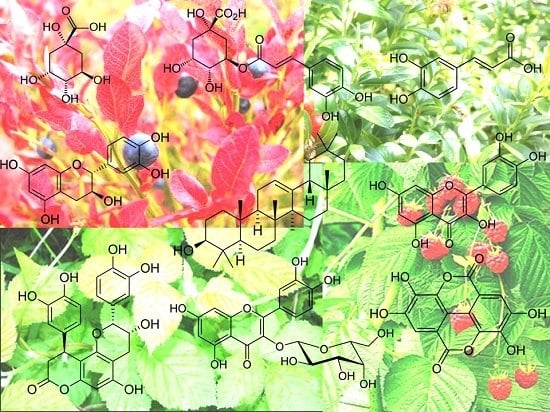
Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value †
Abstract
:1. Introduction
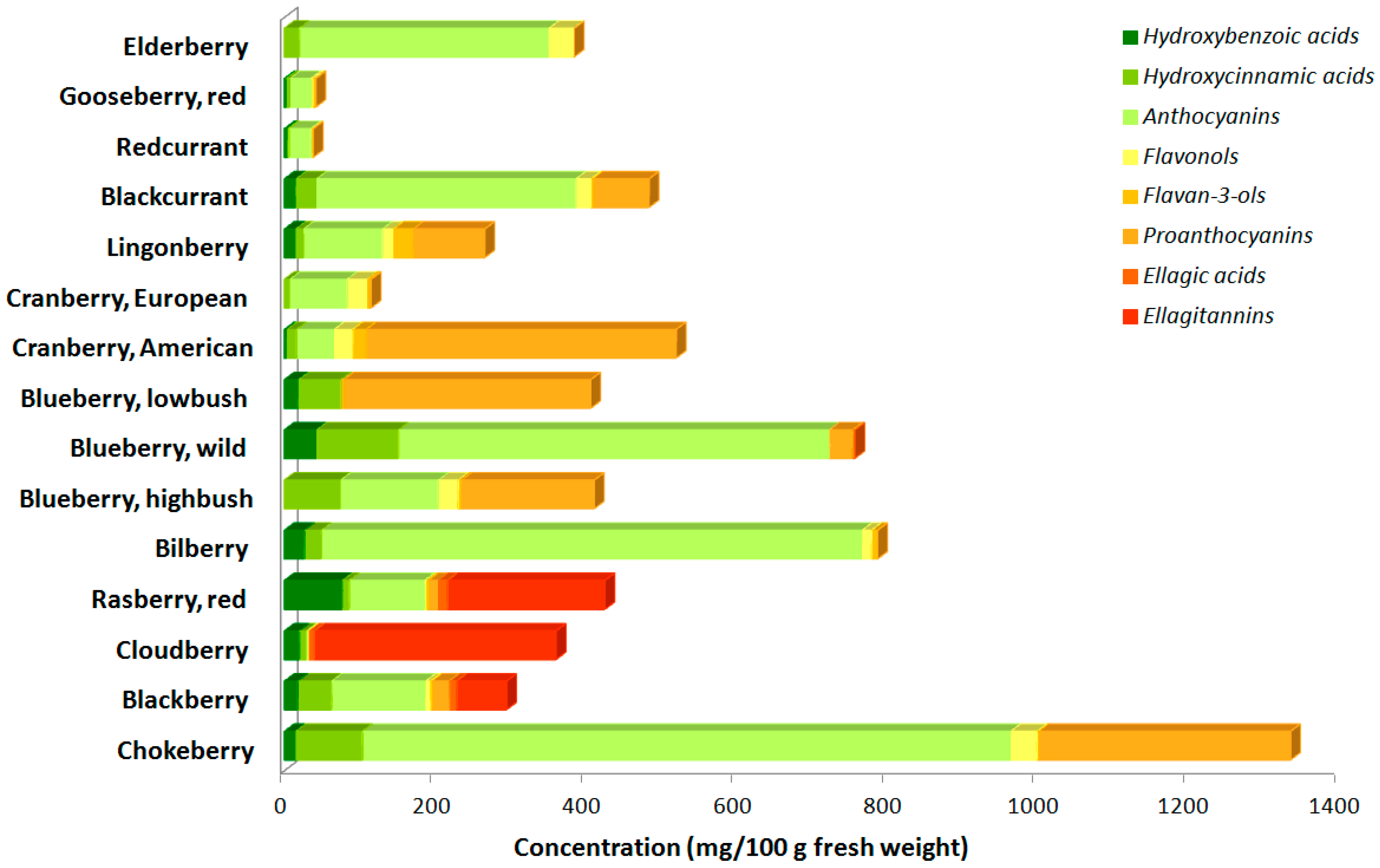
2. Blackcurrant (Ribes nigrum) Leaves
3. Blackberry (Rubus fruticosus) Leaves
4. Raspberry (Rubus idaeus) Leaves
5. Bilberry (Vaccinium myrtillus) Leaves
6. Blueberry (Vaccinium sp.) Leaves
7. Cranberry (Vaccinium sp.) Leaves
8. Lingonberry (Vaccinium vitis-idaea) Leaves
9. Conclusions
Conflicts of Interest
Abbreviations
| DAD | Diode Array Detection |
| EGF | Epidermal Growth Factor |
| EMA | European Medicines Agency |
| ESI | Electronspray ionization |
| HMG | Hydroxymethylglutaric acid |
| HPLC | High Performance Liquid Chromatography |
| HPTLC | High Performance Thin Layer Chromatography |
| IR | Infrared spectroscopy |
| LC | Liquid Chromatography |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| TOF | Time-of-flight |
References
- European Commission. Functional Foods; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2004, 1, 71–86. [Google Scholar] [CrossRef]
- Mattila, P.; Hellstrom, J.; Torronen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; Gonzalez-Paramas, A.M.; Torronen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P. Berry Phenolics: Isolation, Analysis, Identification and Antioxidant Properties. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2011. [Google Scholar]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of northwest Vaccinium berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Call for Scientific Data for Use in HMPC Assessment Work on Fragaria vesca L., Folium. European Medicines Agency: London, UK, 2014. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Rubus idaeus L., Folium. European Medicines Agency: London, UK, 2012. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Ribes nigrum L., Folium. European Medicines Agency: London, UK, 2009. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Arctostaphylos uva-ursi (L.) Spreng., Folium. European Medicines Agency: London, UK, 2009. [Google Scholar]
- Connolly, T.J. Newberry crater: A ten-thousand-year record of human occupation and environmental change in the basin-plateau borderlands. Plains Anthropol. 2003, 48, 168–170. [Google Scholar]
