Evaluation of Antioxidant Status of Two Limoniastrum Species Growing Wild in Tunisian Salty Lands
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Sampling
| Species | Region name | Coordinates UTM | Temperature (°C) | Rainfall (mm/year) |
|---|---|---|---|---|
| L. guyonianum | Oued el Fja (Medenine)
Salt marsh | Latitude: 33°29′33.93″N
Longitude: 10°38′24.70″E | Max: 36.8
Min: 6.2 | 144 |
| L. monopetalum | Hassi Jerbi (Zarzis)
seashore | Latitude: 33°38′13.34″N
Longitude: 11°0′22.49″E | Max: 32
Min: 6 | 200 |
2.2. Ion Analysis
2.3. Methanolic Extract Preparation and Analysis
2.3.1. Polyphenols Contents
2.3.2. Flavonoids Contents
2.3.3. DPPH Radical-Scavenging Assay
2.3.4. Ferrous Ions Chelating Activity
2.3.5. Ferric-Reducing Activity
2.4. Lipid Peroxide Determination
2.5. Proline Contents
2.6. Chlorophyll and Carotenoids Determination
3. Results and Discussion
3.1. Metabolites Contents
| Metabolites | Limoniastrum guyonianum | Limoniastrum monopetalum |
|---|---|---|
| Phenolic content (mg GA/g DW) | 217.82 ± 14.38a | 225.22 ± 8.10a |
| Flavonoids contents (mg QC/g DW) | 50.20 ± 5.23 b | 42.26 ± 5.70b |
| Proline (μg/g DW) | 521.43 ±22c | 642.86 ± 19.20d |
| Sugars (mg/g DW) | 28.70 ±3e | 38.00 ±3.22g |
| Carotenoids (mg/g FW) | 0.22 ± 0.07h | 0.30 ± 0.03i |
| Chl (mg/g FW) | 0.10 ±0.003j | 0.11±0.005j |
| Sodium (μmol/g DW) | 4889.62 ± 29k | 11358.21±41l |
| Potassium (μmol/g DW) | 788.34 ±14m | 1011.14 ±18n |
3.2. Scavenging of DPPH Radicals
| Antioxidant activities | L. guyonianum | L. monopetalum |
|---|---|---|
| DPPH scavenging activity
(IC50 μg/mL) | 14.90 ± 3a | 17.14 ± 2.4a |
| Ferrous ions chelating activity
(IC50 μg/mL) | 191.63 ± 12b | 90.15 ± 9c |
| Reducing power (EC50 μg/mL) | 109.5 ± 11d | 42.33± 5e |
3.3. Ferrous Ions Chelating Activity
3.4. Ferric Reducing Power
3.5. Malonydialdehyde Content (MDA)
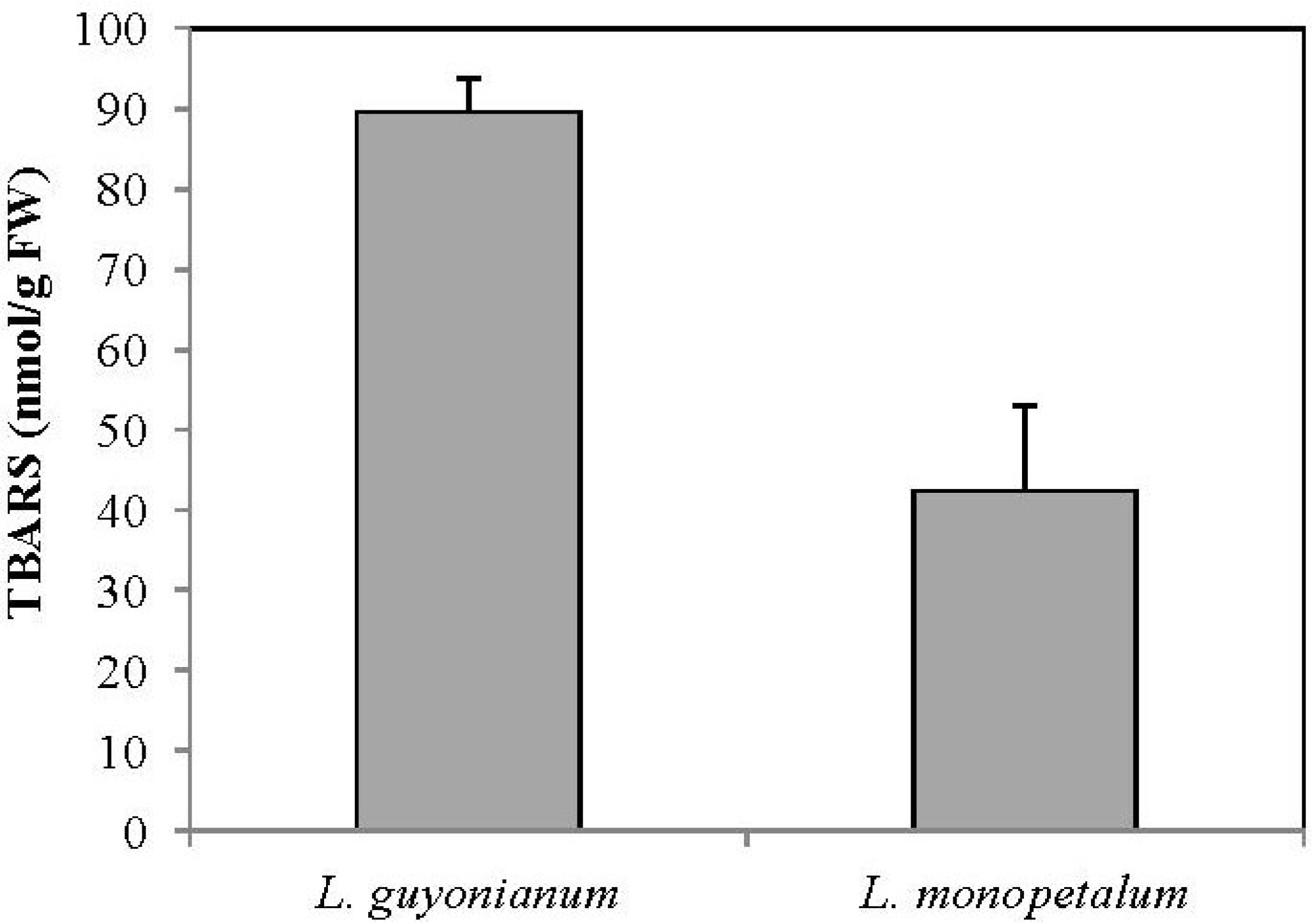
4. Conclusions
Conflict of Interest
References
- Kavitha, K.; George, S.; Venkataraman, G.; Parida, A. A salt inducible chloroplastic monodehydroascorbate reductase from halophyte Avicennia marina confers salt stress tolerance on transgenic plants. Biochimie 2010, 92, 1321–1329. [Google Scholar] [CrossRef]
