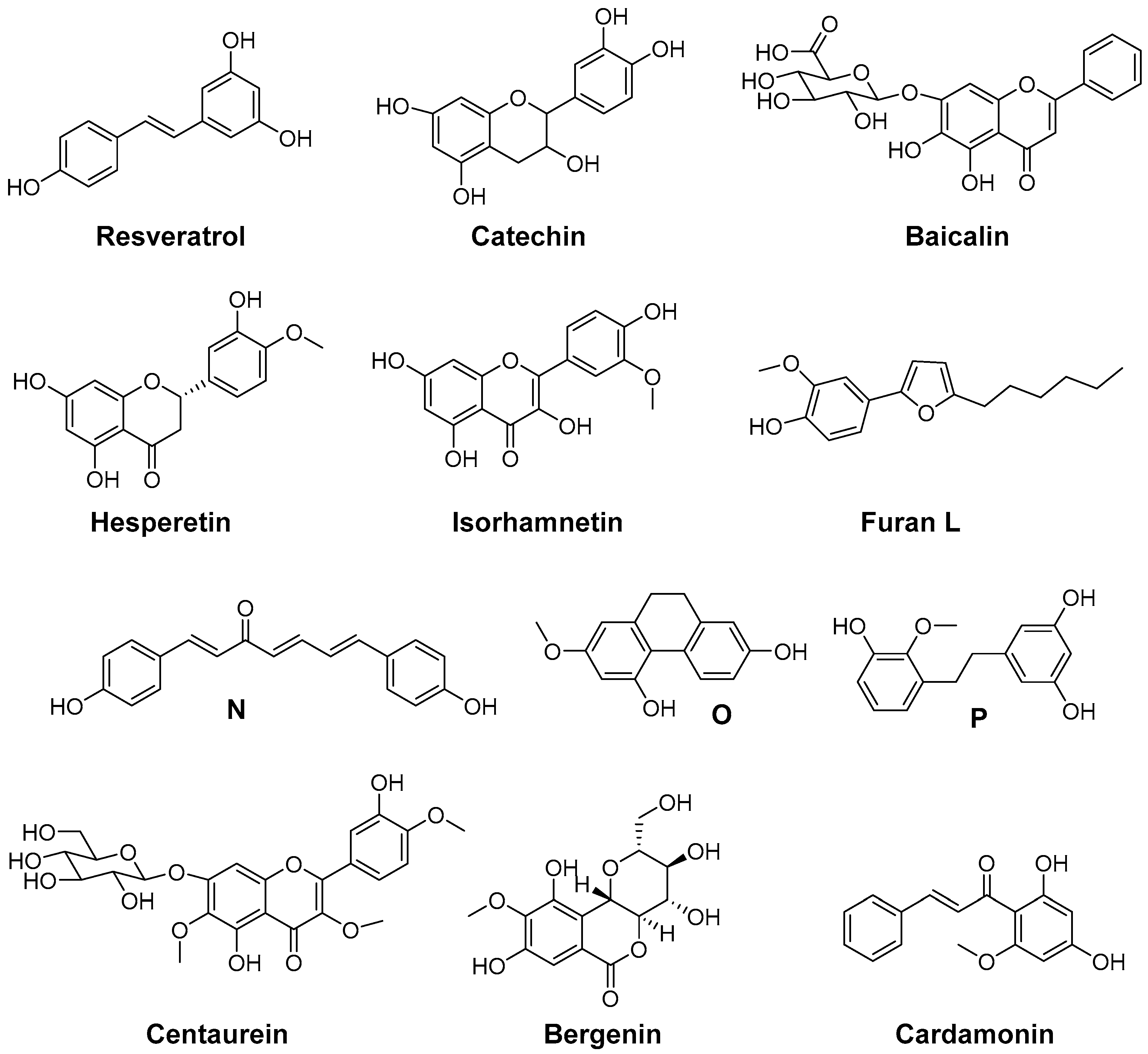
7. Indoles as Prominent Leads for the Development of Antioxidant Agents and Radioprotectors
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adam, A.; Kenny, L.M. Interventional oncology in multidisciplinary cancer treatment in the 21st century. Nat. Rev. Clin. Oncol. 2015, 12, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.L.; Connell, P.P.; Weichselbaum, R.R. New paradigms and future challenges in radiation oncology: An update of biological targets and technology. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Vasin, M.V. Comments on the mechanisms of action of radiation protective agents: Basis components and their polyvalence. SpringerPlus 2014, 3, 414. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Ranjan, A.; Kaur, N.; Sur, S.; Tandon, V. Radioprotective agents: Strategies and translational advances. Med. Res. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, L.J.; Pace, M.B.; Siddiqui, A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am. J. Health Syst. Pharm. 2013, 70, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Robbins, M.E. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: Therapeutic implications. Curr. Med. Chem. 2009, 16, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation-induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Nikitaki, Z.; Mavragani, I.V.; Laskaratou, D.A.; Gika, V.; Moskvin, V.P.; Theofilatos, K.; Vougas, K.; Stewart, R.D.; Georgakilas, A.G. Systemic mechanisms and effects of ionizing radiation: A new “old” paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Pavlopoulou, A.; Louka, M.; Nikitaki, Z.; Vorgias, C.E.; Bagos, P.G.; Michalopoulos, I. Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett. 2015, 368, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Basavaraju, S.R.; Easterly, C.E. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med. Phys. 2002, 29, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Hoving, S.; Russell, N.S. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat. Res. 2010, 174, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I. Radiation damage and radioprotectants: New concepts in the era of molecular medicine. Br. J. Radiol. 2012, 85, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex “wound”. Radiother. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravata, V. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Kura, B.; Ravingerova, T.; Tribulova, N.; Okruhlicova, L.; Barancik, M. Mechanisms of cardiac radiation injury and potential preventive approaches. Can. J. Physiol. Pharmacol. 2015, 93, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Chai, Y.; Ponnaiya, B.; Ivanov, V.N. Radiation induced non-targeted response: Mechanism and potential clinical implications. Curr. Mol. Pharmacol. 2011, 4, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; McCabe, M.T.; Hayward, S.W.; Park, J.M.; Day, M.L. Disruption of Rb/E2F pathway results in increased cyclooxygenase-2 expression and activity in prostate epithelial cells. Cancer Res. 2005, 65, 3633–3642. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Fitzpatrick, S.F.; Frank, T.; Birtwistle, M.; Taylor, C.T.; Cheong, A. NFkappaB and HIF display synergistic behaviour during hypoxic inflammation. Cell. Mol. Life Sci. 2012, 69, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Michalowski, A.S. On radiation-damage to normal-tissues and its treatment. 2. Antiinflammatory drugs. Acta Oncol. 1994, 33, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.L.G. Ionizing radiation and the endothelium. In Late Effects of Cancer Treatment on Normal Tissues; Rubin, P., Constine, L.S., Marks, L.B., Okunieff, P., Eds.; Springer: Berlin, Heidelberg, Germany, 2008; p. 19. [Google Scholar]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. Radioprotection by antioxidants. Ann. N. Y. Acad. Sci. 2000, 899, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Goldberg, J.D.; Perales, M.A.; Berger, D.P.; van den Brink, M.R. Clinical applications of palifermin: Amelioration of oral mucositis and other potential indications. J. Cell. Mol. Med. 2013, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Komaki, R.; Chang, J.; Liao, Z.; Cox, J.D.; Mason, K.; Milas, L. Radioprotectors and chemoprotectors in the management of lung cancer. In Advances in Radiation Oncology in Lung Cancer; Jeremic, B., Ed.; Springer: Berlin, Heidelberg, Germany, 2005; p. 123. [Google Scholar]
- Ullm, S.; Laube, M.; Bechmann, N.; Kniess, T.; Pietzsch, J. Organotypical vascular model for characterization of radioprotective compounds: Studies on antioxidant 2,3-diaryl-substituted indole-based cyclooxygenase-2 inhibitors. Clin. Hemorheol. Microcirc. 2014, 58, 281–295. [Google Scholar] [PubMed]
- Pietzsch, J.; Laube, M.; Bechmann, N.; Pietzsch, F.J.; Kniess, T. Protective effects of 2,3-diaryl-substituted indole-based cyclooxygenase-2 inhibitors on oxidative modification of human low density lipoproteins in vitro. Clin. Hemorheol. Microcirc. 2015, 61, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Masferrer, J.L.; Seibert, K.; Raz, A.; Needleman, P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 1990, 265, 16737–16740. [Google Scholar] [PubMed]
- De Vries, E.F. Imaging of cyclooxygenase-2 (COX-2) expression: Potential use in diagnosis and drug evaluation. Curr. Pharm. Des. 2006, 12, 3847–3856. [Google Scholar] [PubMed]