- Riaz, M.A.; Rahman, N.U. Antimicrobial screening of fruit, leaves, root and stem of Rubus fruticosus L. J. Med. Plants Res. 2011, 5, 5920–5924. [Google Scholar]
- Sarkar, A.; Ganguly, S.N. Rubitic acid, a new triterpene acid from Rubus fruticosus. Phytochemistry 1978, 17, 1983–1985. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, P.; Kaldmae, H.; Kikas, A.; Libek, A.V.; Püssa, T. Nutritional quality of berries and bioactive compounds in the leaves of black currant (Ribes nigrum L.) cultivars evaluated in Estonia. J. Berry Res. 2010, 1, 53–59. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Rubus idaeus L., Folium. European Medicines Agency: London, UK, 2012. [Google Scholar]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shandi, K.; Al-Rawashdeh, A.; Al-Mazaideh, G.; Abu-Nameh, E.; Al-Amro, A.; Al-Soufi, H.; Al-Ma’abreh, A.; Al-Dawdeyah, A. A novel strategy for the identification of the medicinal natural products in Rubus fruticosus plant by using GC/MS technique: A study on leaves, stems and roots of the plant. Adv. Anal. Chem. 2015, 5, 31–41. [Google Scholar]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Riaz, M.; de Feo, V.; Jaafar, H.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.M.; Robinson, R. A survey of anthocyanins. Biochem. J. 1931, 25, 1687–1705. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Ghatak, K.L.; Ganguly, S.N.; Antoulas, S. Rubinic acid, a triterpene acid from Rubus fruticosus. Phytochemistry 1984, 23, 2581–2582. [Google Scholar] [CrossRef]
- Greenway, F.L.; Zhijun, L.; Woltering, E.A. Angiogenic Agents from Plant Extracts, Gallic Acid and Derivatives. U.S. Patent 20,070,031,332, 8 February 2007. [Google Scholar]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee of the British Herbal Medicine Association. Rubus. In British Herbal Pharmacopoeia, 2nd Consolidated; British Herbal Medicine Association: Bournemouth, UK, 1983; pp. 181–182. [Google Scholar]
- Jan, G. Kaemferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Polon. Pharm. 2003, 60, 313–316. [Google Scholar]
- Sadowska, B.; Paszkiewicz, M.; Podsedek, A.; Redzynia, M.; Rozalska, B. Vaccinium myrtillus leaves and Frangula alnus bark derived extracts as potential antistaphylococcal agents. Acta Biochim. Pol. 2014, 61, 163–169. [Google Scholar] [PubMed]
