Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Materials and Reagents
2.2. Extraction Procedure
2.3. Determination of Total Phenolics
2.4. Determination of Antioxidant Activity Using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Method
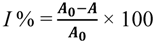
2.5. Determination of Antioxidant Activity Using the ABTS Free Radical Scavenging Method
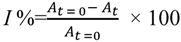
2.6. Rancimat Test

2.7. HPLC Analysis
3. Results and Discussion
3.1. Determination of Total Phenolics
| Family Species | Collection sites | Part examined | Drying method a | Total phenolics b (mg gallic acid/g ds) |
|---|---|---|---|---|
| Origanum dictamnus (A) | Crete | Leaves | Air | 8.2 ±0.3 |
| Eucalyptus globulus (B) | Attiki | Leaves | Air | 10.5 ± 0.3 |
| Sideritis cretica (C) | Crete | Leaves | F/v | 8.6 ± 0.2 |
| Origanum vulgare (D) | Euboea | Leaves | F/v | 19.5 ± 0.2 |
| Phlomis cretica (E) | Crete | Leaves | F/v | 16.2 ± 0.1 |
| Phlomis lanata (F) | Crete | Leaves | F/v | 21.4 ± 0.3 |
| Nepeta melissifolia (G) | Crete | Leaves | F/v | 31.6 ± 0.4 |
| Mentha pulegium (H) | Crete | Leaves | F/v | 13.4 ± 0.2 |
| Thymus vulgaris L. (I) | Attiki | Leaves | Air | 8.0± 0.1 |
| Satureja thymbra (J) | Attiki | Leaves | Air | 9.2± 0.1 |
| Lavandula vera (K) | Attiki | Leaves | Air | 4.9± 0.1 |
| Rosmarinus officinalis (L) | Attiki | Leaves | Air | 8.5± 0.1 |
| Lippia triphylla (M) | Attiki | Leaves | Air | 7.7± 0.1 |
| Matricaria chamomilla (N) | Attiki | Leaves | Air | 6.1± 0.1 |
| Mellisa officinalis L.(O) | Attiki | Leaves | Air | 15.1 ± 0.1 |
| Salvia officinalis (P) | Attiki | Leaves | Air | 15.6 ± 0.1 |
| Geranium purpureum (Q) | Attiki | Leaves | Air | 4.0 ± 0.1 |
| Family Species | Total phenolics a (mg gallic acid/g ds) |
|---|---|
| Thymus vulgaris L. | 18.0 ± 0.9 |
| Salvia officinalis | 12.1 ± 0.1 |
| Rosmarinus officinalis | 9.2 ± 0.1 |
| Origanum vulgare | ±0.2 |
3.2. Determination of Antioxidant Activity Using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Method

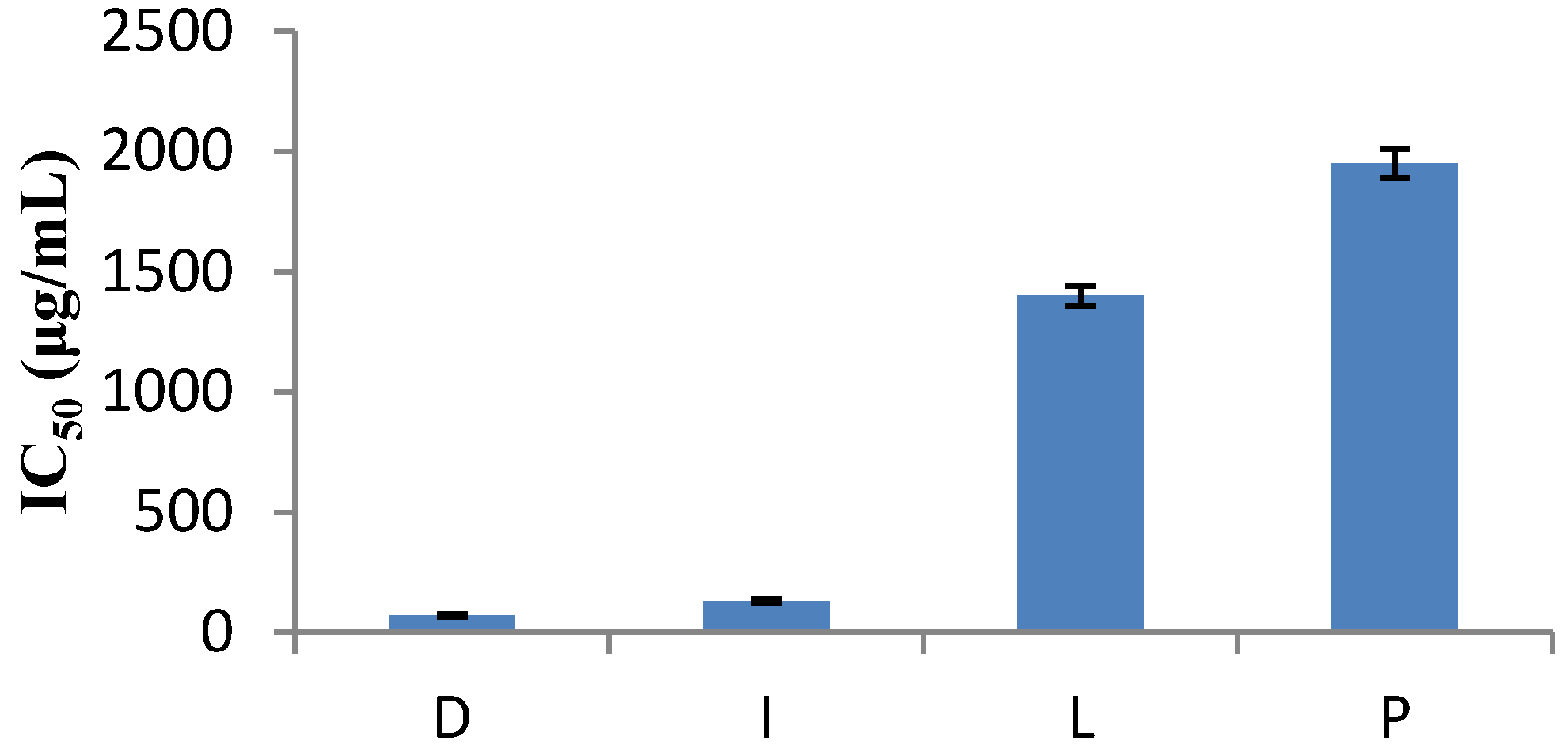
| Family species | Antioxidant Capacity (mg ascorbic acid/g dried sample) |
|---|---|