- Hawkey, C.J. COX-2 chronology. Gut 2005, 54, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity—A critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.H.; Olschowka, J.A.; Williams, J.P.; Paige, S.L.; O’Banion, M.K. Radiation-induced edema is dependent on cyclooxygenase 2 activity in mouse brain. Radiat. Res. 2004, 161, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Cerella, C.; Dicato, M.; Ghibelli, L.; Diederich, M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int. J. Cell. Biol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.; Milas, L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: A rational advance? J. Natl. Cancer Inst. 2003, 95, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Kim, D.; Lee, K.W.; Kim, J.B.; Lee, J.K.; Kim, D.S.; Lee, Y.I.; Ha, K.S.; Joe, C.O.; Han, P.L. COX-2 regulates the insulin-like growth factor I-induced potentiation of Zn2+-toxicity in primary cortical culture. Mol. Pharmacol. 2004, 66, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Kim, D.; Paik, S.G.; Han, P.L. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic. Biol. Med. 2006, 41, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Van der Donk, W.A.; Tsai, A.-L.; Kulmacz, R.J. The cyclooxygenase reaction mechanism. Biochemistry 2002, 41, 15451–15458. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Rao, R.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharmacet. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Jacob, R.F.; Day, C.A.; Dahlborg, R.; Weng, Y.; Mason, R.P. Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: Comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis 2004, 177, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.R.; Corey, E.J. Facile air oxidation of the conjugate base of rofecoxib (Vioxx™), a possible contributor to chronic human toxicity. Tetrahedron Lett. 2005, 46, 927–929. [Google Scholar] [CrossRef]
- Mason, R.P.; Walter, M.F.; McNulty, H.P.; Lockwood, S.F.; Byun, J.; Day, C.A.; Jacob, R.F. Rofecoxib increases susceptibility of human LDL and membrane lipids to oxidative damage: A mechanism of cardiotoxicity. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 1), S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. The COXIB experience: A look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Nel, D.; Adam, S. Reaching and influencing consumers in the prescription medicine market. Mark. Intell. Plan. 2009, 27, 909–925. [Google Scholar] [CrossRef]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J., 2nd. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Lawson, J.A.; Rokach, J.; FitzGerald, G.A. The isoprostanes in biology and medicine. Trends Endocrinol. Metab. 2001, 12, 243–247. [Google Scholar] [CrossRef]
- Praticò, D. Prostanoid and isoprostanoid pathways in atherogenesis. Atherosclerosis 2008, 201, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., 2nd. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Choy, H. Potential for combined modality therapy of cyclooxygenase inhibitors and radiation. Prog. Exp. Tumor Res. 2003, 37, 193–209. [Google Scholar] [PubMed]
- Furuta, Y.; Hunter, N.; Barkley, T., Jr.; Hall, E.; Milas, L. Increase in radioresponse of murine tumors by treatment with indomethacin. Cancer Res. 1988, 48, 3008–3013. [Google Scholar] [PubMed]
- Davis, T.W.; Hunter, N.; Trifan, O.C.; Milas, L.; Masferrer, J.L. COX-2 inhibitors as radio sensitizing agents for cancer therapy. Am. J. Clin. Oncol. 2003, 26, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Crokart, N.; Radermacher, K.; Jordan, B.F.; Baudelet, C.; Cron, G.O.; Gregoire, V.; Beghein, N.; Bouzin, C.; Feron, O.; Gallez, B. Tumor radiosensitization by anti-inflammatory drugs: Evidence for a new mechanism involving the oxygen effect. Cancer Res. 2005, 65, 7911–7916. [Google Scholar] [PubMed]
- Kishi, K.; Petersen, S.; Petersen, C.; Hunter, N.; Mason, K.; Masferrer, J.L.; Tofilon, P.J.; Milas, L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000, 60, 1326–1331. [Google Scholar] [PubMed]
- Milas, L.; Kishi, K.; Hunter, N.; Mason, K.; Masferrer, J.L.; Tofilon, P.J. Enhancement of tumor response to γ-radiation by an inhibitor of cyclooxygenase-2 enzyme. J. Natl. Cancer Inst. 1999, 91, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Petersen, S.; Milas, L.; Lang, F.F.; Tofilon, P.J. Enhancement of intrinsic tumor cell radiosensitivity induced by a selective cyclooxygenase-2 inhibitor. Clin. Cancer Res. 2000, 6, 2513–2520. [Google Scholar] [PubMed]
- Kang, K.B.; Wang, T.T.; Woon, C.T.; Cheah, E.S.; Moore, X.L.; Zhu, C.; Wong, M.C. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: Inhibition of tumor angiogenesis with extensive tumor necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.I.; Chiou, S.H.; Hueng, D.Y.; Tai, L.K.; Huang, P.I.; Kao, C.L.; Chen, Y.W.; Sytwu, H.K. Celecoxib and radioresistant glioblastoma-derived CD133+ cells: Improvement in radiotherapeutic effects. Laboratory investigation. J. Neurosurg. 2011, 114, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wagemakers, M.; van der Wal, G.E.; Cuberes, R.; Álvarez, I.; Andrés, E.M.; Buxens, J.; Vela, J.M.; Moorlag, H.; Mooij, J.J.A.; Molema, G. COX-2 inhibition combined with radiation reduces orthotopic glioma outgrowth by targeting the tumor vasculature. Transl. Oncol. 2009, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Klenke, F.M.; Abdollahi, A.; Bischof, M.; Gebhard, M.M.; Ewerbeck, V.; Huber, P.E.; Sckell, A. Celecoxib enhances radiation response of secondary bone tumors of a human non-small cell lung cancer via antiangiogenesis in vivo. Strahlenther. Onkol. 2011, 187, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Won, J.; Maeng, K.A.; Han, Y.S.; Yun, Y.S.; Hong, S.H. Nimesulide, a selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int. J. Oncol. 2009, 34, 1467–1473. [Google Scholar] [PubMed]
- Sminia, P.; Kuipers, G.; Geldof, A.; Lafleur, V.; Slotman, B. COX-2 inhibitors act as radiosensitizer in tumor treatment. Biomed. Pharmacother. 2005, 59, S272–S275. [Google Scholar] [CrossRef]
- Davis, T.W.; O’Neal, J.M.; Pagel, M.D.; Zweifel, B.S.; Mehta, P.P.; Heuvelman, D.M.; Masferrer, J.L. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2-derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004, 64, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.H.; Tian, X.Y.; Yu, J.J.; Mi, J.Q.; Liu, H.; Wang, R.F. Celecoxib radiosensitizes the human cervical cancer HeLa cell line via a mechanism dependent on reduced cyclo-oxygenase-2 and vascular endothelial growth factor C expression. J. Int. Med. Res. 2012, 40, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Raju, U.; Ariga, H.; Dittmann, K.; Nakata, E.; Ang, K.K.; Milas, L. Inhibition of DNA repair as a mechanism of enhanced radioresponse of head and neck carcinoma cells by a selective cyclooxygenase-2 inhibitor, celecoxib. Int. J. Radiat. Oncol. 2005, 63, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Che, S.M.; Zhang, X.Z.; Hou, L.; Song, T.B. Cyclooxygenase-2 inhibitor NS398 enhances radiosensitivity of radioresistant esophageal cancer cells by inhibiting AKT activation and inducing apoptosis. Cancer Investig. 2010, 28, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, K.H.; Mayer, C.; Ohneseit, P.A.; Raju, U.; Andratschke, N.H.; Milas, L.; Rodemann, H.P. Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: A COX-2 independent mechanism. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ohneseit, P.A.; Krebiehl, G.; Dittmann, K.; Kehlbach, R.; Rodemann, H.P. Inhibition of cyclooxygenase-2 activity by celecoxib does not lead to radiosensitization of human prostate cancer cells in vitro. Radiother. Oncol. 2007, 82, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.K.; Gibbs, I.; Ning, S.; French, J.N.; Armstrong, J.; Knox, S.J. Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 325–328. [Google Scholar] [CrossRef]
