Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Bread-Making Method
2.3. Sample Preparation
2.4. Extraction of Soluble Phenolic Compounds
2.5. Total Phenolic Content Determination
2.6. DPPH Radical Scavenging Activity Assay
2.7. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.8. Extraction of Insoluble Phenolic Compounds after Alkaline Hydrolysis
2.9. High Performance Liquid Chromatography (HPLC) Analysis
2.10. LC-MS/MS
2.11. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content of Solvent Extractible Compounds
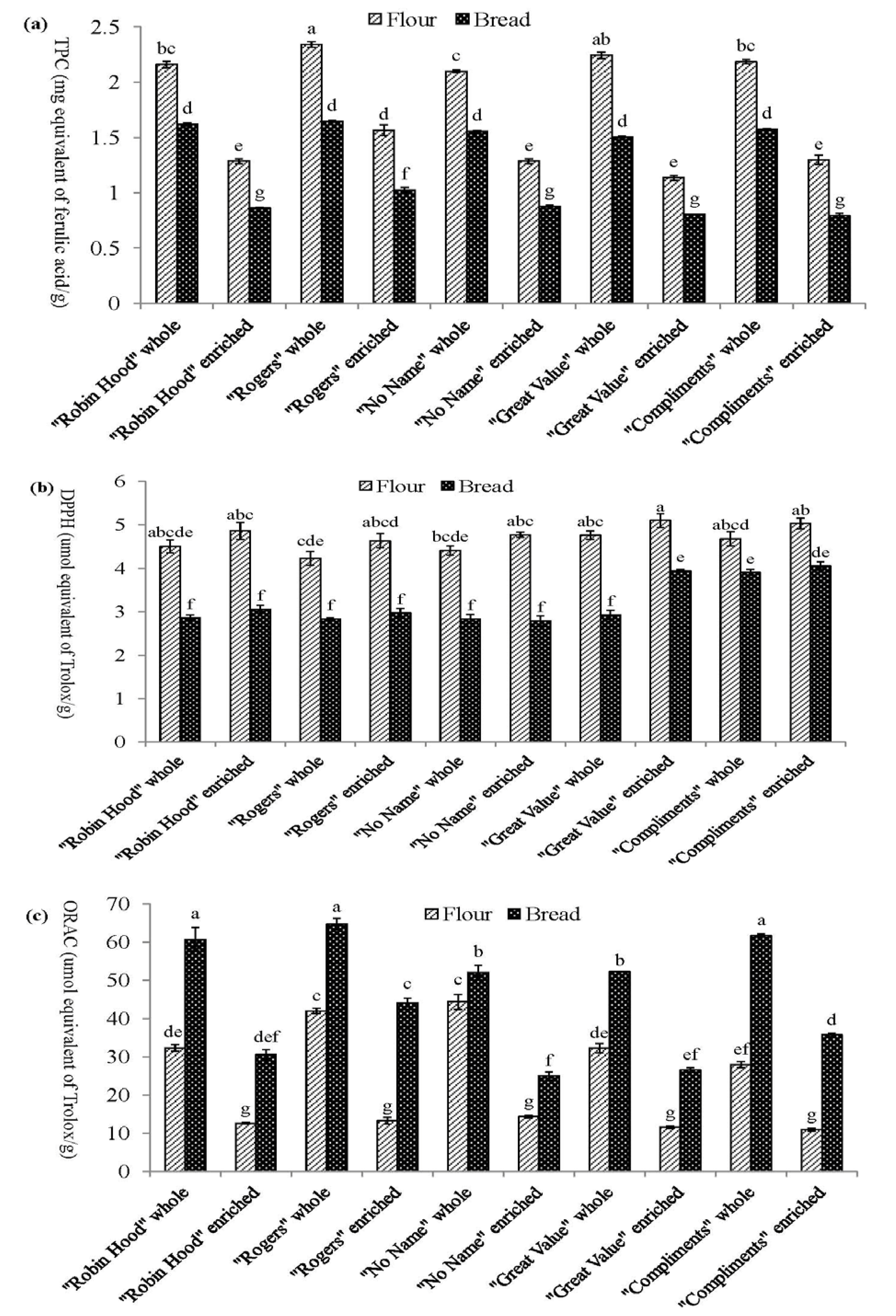
3.2. DPPH Radical Scavenging Activity of Soluble Phenolic Compounds
3.3. Oxygen Radical Absorbance Capacity of Soluble Phenolic Compounds
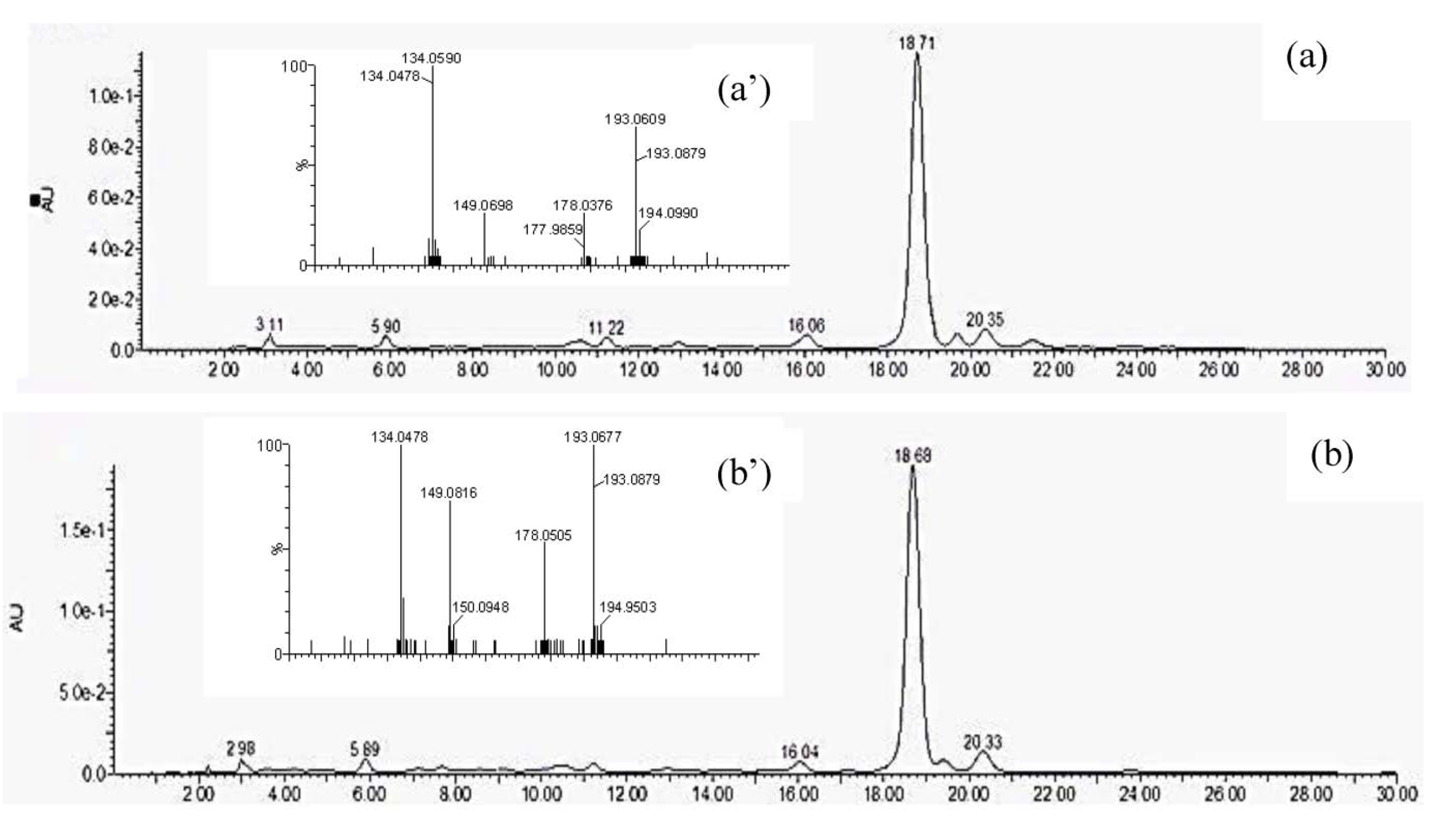
3.4. Ferulic Acid Determination and Quantification Using HPLC
| Sample Name | Whole Wheat Flour | Refined Flour | Bread Made from Whole Wheat Flour | Bread Made from Refined Flour |
|---|---|---|---|---|
| Robin Hood | 247.8 ef | 14.5 g | 316.2 cd | 20.0 g |
| Rogers | 275.7 def | 22.7 g | 374.6 ab | 46.2 g |
| No Name | 290.2 cde | 20.8 g | 393.5 a | 29.2 g |
| Great Value | 313.1 cd | 11.5 g | 334.7 bc | 17.7 g |
| Compliments | 230.5 f | 12.8 g | 231.4 f | 13.6 g |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Okarter, N.; Liu, C.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 199, 249–257. [Google Scholar] [CrossRef]