- Mechikova, G.Y.; Kuzmich, A.S.; Ponomarenko, L.P.; Kalinovsky, A.I.; Stepanova, T.A.; Fedorov, S.N.; Stonik, V.A. Cancer-preventive activities of secondary metabolites from leaves of the bilberry Vaccinium smallii A. Gray. Phytother. Res. 2010, 24, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttila, A.M.; Tolonen, A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef] [PubMed]
- Riihinen, K.; Jaakola, L.; Karenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Paczkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Maatta-Riihinen, K.; Karenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2004, 218, 721–728. [Google Scholar] [PubMed]
- Martz, F.; Jaakola, L.; Julkunen-Tiitto, R.; Stark, S. Phenolic composition and antioxidant capacity of bilberry (Vaccinium myrtillus) leaves in Northern Europe following foliar development and along environmental gradients. J. Chem. Ecol. 2010, 36, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Helmstadter, A.; S chuster, N. Vaccinium myrtillus as an antidiabetic medicinal plant—Research through the ages. Parmazie 2010, 65, 315–321. [Google Scholar]
- Wang, L.J.; Wu, J.; Wang, H.X.; Li, S.S.; Zheng, X.C.; Du, H.; Xu, Y.J.; Wang, L.S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Hänsel, R.; Keller, K.; Rimpler, H.; Schneider, G. Drogen p–z. In Hager’s Handbuch der Pharmazeutischen Praxis, 5th ed.; Springer-Verlag GmbH: Berlin Heidelberg, Germany, 1996; pp. 1051–1067. [Google Scholar]
- Matsuo, Y.; Fujita, Y.; Ohnishi, S.; Tanaka, T.; Hirabaru, H.; Kai, T.; Sakaida, H.; Nishizono, S.; Kouno, I. Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and a-type linkages. Food Chem. 2010, 121, 1073–1079. [Google Scholar]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.; Arnason, J.T. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [PubMed]
- Ferlemi, A.V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Hasnat, M.A.; Lim, B.O. Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [Google Scholar] [CrossRef]
