Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries
Abstract
:1. Introduction
2. Preventive and Normalizing Effect on Obesity and Diabetes
2.1. Anti-Obesity
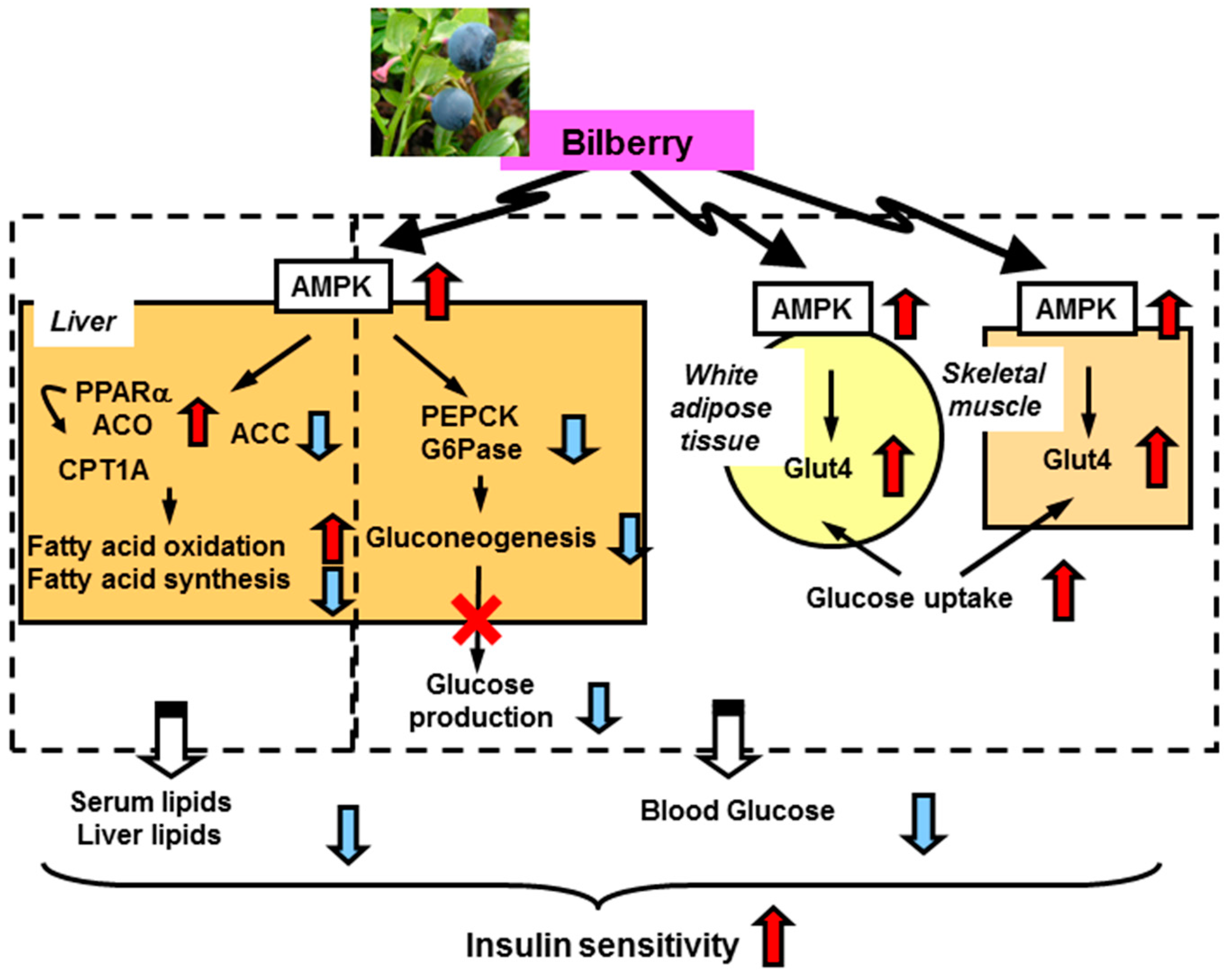
2.2. Anti-Diabetes
3. Health Benefits of Berries and Involvement of Their Metabolites
4. Interventional Studies in Humans
5. Future Research Needs and Prospects
Acknowledgements
Conflicts of Interest
References
- Harborne, J.B.; Grayer, R.J. The Flavonoids; Chapman and Hall: London, UK, 1988; pp. 1–20. [Google Scholar]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama., M. Effects of blackcurrant anthocyanosides intake on dark adaptation and VDT work-induced transient refractive alternation in healthy humans. Altern. Med. Rev. 2000, 5, 553–562. [Google Scholar] [PubMed]
- Iida, H.; Nakamura, Y.; Matsumoto, H.; Takeuchi, Y.; Harano, S.; Ishihara, M.; Katsumi, O. Effect of black-currant extract on negative lens-induced ocular growth in chicks. Ophthalmic. Res. 2010, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.H.; Voutilainen, S.; Virtanen, J.K.; Venho, B.; Vanharanta, M.; Mursu, J.; Salonen, J.T. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J. Nutr. 2003, 133, 199–204. [Google Scholar] [PubMed]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [PubMed]
- Ellingsen, I.; Hjerkinn, E.M.; Seljeflot, I.; Arnesen, H.; Tonstad, S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. Br. J. Nutr. 2008, 99, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Teller, N.; Cai, H.; Marko, D.; Berry, D.P.; Steward, W.P.; Gescher, A.J. Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother. Pharmacol. 