- Czembirek, C.; Eder-Czembirek, C.; Erovic, B.; Turhani, D.; Spittler, A.; Selzer, E.; Pötter, R.; Thurnher, D. The cyclooxygenase-2 inhibitor nimesulide, a nonsteroidal analgesic, decreases the effect of radiation therapy in head-and-neck cancer cells. Strahlenther. Onkol. 2009, 185, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Nakata, E.; Mason, K.A.; Hunter, N.; Husain, A.; Raju, U.; Liao, Z.; Ang, K.K.; Milas, L. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Handrick, R.; Ganswindt, U.; Faltin, H.; Goecke, B.; Daniel, P.T.; Budach, W.; Belka, C.; Jendrossek, V. Combined action of celecoxib and ionizing radiation in prostate cancer cells is independent of pro-apoptotic bax. Radiother. Oncol. 2009, 90, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Gerelchuluun, A.; Hong, Z.S.; Sun, L.; Zenkoh, J.; Moritake, T.; Tsuboi, K. Celecoxib enhances radiosensitivity of hypoxic glioblastoma cells through endoplasmic reticulum stress. Neuro-Oncology 2013, 15, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Raju, U.; Nakata, E.; Yang, P.Y.; Newman, R.A.; Ang, K.K.; Milas, L. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: Mechanistic considerations. Int. J. Radiat. Oncol. 2002, 54, 886–894. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; van den Berg, J.; Kuipers, G.K.; Wedekind, L.E.; Slotman, B.J.; van Rijn, J.; Lafleur, M.V.M.; Sminia, P. Radiosensitizing potential of the selective cyclooygenase-2 (COX-2) inhibitor meloxicam on human glioma cells. J. Neuro-Oncol. 2007, 85, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, A.; McCarthy, K.; Jawahar, A.; Smith, D.; Willis, B.; Nanda, A. Differential cyclooxygenase-2 enzyme expression in radiosensitive versus radioresistant glioblastoma multiforme cell lines. Anticancer Res. 2005, 25, 675–680. [Google Scholar] [PubMed]
- Ganswindt, U.; Budach, W.; Jendrossek, V.; Becker, G.; Bamberg, M.; Belka, C. Combination of celecoxib with percutaneous radiotherapy in patients with localised prostate cancer—A phase I study. Radiat. Oncol. 2006, 1. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.A.; Shepard, R. COX-2 inhibitors as radiation sensitizers for upper GI tract cancers: Esophagus, stomach, and pancreas. Am. J. Clin. Oncol. 2003, 26, S110–S113. [Google Scholar] [CrossRef] [PubMed]
- Debucquoy, A.; Roels, S.; Goethals, L.; Libbrecht, L.; Cutsem, E.V.; Geboes, K.; Penninckx, F.; D’Hoore, A.; McBride, W.H.; Haustermans, K. Double blind randomized phase ii study with radiation + 5-fluorouracil ± celecoxib for resectable rectal cancer. Radiother. Oncol. 2009, 93, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Hsiao, C.F.; Chen, W.T.; Lee, H.H.; Lin, T.C.; Chen, H.C.; Chen, H.H.; Chien, C.R.; Lin, T.Y.; Liu, T.W. Celecoxib plus chemoradiotherapy for locally advanced rectal cancer: A phase II tcog study. J. Surg. Oncol. 2014, 109, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Genden, E.M.; Chen, C.T.; Rivera, M.; Tong, C.C.; Misiukiewicz, K.; Gupta, V.; Gurudutt, V.; Teng, M.; Packer, S.H. Phase 1 trial of concurrent erlotinib, celecoxib, and reirradiation for recurrent head and neck cancer. Cancer 2011, 117, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Jalalabadi, Y.; Shirazi, A.; Ghavam-Nasiri, M.-R.; Davood, A.; Sardari, D. The role of celecoxib as a COX-2 inhibitor increasing the radiosensitivity of tumor tissue. Br. J. Med. Med. Res. 2015, 8, 123–139. [Google Scholar] [CrossRef]
- Hofer, M.; Pospisil, M.; Hoferova, Z.; Weiterova, L.; Komurkova, D. Stimulatory action of cyclooxygenase inhibitors on hematopoiesis: A review. Molecules 2012, 17, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M. Treatment of radiation-induced normal tissue lesions. Iran. J. Radiat. Res. 2003, 1, 63–78. [Google Scholar]
- Lalla, R.V.; Schubert, M.M.; Bensadoun, R.J.; Keefe, D. Anti-inflammatory agents in the management of alimentary mucositis. Support. Care Cancer 2006, 14, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Stupans, I. Radioprotection: The non-steroidal anti-inflammatory drugs (NSAIDs) and prostaglandins. J. Pharm. Pharmacol. 2002, 54, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; Stewart, F.A.; Oussoren, Y.; Weening, J.J.; Dewit, L. Amelioration of radiation nephropathy by acetylsalicylic acid. Int. J. Radiat. Biol. 1995, 67, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, E.M.; Te Poele, J.A.M.; Oussoren, Y.G.; van der Wal, A.; Dewi, L.G.H.; Stewartab, F.A. Influence of acetylsalicylic acid on development of radiation-induced nephropathy. Int. J. Radiat. Biol. 2000, 76, 1565–1573. [Google Scholar] [PubMed]
- Demirel, C.; Kilciksiz, S.C.; Gurgul, S.; Erdal, N.; Yigit, S.; Tamer, L.; Ayaz, L. Inhibition of radiation-induced oxidative damage in the lung tissue: May acetylsalicylic acid have a positive role? Inflammation 2015, 39, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Mennie, A.T.; Dalley, V.M.; Dinneen, L.C.; Collier, H.O.J. Treatment of radiation-induced gastrointestinal distress with acetylsalicylate. Lancet 1975, 2, 942–943. [Google Scholar] [CrossRef]
- Ludgate, C.M. Preliminary report: Acetylsalicylic acid therapy in the treatment of complications following abdominal radiation. J. Can. Assoc. Radiol. 1985, 36, 138–140. [Google Scholar] [PubMed]