- Piljac-Zegarac, J.; Belscak, A.; Piljac, A. Antioxidant capacity and polyphenolic content of blueberry (Vaccinium corymbosum L.) leaf infusions. J. Med. Food 2009, 12, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopée Française. Cassis (feuille de) Ribis nigri Folium; Ministere de la Sante: Paris, France, 1996. [Google Scholar]
- Trajkovski, V. Resistance to Sphaerotheca mors-uvae (Schw.) Berk. in Ribes nigrum L. Swed. J. Agric. Res. 1974, 4, 99–108. [Google Scholar]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic compounds in blackcurrant (Ribes nigrum L.) leaves relative to leaf position and harvest date. Food Chem 2015, 172, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An optimized method for analysis of phenolic compounds in buds, leaves, and fruits of black currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef] [PubMed]
- Tits, M.; Poukens, P.; Angenot, L.; Dierckxsens, Y. Thin-layer chromatographic analysis of proanthocyanidins from Ribes nigrum leaves. J. Pharm. Biomed. Anal. 1992, 10, 1097–1100. [Google Scholar] [CrossRef]
- Tits, M.; Angenot, L.; Poukens, P.; Warin, R.; Dierckxsens, Y. Prodelphinidins from Ribes nigrum. Phytochemistry 1992, 31, 971–973. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Ribes nigrum L., Folium; European Medicines Agency: London, UK, 2010. [Google Scholar]
- Andersson, J.; Bosvik, R.; Von Sydow, E. The composition of the essential oil of black currant leaves (Ribes nigrum L.). J. Sci. Food Agric. 1963, 14, 834–840. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kokubun, T. Plant–fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Chen, M. Effective Fractions of Rubus fruticosus Leaf, Its Pharmaceutical Composition and Uses for Prevention and Treatment of Diabetes. China Patent CN1788755 A, 21 June 2006. [Google Scholar]
- Alonso, R.; Cadavid, I.; Calleja, J.M. A preliminary study of hypoglycemic activity of Rubus fruticosus. Planta Med. 1980, 40, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Osório e Castro, V.R. Chromium and zinc in a series of plants used in Portugal in the herbal treatment of non-insulinized diabetes. Acta Aliment. 2001, 30, 333–342. [Google Scholar] [CrossRef]
- Durgo, K.; Belscak-Cvitanovic, A.; Stancic, A.; Franekic, J.; Komes, D. The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon adenocarcinoma. J. Med. Food 2012, 15, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Brandely, P. Raspberry leaf. In British Herbal Compendium. A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Bournemouth, UK, 2006; pp. 328–331. [Google Scholar]