2009, 64, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Phromnoi, K.; Yadav, V.R.; Chaturvedi, M.M.; Aggarwal, B.B. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010, 76, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40–S47. [Google Scholar] [CrossRef] [PubMed]
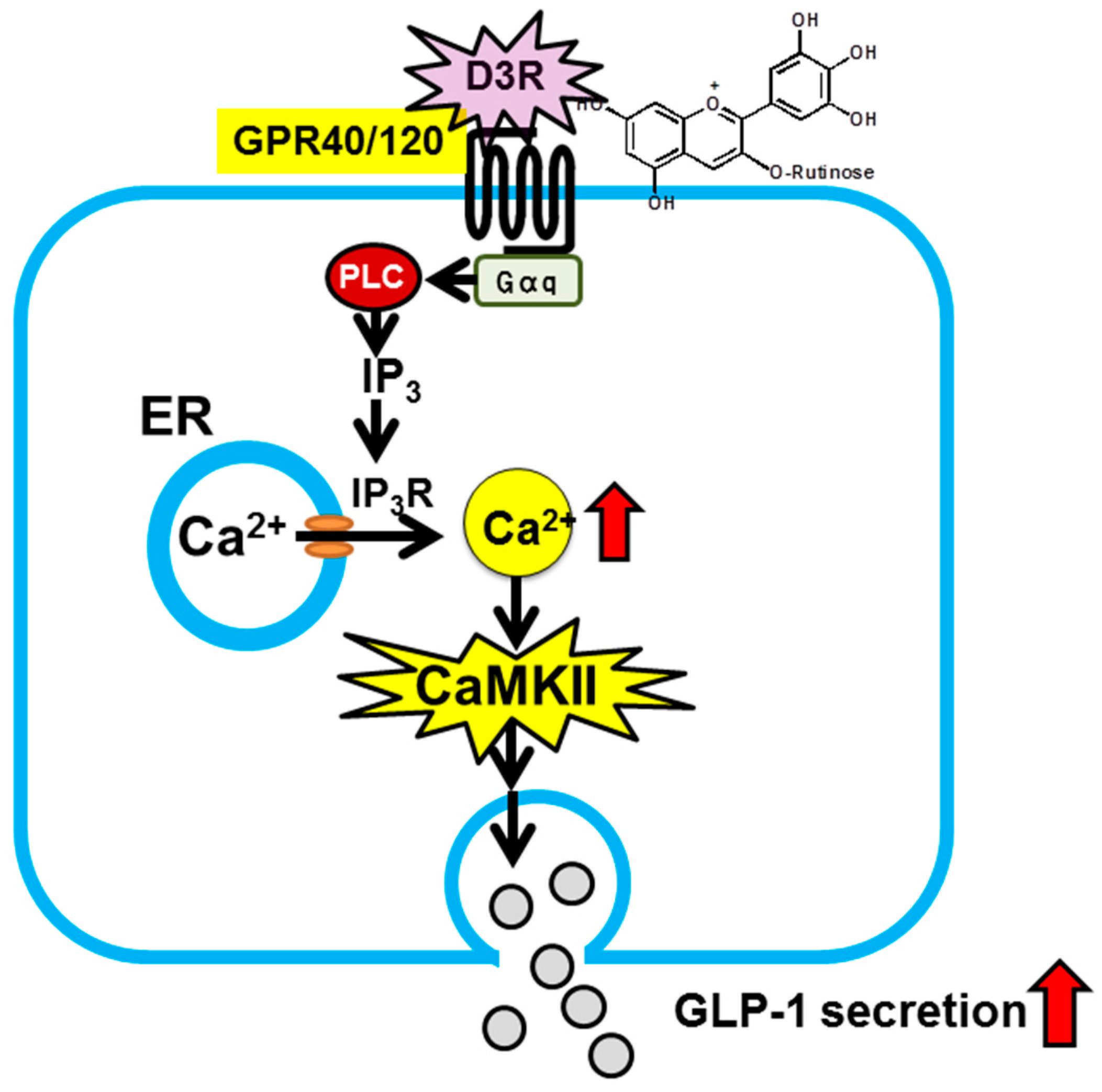
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The anthocyanin delphinidin 3-rutinoside stimulates glucagon-like peptide-1 secretion in murine GLUTag cell line via the Ca2+/calmodulin-dependent kinase II pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar]
- Tsuda, T. Regulation of adipocyte function by anthocyanins: Possibility of preventing the metabolic syndrome. J. Agric. Food Chem. 2008, 56, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Yoshikawa, T.; Kojo, H.; Osawa, T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006, 71, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W., 2nd; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food. 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br. J. Nutr. 2014, 111, 194–200. [Google Scholar]
- Vendrame, S.; Zhao, A.; Merrow, T.; Klimis-Zacas, D. The effects of wild blueberry consumption on plasma markers and gene expression related to glucose metabolism in the obese Zucker rat. J. Med. Food. 2015, 18, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.; Wilkes, S.; Howard, L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 2009, 53, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Howard, L.R. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food. 2009, 12, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2011, 108, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, Y.