- Olivotto, I.A.; Kim-Sing, C.; Bajdik, C.D.; Trevisan, C.H.; Ludgate, C.M.; Weir, L.M.; Jackson, S.M.; Basco, V.E. Effect of acetylsalicylic acid on radiation and cosmetic results after conservative surgery for early breast cancer: A randomized trial. Radiother. Oncol. 1996, 41, 1–6. [Google Scholar] [CrossRef]
- Irving, H.J.; Henderson, T.D.; Henderson, R.D.; L.Williams, E.; Willingham, W.M.; Sorenson, J.R.J. Comparison of the radiorecovery activity of copper(II)2(3,5-diisopropylsalicylate)4 and copper(II) (chloride)2. Inflammopharmacology 1995, 3, 251–257. [Google Scholar] [CrossRef]
- Sorenson, J.R. Bis(3,5-diisopropylsalicylato)copper(II), a potent radioprotectant with superoxide dismutase mimetic activity. J. Med. Chem. 1984, 27, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Borowska, A.; Sierakowski, S.; Mackowiak, J.; Wisniewski, K. A prostaglandin-like activity in small intestine and postirradiation gastrointestional syndrome. Experientia 1979, 35, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Tochner, Z.; Barnes, M.; Mitchell, J.B.; Orr, K.; Glatstein, E.; Russo, A. Protection by indomethacin against acute radiation esophagitis. Digestion 1990, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Halter, S.A.; Su, C.M. The effect of indomethacin on acute radiation induced gastrointestinal injury: A morphologic study. J. Surg. Oncol. 1992, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Briggs, D.B.; Knox, A.P.; Strominger, N.L. Radiation-induced emesis in the dog: Effects of lesions and drugs. Radiat. Res. 1986, 108, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Northway, M.G.; Libshitz, H.I.; Osborne, B.M.; Feldman, M.S.; Mamel, J.J.; West, J.H.; Szwarc, I.A. Radiation esophagitis in the opossum: Radioprotection with indomethacin. Gastroenterology 1980, 78, 883–892. [Google Scholar] [PubMed]
- Northway, M.G.; Scobey, M.W.; Geisinger, K.R. Radiation proctitis in the rat: Sequential changes and effects of anti-inflammatory agents. Cancer 1988, 62, 1962–1969. [Google Scholar] [CrossRef]
- Goldberg, R.I. Protection of irradiated parotid by prostaglandin synthesis inhibitors. J. Am. Dent. Assoc. 1986, 112, 179–181. [Google Scholar] [PubMed]
- Kandasamy, S.B.; Hunt, W.A. Involvement of prostaglandins and histamine in radiation-induced temperature responses in rats. Radiat. Res. 1990, 121, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.B.; Hunt, W.A.; Mickley, G.A. Implication of prostaglandins and histamine H1 and H2 receptors in radiation-induced temperature responses of rats. Radiat. Res. 1988, 114, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Drescher, J.J.; Tabachnick, J. Effect of anti-inflammatory compounds on beta-irradiation-induced increase in vascular peremeability. Radiat. Res. 1968, 34, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulos, N.; Mantidis, A.; Stathopoulos, E.; Papaodysseas, S.; Kouvaris, J.; Varveris, H.; Papavasiliou, C. Prophylactic administration of indomethacin for irradiation esophagitis. Radiother. Oncol. 1985, 3, 23–25. [Google Scholar] [CrossRef]
- Pillsbury, H.C., 3rd; Webster, W.P.; Rosenman, J. Prostaglandin inhibitor and radiotherapy in advanced head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Bito, L.Z.; Klein, E.M. The role of the arachidonic acid cascade in the species-specific X-ray-induced inflammation of the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1982, 22, 579–587. [Google Scholar]
- Stokman, M.A.; Spijkervet, F.K.; Burlage, F.R.; Roodenburg, J.L. Clinical effects of flurbiprofen tooth patch on radiation-induced oral mucositis. A pilot study. Support. Care Cancer 2005, 13, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kozubik, A.; Hofmanova, J.; Pospisil, M.; Netikova, J.; Hola, J.; Lojek, A. Effects of drugs inhibiting prostaglandin or leukotriene biosynthesis on postirradiation hematopoiesis in mouse. Int. J. Radiat. Biol. 1994, 65, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospisil, M.; Tkadlecek, L.; Viklicka, S.; Pipalova, I.; Hola, J. Low survival of mice following lethal gamma-irradiation after administration of inhibitors of prostaglandin synthesis. Physiol. Res. 1992, 41, 157–161. [Google Scholar] [PubMed]
- Floersheim, G.L. Allopurinol, indomethacin and riboflavin enhance radiation lethality in mice. Radiat. Res. 1994, 139, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Juchelkova, L.; Hofer, M.; Pospisil, M.; Pipalova, I. Radioprotective effects of flurbiprofen and its nitroderivative. Physiol. Res. 1998, 47, 73–80. [Google Scholar] [PubMed]
- Hofer, M.; Pospisil, M.; Znojil, V.; Hola, J.; Vacek, A.; Weiterova, L.; Streitova, D.; Kozubik, A. Meloxicam, a cyclooxygenase 2 inhibitor, supports hematopoietic recovery in gamma-irradiated mice. Radiat. Res. 2006, 166, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospisil, M.; Dusek, L.; Hoferova, Z.; Weiterova, L. A single dose of an inhibitor of cyclooxygenase 2, meloxicam, administered shortly after irradiation increases survival of lethally irradiated mice. Radiat. Res. 2011, 176, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospisil, M.; Dusek, L.; Hoferova, Z.; Komurkova, D. Combined pharmacological therapy of the acute radiation disease using a cyclooxygenase-2 inhibitor and an adenosine A3 receptor agonist. Cent. Eur. J. Biol. 2014, 9, 642–646. [Google Scholar] [CrossRef]
- Fedorocko, P.; Mackova, N.O. Combined modality radioprotection: Enhancement of survival and hematopoietic recovery in gamma-irradiated mice by the joint use of liposomal muramyl tripeptide phosphatidylethanolamine (MTP-PE) and indomethacin. Int. J. Immunopharmacol. 1996, 18, 329–337. [Google Scholar] [CrossRef]
- Fedorocko, P.; Mackova, O. Radioprotective effects of combination broncho-vaxom, a macrophage activator, and indomethacin, an inhibitor of prostaglandin production: Relationship to myelopoiesis. Eur. J. Haematol. 1996, 56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, G.; Charest, G.; Fortin, D.; Bujold, R.; Mathieu, D.; Paquette, B. Cyclooxygenase-2 inhibitor prevents radiation-enhanced infiltration of F98 glioma cells in brain of fischer rat. Int. J. Radiat. Biol. 2015, 91, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Cihan, Y.; Orhan, O.; Yilmaz, S.; Akgun, H.; Saraymen, R. COX-2 as inhibitors to protect radiation induced pneumonia. Asian J. Chem. 2009, 21, 6167–6175. [Google Scholar]