- Parsons, M.; Simpson, M.; Ponton, T. Raspberry leaf and its effect on labour: Safety and efficacy. Aust. Coll. Midwives Inc. J. 1999, 12, 20–25. [Google Scholar] [CrossRef]
- Jing, Z.; Pistilli, M.J.; Holloway, A.C.; Crankshaw, D.J. The effects of commercial preparations of red raspberry leaf on the contractility of the rat’s uterus in vitro. Reprod. Sci. 2010, 17, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M. Raspberry leaf in pregnancy: Its safety and efficacy in labor. J. Midwifery Women's Health 2001, 46, 51–59. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, A.; Nastasi, M.; Cavalli, E.; Puglisi, L. Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic treatment, in several models of rat dyslipidaemia: A comparison with ciprofibrate. Thromb. Res. 1996, 84, 311–322. [Google Scholar] [CrossRef]
- Petlevski, R.; Hadžija, M.; Slijepčević, M.; Juretić, D. Effect of “antidiabetis” herbal preparation on serum glucose and fructosamine in NOD mice. J. Ethnopharmacol. 2001, 75, 181–184. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Pharmazie 2006, 61, 952–956. [Google Scholar] [PubMed]
- Ferlemi, A.V.; Mermigki, P.G.; Makri, O.E.; Anagnostopoulos, D.; Koulakiotis, N.S.; Margarity, M.; Tsarbopoulos, A.; Georgakopoulos, C.D.; Lamari, F.N. Cerebral area differential redox response of neonatal rats to selenite-induced oxidative stress and to concurrent administration of highbush blueberry leaf polyphenols. Neurochem. Res. 2015, 40, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Chambers, B.K.; Camire, M.E. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care 2003, 26, 2695–2696. [Google Scholar] [CrossRef] [PubMed]
- Jellin, J.M.; Gregory, P.J.; Batz, F.; Hitchens, K. Natural medicines comprehensive database. Pharm. Lett./Prescr. Lett. 2005, 2239. [Google Scholar]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Neto, C.C.; Salvas, M.R.; Autio, W.R.; van den Heuvel, J.E. Variation in concentration of phenolic acid derivatives and quercetin glycosides in foliage of cranberry that may play a role in pest deterrence. J. Am. Soc. Hortic. Sci. 2010, 135, 494–500. [Google Scholar]