- Miller, H.E.; Rigelhof, F.; Marquart, L.; Prakash, A.; Kanter, M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J. Am. Coll. Nutr. 2000, 19, 312S–319S. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemical and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Jung, M.Y.; Jeon, B.S.; Bock, J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer). Food Chem. 2002, 79, 105–111. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Serpen, A.; Gokmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Waldron, K.; Parr, A.J.; Ng, A.; Ralph, J. Cell wall esterified phenolic dimers: Identification and quantification by reverse phase high performance liquid chromatography and diode array detection. Phytochem. Anal. 1996, 7, 305–312. [Google Scholar] [CrossRef]
- Rubin, K.W. Whole grains. Food Serv. Dir. 2002, 15, 48. [Google Scholar]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanlliaud, E.; Remesy, C. Wheat lipoxygenase activity induces greater loss of carotenoids than vitamin E during breadmaking. J. Agric. Food Chem. 2006, 54, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Koh, B.K. Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J. Sci. Food Agric. 2011, 91, 604–608. [Google Scholar] [CrossRef]
- Gelinas, P.; McKinnon, C.M. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int. J. Food Sci. Technol. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- AACC International. Optimized Straight-Dough Bread-Making Method; AACC International Method 10–10.03; AACC International: St. Paul, MN, USA.