- Khayyal, M.T.; El-Ghazaly, M.A.; El-Hazek, R.M.; Nada, A.S. The effects of celecoxib, a COX-2 selective inhibitor, on acute inflammation induced in irradiated rats. Inflammopharmacology 2009, 17, 255–266. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazaly, M.; Nada, A.S.; El-Hazek, R.; Khayyal, M.T. Effect of selective COX-2 inhibitor, celecoxib on adjuvant-induced arthritis model in irradiated rats. Int. J. Radiat. Biol. 2010, 86, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Hu, D.; Liu, W.; Williams, J.P.; Okunieff, P.; Ding, I. Celecoxib reduces skin damage after radiation: Selective reduction of chemokine and receptor mrna expression in irradiated skin but not in irradiated mammary tumor. Am. J. Clin. Oncol. 2003, 26, S114–S121. [Google Scholar] [CrossRef] [PubMed]
- Haagen, J.; Krohn, H.; Röllig, S.; Schmidt, M.; Wolfram, K.; Dörr, W. Effect of selective inhibitors of inflammation on oral mucositis: Preclinical studies. Radiother. Oncol. 2009, 92, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Choquette, L.E.; Curley, K.F.; Dowsett, R.J.; Feinn, R.S.; Hegde, U.P.; Pilbeam, C.C.; Salner, A.L.; Sonis, S.T.; Peterson, D.E. Randomized double-blind placebo-controlled trial of celecoxib for oral mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol. 2014, 50, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Kiang, J.G.; Cary, L.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. COX-2 inhibitors are contraindicated for treatment of combined injury. Radiat. Res. 2009, 172, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Paquette, B.; Therriault, H.; Desmarais, G.; Wagner, R.; Royer, R.; Bujold, R. Radiation-enhancement of MDA-MB-231 breast cancer cell invasion prevented by a cyclooxygenase-2 inhibitor. Br. J. Cancer 2011, 105, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.N.; Ivanov, V.N.; Gillespie, J.; Geard, C.R.; Amundson, S.A.; Brenner, D.J.; Yu, Z.L.; Lieberman, H.B.; Hei, T.K. Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14641–14646. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, S.; Wang, Z.; Zhou, W.; Luan, M.; Yang, X.; Chen, N. Induction of apoptosis and cell cycle arrest by NS398 in oral squamous cell carcinoma cells via downregulation of E2 promoter-binding factor-1. Oncol. Rep. 2008, 20, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, J.; Laube, M.; Pietzsch, F.J.; Bergmann, R.; Kniess, T. Concomitant targeting of cyclooxygenase-2 and oxidant stress pathways for radioprotection of endothelial cells. In Coronary Artery Disease: 2011 Update: From Prevention to Intervention; Medimond s.r.l.: Bologna, Italy, 2011; pp. 107–110. [Google Scholar]
- Hu, W.; Guo, Z.; Yi, X.; Guo, C.; Chu, F.; Cheng, G. Discovery of 2-phenyl-3-sulfonylphenyl-indole derivatives as a new class of selective COX-2 inhibitors. Bioorg. Med. Chem. 2003, 11, 5539–5544. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Guo, Z.; Chu, F.; Bai, A.; Yi, X.; Cheng, G.; Li, J. Synthesis and biological evaluation of substituted 2-sulfonyl-phenyl-3-phenyl-indoles: A new series of selective COX-2 inhibitors. Bioorg. Med. Chem. 2003, 11, 1153–1160. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, G.; Chu, F. Sulfonyl-Containing 2,3-Diarylindole Compounds, Methods for Making Same, and Methods of Use Thereof. U.S. Patent 2004/58977, 25 March 2004. [Google Scholar]
- Kniess, T.; Laube, M.; Bergmann, R.; Sehn, F.; Graf, F.; Steinbach, J.; Wuest, F.; Pietzsch, J. Radiosynthesis of a 18F-labeled 2,3-diarylsubstituted indole via mcmurry coupling for functional characterization of cyclooxygenase-2 (COX-2) in vitro and in vivo. Bioorg. Med. Chem. 2012, 20, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Tondera, C.; Laube, M.; Wimmer, C.; Kniess, T.; Mosch, B.; Grossmann, K.; Pietzsch, J. Visualization of cyclooxygenase-2 using a 2,3-diarylsubstituted indole-based inhibitor and confocal laser induced cryofluorescence microscopy at 20K in melanoma cells in vitro. Biochem. Biophys. Res. Commun. 2013, 430, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tondera, C.; Ullm, S.; Laube, M.; Meister, S.; Neuber, C.; Mosch, B.; Kniess, T.; Pietzsch, J. Optical imaging of COX-2: Studies on an autofluorescent 2,3-diaryl-substituted indole-based cyclooxygenase-2 inhibitor. Biochem. Biophys. Res. Commun. 2015, 458, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Tondera, C.; Sharma, S.K.; Bechmann, N.; Pietzsch, F.-J.; Pigorsch, A.; Kockerling, M.; Wuest, F.; Pietzsch, J.; Kniess, T. 2,3-Diaryl-substituted indole based COX-2 inhibitors as leads for imaging tracer development. RSC Adv. 2014, 4, 38726–38742. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Damiani, E.; Jassem, W.; Wozniak, M.; Orena, M.; Greci, L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Radic. Biol. Med. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- Suzen, S.; Bozkaya, P.; Coban, T.; Nebiogu, D. Investigation of the in vitro antioxidant behaviour of some 2-phenylindole derivatives: Discussion on possible antioxidant mechanisms and comparison with melatonin. J. Enzyme Inhib. Med. Chem. 2006, 21, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, J.; Pietzsch, F.J.; Laube, M.; Bergmann, R.; Kniess, T.; Wuest, F. 837 concomitant targeting of cyclooxygenase-2 and oxidant stress pathways for radioprotection of normal vascular tissue. EJC Suppl. 2010, 8. [Google Scholar] [CrossRef]
- Ullm, S.; Sehn, F.; Laube, M.; Tondera, C.; Bechmann, N.; Mosch, B.; Kniess, T.; Pietzsch, J. Targeting cyclooxygenase-2 and oxidant stress pathways for attenuation of radiation-induced vascular dysfunction. In Proceedings of the 6th European Congress of Pharmacology, Granada, Spain, 17–20 July 2012; Zarzuelo, A., Jimenez, R., Eds.; Medimond S.r.l—Monduzzi Editore International: Pianoro, Italy, 2013; p. 87. [Google Scholar]