| Thymus vulgaris L. | 0.6 ± 0.3 |
| Lavandula vera | 0.6 ± 0.4 |
| Rosmarinus officinalis | 0.5 ± 0.1 |
| Origanum dictamnus | 0.2 ± 0.2 |
| Sideritis cretica | 0.8 ± 0.1 |
| Salvia officinalis | 0.4 ± 0. 1 |
| Origanum vulgare | 0.5 ± 0.1 |
| Geranium purpureum | ±0.2 |
3.3. Determination of Antioxidant Activity Using the ABTS Free Radical Scavenging Method
| Family species | % Inhibition (0–5 min) | % Inhibition (5–10 min) |
|---|---|---|
| Thymus vulgaris L. | 21.1 ± 0.,2 | 10.9 ± 0.3 |
| Satureja thymbra | 19.2 ± 0.3 | 20.42 ± 0.4 |
| Lavandula vera | 18.2 ± 0.1 | 15.7 ± 0.3 |
| Rosmarinus officinalis | 15.6 ± 0.2 | 9.8 ± 0.5 |
| Lippia triphylla | 14.3 ± 0.3 | 7.2 ± 0.2 |
| Matricaria chamomilla | 12.4 ± 0.1 | 11.9 ± 0.5 |
| Origanum dictamnus | 22.7 ± 0.1 | 24.2 ± 0.3 |
| Melissa officinalis L. | 11.8 ± 0.2 | 38.6 ± 0.2 |
| Sideritis spp. | 12.2 ± 0.1 | 8.7 ± 0.4 |
| Salvia officinalis | 13.2 ± 0.3 | 9.6 ± 0.4 |
| Origanum vulgare | 25.5 ± 0.3 | 14.7 ± 0.3 |
| Geranium purpureum | 10.8 ± 0.2 | 12.5 ± 0.1 |
3.4. Rancimat Test—Method of Assessment of Oil Oxidation and Stability
| Family Species | PF α (ground material) | PF α (methanol extracts) |
|---|---|---|
| Thymus vulgaris L. | ND b | 1.7 ± 0.05 |
| Rosmarinus officinalis | ND | 2.0 ± 0.06 |
| Origanum vulgare | 1.8 ± 0.09 | 1.9 ± 0.06 |
| Origanum dictamnus | 1.3 ± 0.08 | 1.2 ± 0.09 |
| Eucalyptus globulus | 1.5 ± 0.07 | 1.4 ± 0.08 |
| Sideritis cretica | 1 ± 0.06 | 1.1 ± 0.07 |
| Phlomis cretica | 2.1 ± 0.07 | 1.9 ± 0.07 |
| Phlomis lanata | 2.4 ± 0.09 | 2.1 ± 0.06 |
| Nepeta melissifolia | 3.1 ± 0.06 | 2.9 ± 0.09 |
| Mentha pulegium | 1.9 ± 0.05 | 1.7 ± 0.07 |
3.5. HPLC Analysis
| Family species | gallic acid | gentisic acid | caffeic acid | p-coumaric acid | vanillic acid | syringic acid | ferulic acid | p-hydroxybenzoic acid | |
|---|---|---|---|---|---|---|---|---|---|
| Origanum dictamnus (A) | 4.9 ± 0.03 | ND | 13.5 ± 0.02 | 13.9 ± 0.04 | 18.5 ± 0.02 | ND | 16.9 ± 0.04 | ND | |
| Eucalyptus globulus (B) | ND | ND | 8.1 ± 0.01 | 6.6 ± 0.02 | ND | ND | 12.3 ± 0.03 | ND | |
| Sideritis cretica (C) | 1.1 ± 0.02 | ND | 3.3 ± 0.02 | ND | ND | ND | 6.8 ± 0.02 | 2.5 ± 0.01 | |