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, Y.; Shibayama, S.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; Tsuda, T. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Elks, C.M.; Terrebonne, J.D.; Ingram, D.K.; Stephens, J.M. Blueberries improve glucose tolerance without altering body composition in obese postmenopausal mice. Obesity 2015, 23, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Herman, G.A.; Bergman, A.; Stevens, C.; Kotey, P.; Yi, B.; Zhao, P.; Dietrich, B.; Golor, G.; Schrodter, A.; Keymeulen, B.; et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 4612–4619. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Brock, B.; Perrild, H.; Levin, K.; Lervang, H.H.; Kølendorf, K.; Krarup, T.; Schmitz, O.; Zdravkovic, M.; Le-Thi, T.; et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet. Med. 2008, 25, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Possible abilities of dietary factors to prevent and treat diabetes via the stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2015, 59, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-β-d-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanis and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.-A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magnuson, B.A.; Giusti, M.M. Analysis of anthocyanins in rat intestinal contents—Impact of anthocyanin chemical structure on fecal excretion. J. Agric. Food Chem. 2005, 53, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Identification of Cabernet Sauvignon Anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J. Agric. Food Chem. 2010, 58, 5320–5327. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Hidalgo, M.; Sanchez-Moreno, C.; Pelaez, C.; Requena, T.; de Pascual-Teresa, S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Cheynier, V.; Donovan, J.L.; Manach, C.; Morand, C.; Mila, I.; Lapierre, C.; Rémésy, C.; Scalbert, A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J. Nutr. 2003, 133, 461–467. [Google Scholar] [PubMed]
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.; Crozier, A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-MecaIntake, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Liu, Y.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A. Anthocyanin metabolites are abundant and persistent in human urine. J. Agric. Food Chem. 2014, 62, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Wright, O.R.; Netzel, G.A.; Sakzewski, A.R. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the QUENCH trial. Can. J. Physiol. Pharmacol. 2013, 91, 480–488. [Google Scholar] [CrossRef] [PubMed]
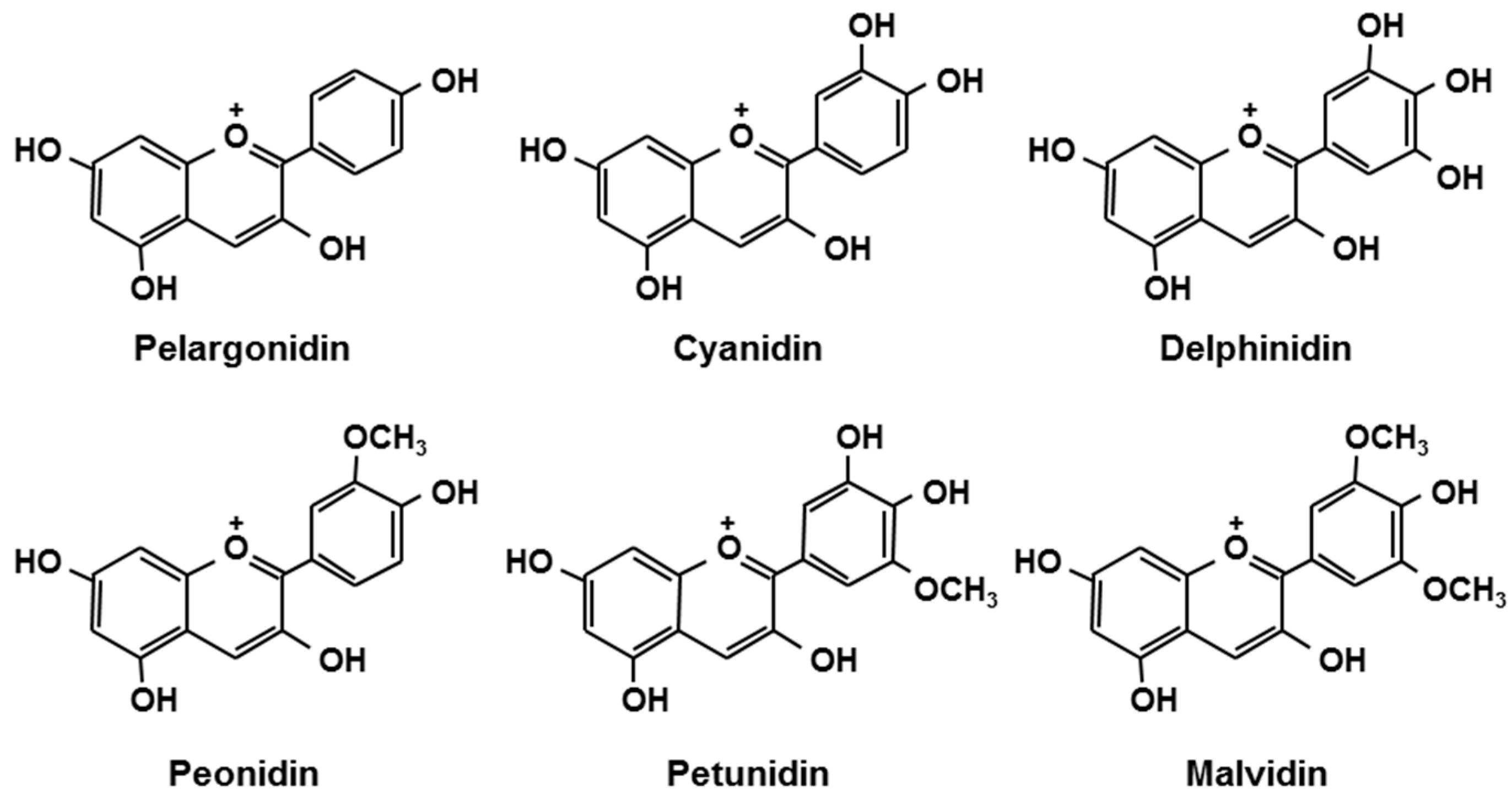

| Berries | Anthocyanins | mg/g Extract a |
|---|---|---|
| Bilberry | cyanidin 3-galactoside | 3.70 |
| cyanidin 3-glucoside | 4.05 | |
| cyanidin 3-arabinoside | 2.54 | |
| delphinidin 3-galactoside | 4.58 | |
| delphinidin 3-glucoside | 4.73 | |
| delphinidin 3-arabinoside | 3.53 | |
| peonidin 3-galactoside | 0.46 | |
| petunidin 3-halactoside | 1.52 | |
| petunidin 3-glucoside | 2.94 | |
| petunidin 3-arabinoside | 0.84 | |
| malvidin 3-arabinoside | 0.81 | |
| peonidin 3-glucoside/malvidin 3-galactoside | 3.48 | |