- Wuest, F.; Kniess, T.; Bergmann, R.; Pietzsch, J. Synthesis and evaluation in vitro and in vivo of a 11C-labeled cyclooxygenase-2 (COX-2) inhibitor. Bioorg. Med. Chem. 2008, 16, 7662–7670. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Sanchez, S.C.; Musiek, E.S.; Morrow, J.D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007, 2, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Gassner, C.; Sharma, S.K.; Gunther, R.; Pigorsch, A.; Konig, J.; Kockerling, M.; Wuest, F.; Pietzsch, J.; Kniess, T. Diaryl-substituted (dihydro)pyrrolo[3,2,1-hi]indoles, a class of potent COX-2 inhibitors with tricyclic core structure. J. Org. Chem. 2015, 80, 5611–5624. [Google Scholar] [CrossRef] [PubMed]
- Süzen, S. Antioxidant activities of synthetic indole derivatives and possible activity mechanisms. Top. Heterocycl. Chem. 2007, 11, 145–178. [Google Scholar]
- Burnett, B.P.; Bitto, A.; Altavilla, D.; Squadrito, F.; Levy, R.M.; Pillai, L. Flavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), and 5-lipoxygenase, modifies COX-2 gene expression, and acts as an antioxidant. Mediat. Inflamm. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Moutinho, L.; Lima, J.L.; Fernandes, E. Antioxidant activity and inhibition of human neutrophil oxidative burst mediated by arylpropionic acid non-steroidal anti-inflammatory drugs. Biol. Pharm. Bull. 2006, 29, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Twomey, B.M.; Dale, M.M. Cyclooxygenase-independent effects of nonsteroidal anti-inflammatory drugs on the neutrophil respiratory burst. Biochem. Pharmacol. 1992, 43, 413–418. [Google Scholar] [CrossRef]
- Wasil, M.; Halliwell, B.; Moorhouse, C.P.; Hutchison, D.C.; Baum, H. Biologically-significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by some anti-inflammatory drugs. Biochem. Pharmacol. 1987, 36, 3847–3850. [Google Scholar] [CrossRef]
- Fernandes, E.; Toste, S.A.; Lima, J.L.; Reis, S. The metabolism of sulindac enhances its scavenging activity against reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2003, 35, 1008–1017. [Google Scholar] [CrossRef]
- Neve, J.; Parij, N.; Moguilevsky, N. Inhibition of the myeloperoxidase chlorinating activity by non-steroidal anti-inflammatory drugs investigated with a human recombinant enzyme. Eur. J. Pharmacol. 2001, 417, 37–43. [Google Scholar] [CrossRef]
- Van Antwerpen, P.; Nève, J. In vitro comparative assessment of the scavenging activity against three reactive oxygen species of non-steroidal anti-inflammatory drugs from the oxicam and sulfoanilide families. Eur. J. Pharmacol. 2004, 496, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Facino, R.M.; Carini, M.; Aldini, G. Antioxidant activity of nimesulide and its main metabolites. Drugs 1993, 46 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Saibene, L.; Morelli, R. Differential inhibition of superoxide, hydroxyl and peroxyl radicals by nimesulide and its main metabolite 4-hydroxynimesulide. Arzneimittelforschung 1995, 45, 1102–1109. [Google Scholar] [PubMed]
- Nam, T.G.; Nara, S.J.; Zagol-Ikapitte, I.; Cooper, T.; Valgimigli, L.; Oates, J.A.; Porter, N.A.; Boutaud, O.; Pratt, D.A. Pyridine and pyrimidine analogs of acetaminophen as inhibitors of lipid peroxidation and cyclooxygenase and lipoxygenase catalysis. Org. Biomol. Chem. 2009, 7, 5103–5112. [Google Scholar] [CrossRef] [PubMed]
- Kullich, W.; Fagerer, N.; Schwann, H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr. Med. Res. Opin. 2007, 23, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M.; Uguz, A.C.; Gokcimen, A.; Bulbul, M.; Karatopuk, D.U.; Turker, Y.; Cerçi, C. Tenoxicam modulates antioxidant redox system and lipid peroxidation in rat brain. Neurochem. Res. 2008, 33, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Ardicoglu, O.; Erdogan, H.; Fadillioglu, E.; Gudul, H. In vivo effect of celecoxib and tenoxicam on oxidant/anti-oxidant status of patients with knee osteoarthritis. Ann. Clin. Lab. Sci. 2005, 35, 137–143. [Google Scholar] [PubMed]
- Hiller, K.O.; Hodd, P.L.; Willson, R.L. Antiinflammatory drugs: Protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem. Biol. Interact. 1983, 47, 293–305. [Google Scholar] [CrossRef]
- Fernandes, E.; Costa, D.; Toste, S.A.; Lima, J.L.; Reis, S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic. Biol. Med. 2004, 37, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Gul, S.; Gul, H.; Zia-Ul-Haq, M.; Ercisli, S. Cyclooxygenase-2 inhibition improves antioxidative defense during experimental hypercholesterolemia. Bosn. J. Basic Med. Sci. 2014, 14, 63–69. [Google Scholar] [PubMed]
- Gupta, S.; Sarotra, P.; Aggarwal, R.; Dutta, N.; Agnihotri, N. Role of oxidative stress in celecoxib-induced renal damage in wistar rats. Dig. Dis. Sci. 2007, 52, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Demiryilmaz, I.; Turan, M.I.; Kisaoglu, A.; Gulaboglu, M.; Yilmaz, I.; Suleyman, H. Protective effect of nimesulide against hepatic ischemia/reperfusion injury in rats: Effects on oxidant/antioxidants, DNA mutation and COX-1/COX-2 levels. Pharmacol. Rep. 2014, 66, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Adsul, L.K.; Chavan, H.V.; Jalde, S.S.; Shringare, S.N.; Shaikh, R.; Meshram, R.J.; Gacche, R.N.; Masand, V. Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5-(9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Chavan, H.V.; Adsul, L.K.; Thakare, V.N.; Shringare, S.N.; Shaikh, R.; Gacche, R.N. Design, synthesis, characterization and biological evaluation of novel pyrazole integrated benzophenones. Bioorg. Med. Chem. Lett. 2013, 23, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Ulbrich, H.K.; Soehnlein, O.; Lindbom, L.; Mattern, A.; Dannhardt, G. Diaryl-dithiolanes and -isothiazoles: COX-1/COX-2 and 5-LOX-inhibitory, •OH scavenging and anti-adhesive activities. Bioorg. Med. Chem. 2009, 17, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H.; Shaari, K.; Mohamed, H.; Wahab, A.A.; Ariffin, K.T.; Hoo, W.Y.; Aziz, N.A.; Kadir, A.A.; et al. Cardamonin, inhibits pro-inflammatory mediators in activated raw 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherif, Y.E.-S.; El-Asmy, A.A.-H.; Lotfy, M. 4-hydroxy-2-methyl-N-(2-thiazole)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide (EX15) and its cu(II) complex as new oxicam selective cyclooxygenase-2 inhibitors. Croat. Chem. Acta 2012, 85, 19–26. [Google Scholar] [CrossRef]