| Origanum vulgare (D) | ND | ND | 6.4 ± 0.02 | ND | ND | ND | 10.4 ± 0.03 | ND | |
| Phlomis cretica (E) | ND | ND | 2.2 ± 0.01 | ND | ND | ND | 5.1 ± 0.02 | ND | |
| Phlomis lanata (F) | 14 ± 0.02 | 3.2 ± 0.03 | 20 ± 0.03 | 4.1 ± 0.02 | 2 ± 0.02 | 1.1 ± 0.02 | ND | 1.5 ± 0.01 | |
| Nepeta melissifolia (G) | 20 ± 0.02 | 4.3 ± 0.03 | 26 ± 0.03 | 5.2 ± 0.02 | 2.7 ± 0.02 | 2.6 ± 0.02 | 22.4 ± 0.03 | 5.4 ± 0.01 | |
| Mentha pulegium (H) | ND | ND | 13.5 ± 0.02 | ND | 13.5 ± 0.02 | ND | 13.5 ± 0.02 | ND | |
| Family species | quercetin | apigenin | luteolin | naringenin | myricetin | rutin | (+)-catechin hydrated | (−)-epicatechin |
|---|---|---|---|---|---|---|---|---|
| Origanum dictamnus | 52 ± 0.09 | ND | ND | ND | ND | ND | 1.9 ± 0.01 | ND |
| Eucalyptus globulus | ND | ND | ND | ND | ND | 10 ± 0.03 | ND | ND |
| Sideritis cretica | ND | ND | ND | ND | ND | ND | 6.9 ± 0.02 | 2.8 ± 0.01 |
| Origanum vulgare | 7.3 ± 0.02 | ND | ND | ND | ND | 2.3 ± 0.01 | 2.5 ± 0.01 | ND |
| Phlomis cretica | 1.2 ± 0.01 | ND | ND | ND | ND | ND | 1.5 ± 0.01 | 2.6 ± 0.01 |
| Phlomis lanata | 2.2 ± 0.01 | ND | ND | ND | ND | 4.5 ± 0.01 | ND | ND |
| Nepeta melissifolia | 11.2 ± 0.02 | 5.6 ± 0.02 | ND | ND | 1.8 ± 0.01 | 2.4 ± 0.01 | 5.5 ± 0.02 | ND |
| Mentha pulegium | ND | 6.9 ± 0.02 | ND | 4.7 ± 0.02 | ND | ND | 3.5 ± 0.01 | ND |
4. Conclusions
Acknowledgments
References
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Halliwell, B.; Guteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Heikki, J.V.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Packer, L. Flavonoids in Health and Disease, 2nd ed; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuolera, H.; Vuolera, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gardeli, C.; Papageorgiou, V.; Mallouchos, A.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Bantoniene, D.; Pukalskas, A.; Venskutonis, P.R.; Gruzdiene, D. Preliminary screening of antioxidant activity of some plant extracts in rapeseed oil. Food Res. Int. 2000, 33, 785–791. [Google Scholar] [CrossRef]