- Ghnaya, T.; Nouairi, I.; Slama, I.; Messedi, D.; Grignon, C.; Abdelly, C.; Ghorbel, M.H. Cadmium effects on growth and mineral nutrition of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum. J. Plant Physiol. 2005, 162, 1133–1140. [Google Scholar] [CrossRef]
- Lopez-Chuken, U.J.; Young, S.D. Plant screening of halophyte species for cadmium phytoremediation. Z. Naturforsch. C 2005, 60, 236–243. [Google Scholar] [PubMed]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Chaieb, M.; Boukhris, M. Flore Suscinte et Illustrée des Zones Arides et Sahariennes de Tunisie; Association de la Protection de la Nature et de l’Environnement: Sfax, Tunisia, 1998; pp. 204–205. [Google Scholar]
- Laudadio, V.; Dario, M.; Hammadi, M.; Tufarelli, V. Nutritional composition of three fodder species browsed by camels (Camelus dromedarius) on arid area of Tunisia. Trop. Anim. Health Prod. 2008, 41, 1219–1224. [Google Scholar] [PubMed]
- Nieukerken, J.V.E. Acalyptris Meyrick: Revision of the platani and staticis groups in Europe and the Mediterranean (Lepidoptera: Nepticulidae). Zootaxa 2007, 1436, 1–48. [Google Scholar]
- Heimler, D.; Vignolini, P.; Dini, M.; Vincieri, F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Dewanto, V.X.; Wu, K.; Adom, K.; Liu, D.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Zhao, F.; Yang, J.; Schoneich, C. Effects of polyaminocarboxylate metal chelators on iron-thiolate induced oxidation of methionine- and histidine-containing peptides. Pharm. Res. 1996, 13, 931–938. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dhindsa, R.A.; Plumb-Dhindsa, P.; Thorpe, P.A. Leaf senescence: Correlated with increased permeability and lipid peroxidation, and decreases levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 126, 93–101. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- MacKinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 144, 315–323. [Google Scholar]
- Hajlaoui, H.; El Ayeb, N.; Garrec, J.P.; Denden, M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.). Ind. Crops Prod. 2010, 31, 122–130. [Google Scholar] [CrossRef]