- Turner, A.; Chen, S.N.; Nikolic, D.; van Breemen, R.; Farnsworth, N.R.; Pauli, G.F. Coumaroyl iridoids and a depside from cranberry (Vaccinium macrocarpon). J. Nat. Prod. 2007, 70, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.D.; Kimble, L.L.; Kaspar, K.L.; Khoo, C.; Chew, B.P. Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: A randomized controlled trial. Nutr. Res. 2014, 34, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Fan, Y.; Li, L.; Makino, T.; Kano, Y. Analysis and bioactive evaluation of the compounds absorbed into blood after oral administration of the extracts of Vaccinium vitis-idaea in rat. Biol. Pharm. Bull. 2005, 28, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Med. Inflamm. 2007, 2007, 1–10. [Google Scholar]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [PubMed]
- Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
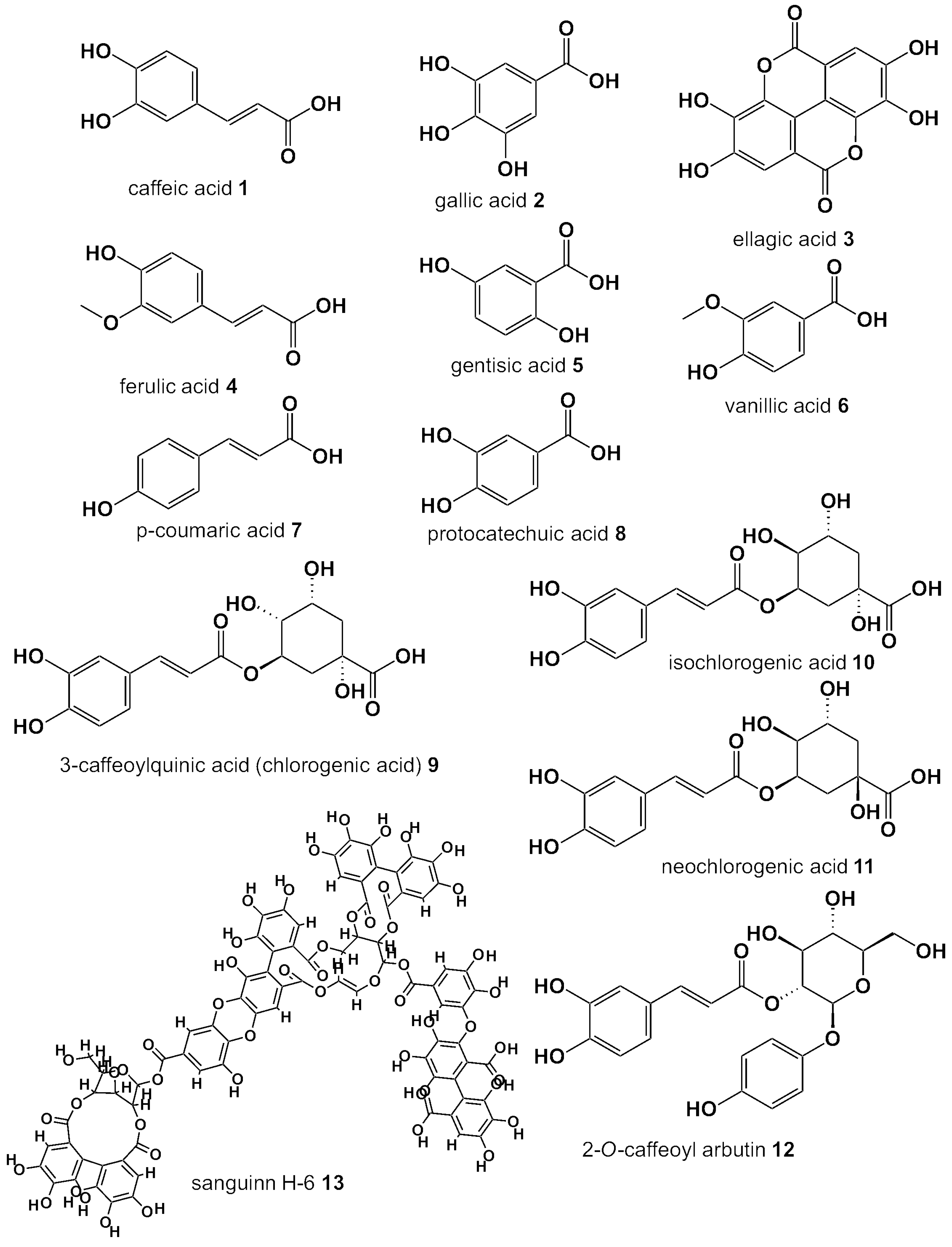
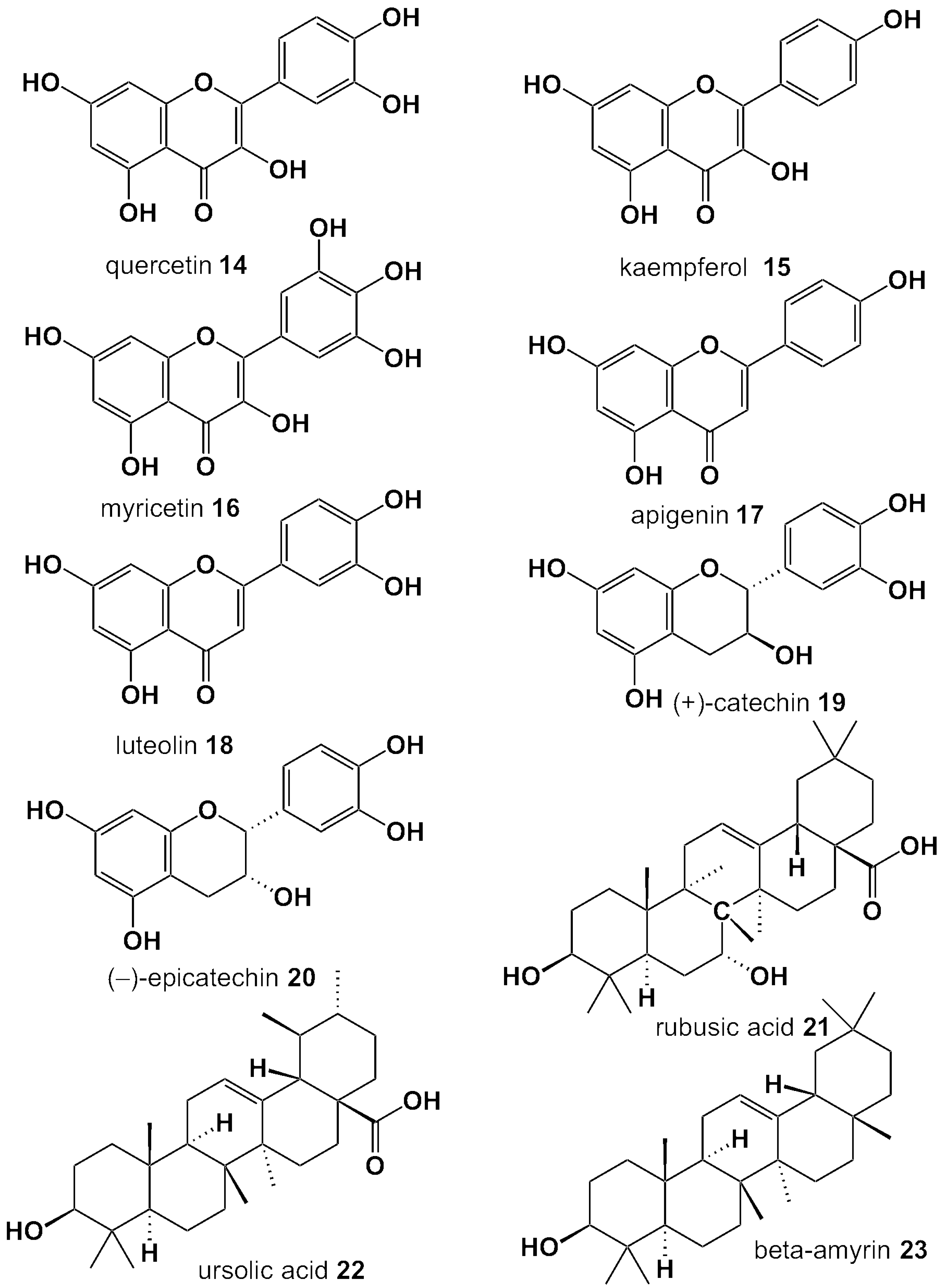
| Compound Name | Berry Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | Chlorogenic acid | BC | RB | BI | H-BL | L-BL | CB | LGB | |
| Neo-chlorogenic acid | BC | BB | H-BL | L-BL | CB | ||||
| Iso-chlorogenic acid | BC | H-BL | |||||||
| Caffeic acid | BC | BB | RB | BI | H-BL | L-BL | LGB | ||
| Gallic acid | BC | BB | |||||||
| Ferulic acid | BC | H-BL | |||||||
| Quinic acid | H-BL | ||||||||
| p-coumaric acid | BC | BB | RB | H-BL | LGB | ||||
| Coumaroyl-quinic acid | L-BL | CB | LGB | ||||||
| Caffeoyl-shikimic acid/ Ferroyl-quinic acid isomer | BI | LGB | |||||||
| Gentisic acid | BC | ||||||||
| p-hydroxybenzoic acid/ vanillinic acid | RB | ||||||||
| 2-O-caffeoylarbutin | LGB | ||||||||
| Coumaroyl/caffeoyl-hexose hydroxyphenols | LGB | ||||||||
| Ellagic acid | BB | RB | |||||||
| Flavonols | Quercetin | BB | RB | BI | H-BL | L-BL | |||
| Quercetin-3-O-rutinoside | BC | RB | H-BL | LGB | |||||
| Quercetin-3-O-galactoside | BC | BB | RB | BI | H-BL | L-BL | CB | LGB | |
| Quercetin-3-O-glucoside | BC | RB | BI | H-BL | L-BL | LGB | |||
| Quercetin-3-O-glucuronide | BB | RB | BI | ||||||
| Quercetin-3-O-a-L-rhamnoside | BI | H-BL | L-BL | CB | LGB | ||||