- Bengmark, S. Curcumin, an atoxic antioxidant and natural NFκB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic diseases. JPEN J. Parenter. Enter. Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Kinkar, S.N.; Chavan, H.V.; Jalde, S.S.; Shaikh, R.U.; Gacche, R.N. Synthesis and biological evaluation of asymmetric indole curcumin analogs as potential anti-inflammatory and antioxidant agents. J. Enzyme Inhib. Med. Chem. 2014, 29, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M.; Kumar, V.; Mohan, C.G. Synthesis, biological evaluation and molecular docking studies of stellatin derivatives as cyclooxygenase (COX-1, COX-2) inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2011, 21, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, G.N.; Rekka, E.A.; Gavalas, A.M.; Eleftheriou, P.T.; Kourounakis, P.N. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2006, 14, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.H.; Kim, S.H.; Lee, S.; Lim, S.J. Comparison of three tocopherol analogs as an inhibitor of production of proinflammatory mediators in macrophages. J. Pharmacol. Sci. 2012, 118, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; de Oliveira, A.C.P.; Graef, S.; Bhatia, H.S.; Huell, M.; Munoz, E.; Fiebich, B.L. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflamm. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Squadrito, F.; Irrera, N.; Pizzino, G.; Pallio, G.; Mecchio, A.; Galfo, F.; Altavilla, D. Flavocoxid, a nutraceutical approach to blunt inflammatory conditions. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar] [PubMed]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The antioxidant effects of isorhamnetin contribute to inhibit COX-2 expression in response to inflammation: A potential role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Fukuhara, A.; Omata, Y.; Saito, Y.; Yamaguchi, S.; Kato, H.; Yoshida, Y.; Niki, E. Characterization of novel furan compounds on the basis of their radical scavenging activity and cytoprotective effects against glutamate- and lipopolysaccharide-induced insults. Bioorg. Med. Chem. 2008, 16, 10332–10337. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Yoon, K.D.; Chin, Y.-W.; Park, J.H.; Kim, J. Phenolic compounds with radical scavenging and cyclooxygenase-2 (COX-2) inhibitory activities from dioscorea opposita. Bioorg. Med. Chem. 2009, 17, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Jachak, S.M.; Gautam, R.; Selvam, C.; Madhan, H.; Srivastava, A.; Khan, T. Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of tridax procumbens l. Fitoterapia 2011, 82, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.D.; Barreiro, E.J.; Fraga, C.A.M. From nature to drug discovery: The indole scaffold as a “privileged structure”. Mini Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef]
- Aiello, F.V. Guiseppe. Synthesis and evaluation of indole based molecules for treatment of oxidative stress related diseases. Curr. Top. Med. Chem. 2014, 14, 2576–2589. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, K.; Madhumitha, G.; Roopan, S.M. Indole as a core anti-inflammatory agent—A mini review. Chem. Sci. Rev. Lett. 2013, 2, 287–292. [Google Scholar]
- Lal, S.; Snape, T.J. 2-Arylindoles: A privileged molecular scaffold with potent, broad-ranging pharmacological activity. Curr. Med. Chem. 2012, 19, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.; Ghobadi, G.; Ghazi-Khansari, M. A radiobiological review on melatonin: A novel radioprotector. J. Radiat. Res. 2007, 48, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Walters-Laporte, E.; Furman, C.; Fouquet, S.; Martin-Nizard, F.; Lestavel, S.; Gozzo, A.; Lesieur, D.; Fruchart, J.C.; Duriez, P.; Teissier, E. A high concentration of melatonin inhibits in vitro LDL peroxidation but not oxidized LDL toxicity toward cultured endothelial cells. J. Cardiovasc. Pharmacol. 1998, 32, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Thuermann, S.; Dose, A.; Schoenke, M.; Burkhardt, S.; Hardeland, R. Melatonin’s unique radical scavenging properties—Roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res. 2002, 33, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Silva, C.; Vacondio, F.; Rivara, M.; Plazzi, P.; Franceschinit, D.; et al. Antioxidant and cytoprotective activity of indole derivatives related to melatonin. Adv. Exp. Med. Biol. 2003, 527, 567–575. [Google Scholar] [PubMed]
- Gozzo, A.; Lesieur, D.; Duriez, P.; Fruchart, J.C.; Teissier, E. Structure-activity relationships in a series of melatonin analogues with the low-density lipoprotein oxidation model. Free Radic. Biol. Med. 1999, 26, 1538–1543. [Google Scholar] [CrossRef]
- Lissi, E.A.; Faure, M.; Montoya, N.; Videla, L.A. Reactivity of indole derivatives towards oxygenated radicals. Free Radic. Res. Commun. 1991, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tabor, M.W.; Coats, E.; Sainsbury, M.; Shertzer, H.G. Antioxidation potential of indole compounds—Structure activity studies. Adv. Exp. Med. Biol. 1991, 283, 833–836. [Google Scholar] [PubMed]
- Suzen, S.; Cihaner, S.S.; Coban, T. Synthesis and comparison of antioxidant properties of indole-based melatonin analogue indole amino acid derivatives. Chem. Biol. Drug Des. 2012, 79, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Ferreira, L.M.; Fernandes, E.; Marques, M.M.B. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Kruk, I.; Lichszteld, K.; Michalska, T.; Kladna, A.; Marczynski, S.; Ölgen, S. Scavenging of reactive oxygen species by N-substituted indole-2 and 3-carboxamides. Luminescence 2004, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olgen, S.; Coban, T. Synthesis and antioxidant properties of novel N-substituted indole-2-carboxamide and indole-3-acetamide derivatives. Arch. Pharm. 2002, 335, 331–338. [Google Scholar] [CrossRef]
- Ölgen, S.; Coban, T. Antioxidant activity of n-substituted indole 2- and 3-carboxamides. J. Fac. Pharm Ank. 2004, 33, 109–116. [Google Scholar]
- Olgen, S.; Guner, E.; Fabregat, M.A.; Crespo, M.I.; Nebioglu, D. Syntheses and biological evaluation of indole-2 and 3-carboxamides: New selective cyclooxygenase-2 inhibitors. Pharmazie 2002, 57, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Olgen, S.; Akaho, E.; Nebioglu, D. Synthesis and receptor docking studies of N-substituted indole-2-carboxylic acid esters as a search for COX-2 selective enzyme inhibitors. Eur. J. Med. Chem. 2001, 36, 747–770. [Google Scholar] [CrossRef]