| peonidin 3-arabinoside/malvidin 3-glucoside | 3.62 | |
| Blackberry | cyanidin 3-glucoside | 7.17 |
| cyanidin 3-rutinoside | 0.06 | |
| cyanidin 3-arabinoside | 0.05 | |
| cyanidin 3-xyloside | 0.47 | |
| cyanidin 3-(6-malonoyl)glucoside | 0.3 | |
| cyanidin 3-dioxaloylglucoside | 2.05 | |
| Blackcurrant | cyanidin 3-glucoside | 1.1 |
| cyanidin 3-rutinoside | 7.08 | |
| delphinidin 3-glucoside | 2.94 | |
| delphinidin 3-rutinoside | 9.79 | |
| peonidin 3-rutinoside | 0.11 | |
| petunidin 3-rutinoside | 0.18 | |
| Blueberry | cyanidin 3-galactoside | 0.28 |
| cyanidin 3-glucoside | 0.04 | |
| cyanidin 3-arabinoside | 0.12 | |
| delphinidin 3-galactoside | 1.37 | |
| delphinisin 3-glucoside | 0.13 | |
| delphinidin 3-arabinoside | 0.74 | |
| peonidin 3-galactoside | 0.15 | |
| petunidin 3-galactoside | 1.07 | |
| petunidin 3-glucoside | 0.11 | |
| petunidin 3-arabinoside | 0.46 | |
| marlidin 3-arabinoside | 1.75 | |
| peonidin 3-glucoside/malvidin 3-galactoside | 3.65 | |
| peonidin 3-arabinoside/malvidin 3-glucoside | 0.43 | |
| Strawberry | cyanidin 3-glucoside | 0.09 |
| pelargonidin 3-glucoside | 5.07 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, T. Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries. Antioxidants 2016, 5, 13. https://doi.org/10.3390/antiox5020013
Tsuda T. Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries. Antioxidants. 2016; 5(2):13. https://doi.org/10.3390/antiox5020013
Chicago/Turabian StyleTsuda, Takanori. 2016. "Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries" Antioxidants 5, no. 2: 13. https://doi.org/10.3390/antiox5020013