| Quercetin-3-O-(4’’-HMG)-a-rhamnoside | BI | LGB | |||||||
| Quercetin-3-O-arabinoside | BI | H-BL | L-BL | CB | LGB | ||||
| Quercetin-3-O-xyloside | H-BL | CB | LGB | ||||||
| Quercetin-3-O-malonylglucoside | BC | ||||||||
| Quercetin-3-O-glucosyl-6’’-acetate | H-BL | ||||||||
| Quercetin-3-O-R-arabinofuranoside | LGB | ||||||||
| Kaempferol | BB | H-BL | |||||||
| Kaempferol-3-O-rutinoside | BC | H-BL | L-BL | ||||||
| Kaempferol-3-O-galactoside | BC | ||||||||
| Kaempferol-3-O-glucoside | BC | RB | |||||||
| Kaempferol-3-O-glucuronide | BI | H-BL | |||||||
| Kaempferol-3-O-glucosyl-6’’-acetate | BC | ||||||||
| Kaempferol-3-O-malonylglucoside | BC | ||||||||
| kaempferol-(HMG)-rhamnoside | LGB | ||||||||
| Kaempferol-pentoside | BI | LGB | |||||||
| Myricetin | H-BL | ||||||||
| Myricetin-3-O-malonylglucoside | BC | ||||||||
| Myricetin-3-O-rutinoside/-3- O-galactoside/-3-O-glucoside/-3-O-arabinoside/-3-O-xyloside | H-BL | ||||||||
| Isorhamnetin-3-O-rutinoside/-3-O-glucoside | BC | ||||||||
| Flavan-3-ols | Catechin | BC | BB | BI | L-BL | LGB | |||
| Epicatechin | BC | BB | BI | L-BL | |||||
| Epigallocatechin/ Gallocatechin and its isomers | BC | BI | |||||||
| Epicatechin gallate methyl gallate | BB | RB | |||||||
| ETs | Sanguiin H-6 /Lambertianin C | BB | RB | ||||||
| Lambertianin D | RB | ||||||||
| Casuarinin | BB | ||||||||
| PACs | Cinchonains | BI | L-BL | ||||||
| Proanthocyanidin A1 | LGB | ||||||||
| Proanthocyanidin A2 | CB | LGB | |||||||
| Proanthocyanidin B | BI | ||||||||
| Kandelin A1/A2 | L-BL | ||||||||
| Procyanidins/Prodelphinidins | L-BL | ||||||||
| Anthocyanins | Delphinidin-3-O-glucoside/-3-O-rutinoside | BC | |||||||
| Cyanidin-3-O-glucoside | BC | BB | H-BL | ||||||
| Cyanidin-3-O-rutinoside | BC | ||||||||
| Cyanidin-3-O-arabinoside/-3-O-glucuronide | H-BL | ||||||||
| Blackcurrant | Red Raspberry | Blackberry | ||||
|---|---|---|---|---|---|---|
| EMA | Traditional Medicinal Product Minor articular pain Adjuvant in minor urinary complaints [13] | Traditional Medicinal Product Symptomatic relief of minor spasm associated with menstrual periods Symptomatic treatment of mild inflammation in the mouth or throat Symptomatic treatment of mild diarrhea [16] | ||||