- Suárez, B.; Picinelli, A.; Mangas, J.J. Solid-phase extraction and high-performance liquid chromatographic determination of polyphenols in apple musts and ciders. J. Chromatogr. 1996, 727, 203–209. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.-J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2009, 25, 13–19. [Google Scholar]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Proestos, C. Determination of Phenolic Compounds in Aromatic Plants. Ph.D Thesis, Agricultural University of Athens, Athens, Greece; p. 2005.
- Kintzios, S.; Papageorgiou, K.; Yiakoumettis, I.; Baricevi, D.; Kusar, A. Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. J. Pharm. Biomed. Anal. 2010, 53, 773–776. [Google Scholar] [CrossRef]
- Aruoma, O.; Spencer, J.P.; Rossi, R.; Aeschbach, R.; Khan, A.; Mahmood, N.; Munoz, A.; Murcia, A.; Butler, J.; Halliwell, B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and provencal herbs. Food Chem. Toxicol. 1996, 34, 449–456. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Kammiovirta, K.; Oksman-Caldentey, K.M. Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Torben, T.J. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Finger, A.; Kuhr, S.; Engelhardt, H.U. Chromatography of tea constituents. J. Chromatogr. 1992, 624, 293–315. [Google Scholar] [CrossRef]
- Lee, S.H.; Widmer, W.B. Handbook of Food Analysis, 1st ed; Marcel Dekker Inc.: New York, NY, USA, 1996. [Google Scholar]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11-22. https://doi.org/10.3390/antiox2010011
Proestos C, Lytoudi K, Mavromelanidou OK, Zoumpoulakis P, Sinanoglou VJ. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants. 2013; 2(1):11-22. https://doi.org/10.3390/antiox2010011
Chicago/Turabian StyleProestos, Charalampos, Konstantina Lytoudi, Olga Konstantina Mavromelanidou, Panagiotis Zoumpoulakis, and Vassileia J. Sinanoglou. 2013. "Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils" Antioxidants 2, no. 1: 11-22. https://doi.org/10.3390/antiox2010011
APA StyleProestos, C., Lytoudi, K., Mavromelanidou, O. K., Zoumpoulakis, P., & Sinanoglou, V. J. (2013). Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants, 2(1), 11-22. https://doi.org/10.3390/antiox2010011