- Araujo, S.A.M.; Silveira, J.A.G.; Almeida, T.A.; Rocha, I.M.A.; Morais, D.L.; Viegas, R.A. Salinity tolerance of halophyte Atriplex nummularia L. grown under increasing NaCl levels. Rev. Bras. Eng. Agric. Ambient. 2006, 10, 848–854. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Flerlage, N.; Burillo, J.; Codina, C. Comparison between the radical scavenging activities and antioxidant activity of six distilled and nondistilled maditerranean herbs and aromatic plants. J. Agric. Food Chem. 2002, 50, 6882–6890. [Google Scholar] [CrossRef]
- Galvez, M.; Martin-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of methanol extracts obtained from Plantago species. J. Agric. Food Chem. 2005, 53, 1927–1933. [Google Scholar] [CrossRef]
- Melo, E.A.; Filho, J.M.; Guerra, N.B. Characterization of antioxidant compounds in aqueous coriander extract (Coriander sativum L.). Food Sci. Technol. 2005, 38, 15–19. [Google Scholar]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Hou, W.C.; Lin, R.D.; Cheng, K.T.; Hung, Y.T.; Cho, C.H.; Chen, C.H.; Hwang, S.Y.; Lee, M.H. Free radical scavenging activity of Taiwanese native plants. Phytomedicine 2003, 10, 170–175. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Korus, J.; Gumul, D.; Gibinski, M. Influence of extrusion on polyphenol content and antioxidant activity of common bean (Phaseolus vulgaris L.) seeds. Zywnosc 2006, 13, 102–111. [Google Scholar]
- Matysik, J.; Alia, B.B.; Mohanty, P. Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Li, K.; Pang, C.H.; Ding, F.; Sui, N.; Feng, Z.T.; Wang, B.S. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S. Afr. J. Bot. 2012, 78, 235–245. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Debouba, M.; Zouari, S.; Zouari, N. Evaluation of Antioxidant Status of Two Limoniastrum Species Growing Wild in Tunisian Salty Lands. Antioxidants 2013, 2, 122-131. https://doi.org/10.3390/antiox2030122
Debouba M, Zouari S, Zouari N. Evaluation of Antioxidant Status of Two Limoniastrum Species Growing Wild in Tunisian Salty Lands. Antioxidants. 2013; 2(3):122-131. https://doi.org/10.3390/antiox2030122
Chicago/Turabian StyleDebouba, Mohamed, Sami Zouari, and Nacim Zouari. 2013. "Evaluation of Antioxidant Status of Two Limoniastrum Species Growing Wild in Tunisian Salty Lands" Antioxidants 2, no. 3: 122-131. https://doi.org/10.3390/antiox2030122