- Kruk, I.; Aboul-Enein, H.Y.; Michalska, T.; Lichszteld, K.; Kubasik-Kladna, K.; Ölgen, S. In vitro scavenging activity for reactive oxygen species by N-substituted indole-2-carboxylic acid esters. Luminescence 2007, 22, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.A.; Bordunov, V.; Broka, C.A.; Browner, M.F.; Kress, J.M.; Mirzadegan, T.; Ramesha, C.; Sanpablo, B.F.; Stabler, R.; Takahara, P.; et al. Rational design of 6-methylsulfonylindoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 4741–4745. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Bhardwaj, A.; Huang, Z.; Knaus, E.E. N-1 and C-3 substituted indole schiff bases as selective COX-2 inhibitors: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Estevão, M.S.; Carvalho, L.C.R.; Freitas, M.; Gomes, A.; Viegas, A.; Manso, J.; Erhardt, S.; Fernandes, E.; Cabrita, E.J.; Marques, M.M.B. Indole based cyclooxygenase inhibitors: Synthesis, biological evaluation, docking and NMR screening. Eur. J. Med. Chem. 2012, 54, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Saibene, L.; Macciocchi, A. Antioxidant profile of nimesulide, indomethacin and diclofenac in phosphatidylcholine liposomes (PCL) as membrane model. Int. J. Tissue React. 1993, 15, 225–234. [Google Scholar] [PubMed]
- Purpora, R.; Massad, W.; Ferrari, G.; Reynoso, E.; Criado, S.; Miskoski, S.; Pajares, A.; García, N.A. The NSAIDs indomethacin and diflunisal as scavengers of photogenerated reactive oxygen species. Photochem. Photobiol. 2013, 89, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Marnett, A.B.; Kozak, K.R.; Remmel, R.P.; Marnett, L.J. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: Facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc. Natl. Acad. Sci. USA 2000, 97, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Marnett, A.B.; Crews, B.C.; Remmel, R.P.; Marnett, L.J. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 2000, 43, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Crews, B.C.; Saleh, S.; Prudhomme, D.; Marnett, L.J. Indolyl esters and amides related to indomethacin are selective COX-2 inhibitors. Bioorg. Med. Chem. 2005, 13, 6810–6822. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Crews, B.C.; Ghebreselasie, K.; Marnett, L.J. Design, synthesis, and structure–activity relationship studies of fluorescent inhibitors of cycloxygenase-2 as targeted optical imaging agents. Bioconjug. Chem. 2013, 24, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Blobaum, A.L.; Marnett, L.J.; Hey-Hawkins, E. Ortho-carbaborane derivatives of indomethacin as cyclooxygenase (COX)-2 selective inhibitors. Bioorg. Med. Chem. 2012, 20, 4830–4837. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Crews, B.C.; Blobaum, A.L.; Kingsley, P.J.; Gorden, D.L.; McIntyre, J.O.; Matrisian, L.M.; Subbaramaiah, K.; Dannenberg, A.J.; Piston, D.W.; et al. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010, 70, 3618–3627. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.; Xu, S.; Sarosi, M.B.; Scholz, M.S.; Crews, B.C.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J.; Hey-Hawkins, H.C. Nido-dicarbaborate induces potent and selective inhibition of cyclooxygenase-2. ChemMedChem 2015, 11, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Crews, B.C.; Huda, I.; Ghebreselasie, K.; Daniel, C.K.; Marnett, L.J. Trifluoromethyl fluorocoxib a detects cyclooxygenase-2 expression in inflammatory tissues and human tumor xenografts. ACS Med. Chem. Lett. 2014, 5, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Bayly, C.; Belley, M.; Chan, C.C.; Charleson, S.; Denis, D.; Gauthier, J.Y.; Gordon, R.; Guay, D.; Kargman, S.; et al. From indomethacin to a selective COX-2 inhibitor: Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1996, 6, 725–730. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Uddin, M.J.; Felts, A.S.; Crews, B.C.; Rouzer, C.A.; Marnett, L.J. The 2′-trifluoromethyl analogue of indomethacin is a potent and selective COX-2 inhibitor. ACS Med. Chem. Lett. 2013, 4, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A. Design and synthesis of some 5-substituted-2-(4-(azido or methylsulfonyl)phenyl)-1H-indole derivatives as selective cyclooxygenase (COX-2) inhibitors. Sci. Pharm. 2008, 76, 361–376. [Google Scholar] [CrossRef]
- Laube, M.; Neumann, W.; Scholz, M.; Lonnecke, P.; Crews, B.; Marnett, L.J.; Pietzsch, J.; Kniess, T.; Hey-Hawkins, E. 2-Carbaborane-3-phenyl-1H-indoles—Synthesis via McMurry reaction and cyclooxygenase (COX) inhibition activity. ChemMedChem 2013, 8, 329–335. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.J.; Ghobadi, G.; de Boer, R.A.; Faber, H.; Cannon, M.V.; Nagle, P.W.; Brandenburg, S.; Langendijk, J.A.; van Luijk, P.; Coppes, R.P. Ace inhibition attenuates radiation-induced cardiopulmonary damage. Radiother. Oncol. 2015, 114, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Jelveh, S.; Zaidi, A.; Doctrow, S.R.; Medhora, M.; Hill, R.P. Targeting the renin-angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Kniess, T.; Kockerling, M.; Pigorsch, A.; Steinbach, J.; Pietzsch, J. Novel (pyrazolyl)benzenesulfonamides with a nitric oxide-releasing moiety as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3295–3300. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S. Nitric oxide for cancer therapy. Future Sci. OA 2015, 1, FSO44. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laube, M.; Kniess, T.; Pietzsch, J. Development of Antioxidant COX-2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review. Antioxidants 2016, 5, 14. https://doi.org/10.3390/antiox5020014
Laube M, Kniess T, Pietzsch J. Development of Antioxidant COX-2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review. Antioxidants. 2016; 5(2):14. https://doi.org/10.3390/antiox5020014
Chicago/Turabian StyleLaube, Markus, Torsten Kniess, and Jens Pietzsch. 2016. "Development of Antioxidant COX-2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review" Antioxidants 5, no. 2: 14. https://doi.org/10.3390/antiox5020014