| Traditional uses | Diaphoretic and diuretic agent Against diarrhea Against spasmodic cough Relief of rheumatic pain [14,15] | Labor stimulator [17] Relief of menstrual cramps Relief of diarrhea Astringent agent Anti-inflammatory agent (mouth, throat) Against chronic skin conditions Treatment of conjunctivitis [18] | Mouthwash against thrush, gum inflammations, mouth ulcers, sore throat Against respiratory problems Astringent agent Regulation of anemia, diarrhea, dysentery, cystitis, hemorrhoids [19] | |||
| In vitro/In vivo | Antioxidant, Anti-inflammatory activity [14,20] Analgesic activity [15] | Antioxidant activity [21] | Antidiabetic/Hypoglycemic activity [22,23,24] Antimicrobial activity [25] Analgesic, Anti-inflammatory, Angiogenic activity [19,26,27] | |||
| Clinical trials | Indications that it facilitates labor [28,29,30] | |||||
| Bilberry | Blueberry | Cranberry | Lingonberry | |||
| Traditional uses | Diuretic, astringent and antiseptic agent for the urinary tract Antibacterial Anti-inflammatory Antidiabetic [31] | Antidiabetic agent [32,33] | Diuretic agent Antiseptic in urinary tract [31] | |||
| In vitro/In vivo | Antidiabetic activity [34,35,36] Anti-hyperlipidemic activity [37] Antistaphylococcal activity [38] Antioxidant, Anti-neoplastic activity [39] | Antioxidant, Anticataract [40] Neuroprotective activity [41] Antidiabetic activity [42] Antimicrobial activity [43] | Antioxidant activity [44] | Antitussive, Anti-inflammatory Anti-catarrhal activity [45] Neuroprotective activity [46] | ||
| Clinical trials | Antimicrobial agent—urinary tract protection Antioxidant activity [47] | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferlemi, A.-V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. https://doi.org/10.3390/antiox5020017
Ferlemi A-V, Lamari FN. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants. 2016; 5(2):17. https://doi.org/10.3390/antiox5020017
Chicago/Turabian StyleFerlemi, Anastasia-Varvara, and Fotini N. Lamari. 2016. "Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value" Antioxidants 5, no. 2: 17. https://doi.org/10.3390/antiox5020017
APA StyleFerlemi, A.-V., & Lamari, F. N. (2016). Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants, 5(2), 17. https://doi.org/10.3390/antiox5020017