- Li, W.; Pickard, M.D.; Beta, T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple wheat grain. J. Agric. Food Chem. 2007, 55, 8958–8966. [Google Scholar] [CrossRef]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-mill fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Chiremba, C.; Rooney, L.W.; Beta, T. Microwave-assisted extraction of bound phenolic acids in bran and flour fractions from sorghum and maize cultivars varying in hardness. J. Agric. Food Chem. 2012, 60, 4735–4742. [Google Scholar] [CrossRef]
- Hirawan, R.; Ser, W.Y.; Arntfield, S.D.; Beta, T. Antioxidant properties of commercial, regular- and whole-wheat spaghetti. Food Chem. 2010, 119, 258–264. [Google Scholar] [CrossRef]
- Hirawan, R.; Diehl-Jones, W.; Beta, T. Comparative evaluation of the antioxidant potential of infant cereal produced from purple what and red rice grains and LC-MS analysis of their anthocyanins. J. Agric. Food Chem. 2011, 59, 12330–12341. [Google Scholar] [CrossRef]
- Chandrika, M.L.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Li, W.; Beta, T. Flour and Bread from Black-, Purple-, and Blue-Colored Wheats. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 59–67. [Google Scholar]
- Juhasz, M.; Kitahara, Y.; Takahashi, S.; Fujii, T. Thermal stability of vitamin C: Thermogravimetric analysis and use of total ion monitoring chromatograms. J. Pharm. Biomed. Anal. 2012, 59, 190–193. [Google Scholar] [CrossRef]
- Lavelli, V.; Hidalgo, A.; Pompei, C.; Brandolini, A. Radical scavenging activity of einkorn (Triticum monococcum L. subsp. monococcum) wholemeal flour and its relationship to soluble phenolic and lipophilic antioxidant content. J. Cereal Sci. 2009, 49, 319–321. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Mpofu, A.; Beta, T.; Sapirstein, H.D. Effects of Genotype, Environment and Genotype × Environment Interaction on the Antioxidant Properties of Wheat. In Wheat Antioxidants; Yu, L., Ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2007; pp. 24–41. [Google Scholar]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Lin, L.; Liu, H.; Yu, Y.; Lin, S.; Mau, J. Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem. 2009, 112, 987–991. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, R.C. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2001, 11, 340–346. [Google Scholar]
- Michalska, A.; Amigop-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Moore, J.; Yu, L. Methods for Antioxidant Capacity Estimation of Wheat and Wheat-Based Food Products. In Wheat Antioxidants; Yu, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 118–166. [Google Scholar]
- Revanappa, S.B.; Salimath, P.V. Phenolic acid profiles and antioxidant activities of different wheat (Triticum aestivum L.) varieties. J. Food Biochem. 2011, 35, 759–775. [Google Scholar] [CrossRef]
- Gasztonyi, M.N.; Farkas, R.T.; Berki, M.; Petroczi, I.M.; Daood, H.G. Content of phenols in wheat as affected by varietal and agricultural factors. J. Food Comp. Anal. 2011, 24, 785–789. [Google Scholar] [CrossRef]
- Hung, P.V.; Maeda, T.; Miyatake, K.; Morita, N. Total phenolic compounds and antioxidant capacity of wheat flours by polishing method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Lu, F.; Steinhart, H. Lignins and ferulate-coniferyl alcohol cross-coupling products in cereal grains. J. Agric. Food Chem. 2004, 52, 6496–6502. [Google Scholar] [CrossRef]
- Klepacka, J.; Fornal, L. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. Nutr. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Lin, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2012, 63, 71–74. [Google Scholar] [CrossRef]
- Hung, P.V.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, L.; Nanguet, A.-L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370-383. https://doi.org/10.3390/antiox2040370
Yu L, Nanguet A-L, Beta T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants. 2013; 2(4):370-383. https://doi.org/10.3390/antiox2040370
Chicago/Turabian StyleYu, Lilei, Anne-Laure Nanguet, and Trust Beta. 2013. "Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread" Antioxidants 2, no. 4: 370-383. https://doi.org/10.3390/antiox2040370




