High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest
Abstract
:1. Introduction
2. Experimental Section
2.1. Collection of Plants and Preparation of Extracts
| Extract No. | Plant | Part | Extract | DPPH 1 | FRAP 1 | TPC 2 |
|---|---|---|---|---|---|---|
| 1 | Apama tricornutum | Leaf | EtOH | 2740 | 1617 | 59.6 |
| 2 | EtAc | ≥4000 | ≥4000 | 37.2 | ||
| 3 | Hex | ≥4000 | ≥4000 | 9.0 | ||
| 4 | Bark | EtOH | ≥4000 | 1842 | 58.0 | |
| 5 | EtAc | ≥4000 | 2394 | 33.8 | ||
| 6 | Hex | ≥4000 | ≥4000 | 11.5 | ||
| 7 | Dysoxylum dumosum King | Leaf | EtOH | 476 | 337 | 182.7 |
| 8 | EtAc | ≥4000 | ≥4000 | 5.0 | ||
| 9 | Bark | EtOH | 556 | 580 | 134.1 | |
| 10 | EtAc | ≥4000 | ≥4000 | 40.3 | ||
| 11 | Hex | ≥4000 | ≥4000 | 7.7 | ||
| 12 | Alternanthera sessilis | Whole | EtOH | ≥4000 | ≥4000 | 41.9 |
| 13 | EtAc | ≥4000 | ≥4000 | 33.1 | ||
| 14 | Hex | ≥4000 | ≥4000 | 7.4 | ||
| 15 | Borreria latifolia | Whole | EtOH | ≥4000 | ≥4000 | 37.8 |
| 16 | EtAc | ≥4000 | ≥4000 | 37.4 | ||
| 17 | Hex | ≥4000 | ≥4000 | 8.8 | ||
| 18 | Eclipta alba | Whole | EtOH | 3699 | ≥4000 | 37.7 |
| 19 | EtAc | ≥4000 | ≥4000 | 33.9 | ||
| 20 | Hex | ≥4000 | ≥4000 | 10.7 | ||
| 21 | Calotropis gigantea | Whole | EtOH | ≥4000 | ≥4000 | 35.5 |
| 22 | EtAc | 3674 | 1430 | 72.7 | ||
| 23 | Hex | ≥4000 | ≥4000 | 2.4 | ||
| 24 | Acalypha wilkesiana | Whole | EtOH | 52.2 | 270 | 421.5 |
| 25 | EtAc | 47.6 | 310 | 474.6 | ||
| 26 | Hex | 2170 | ≥4000 | 12.5 | ||
| 27 | Borreria articularis | Whole | EtOH | ≥4000 | 2562 | 39.4 |
| 28 | EtAc | ≥4000 | 3288 | 33.9 | ||
| 29 | Hex | ≥4000 | ≥4000 | 3.7 | ||
| 30 | Euphorbia hirta | Whole | EtOH | ≥4000 | 3918 | 39.2 |
| 31 | EtAc | ≥4000 | ≥4000 | 19.6 | ||
| 32 | Hex | ≥4000 | ≥4000 | 13.1 | ||
| 33 | Gluta wallichii | Whole | EtOH | 736 | 1110 | 134.6 |
| 34 | EtAc | ≥4000 | ≥4000 | 47.0 | ||
| 35 | Hex | ≥4000 | ≥4000 | 25.3 | ||
| 36 | Derris dalbergioides | Whole | EtOH | 115 | 339 | 202.0 |
| 37 | EtAc | 893 | 2293 | 66.8 | ||
| 38 | Hex | ≥4000 | ≥4000 | 17.3 | ||
| 39 | Gnetum gnemon | Whole | EtOH | 3315 | 2731 | 108.3 |
| 40 | EtAc | 3787 | 2783 | 185.9 | ||
| 41 | Hex | ≥4000 | ≥4000 | 9.2 | ||
| 42 | Polyalthia hypoleuca | Whole | EtOH | 661 | 1546 | 70.7 |
| 43 | EtAc | 2609 | 2774 | 53.2 | ||
| 44 | Hex | ≥4000 | ≥4000 | 19.0 | ||
| 45 | Sida acuta | Whole | EtOH | 531 | 800 | 113.1 |
| 46 | EtAc | 1897 | 2600 | 67.6 | ||
| 47 | Hex | ≥4000 | ≥4000 | 17.8 | ||
| 48 | Pseudo-uvaria macrophylla (Oliv.) Merr. | Whole | EtOH | 431 | 1260 | 91.9 |
| 49 | EtAc | ≥4000 | ≥4000 | 30.1 | ||
| 50 | Hex | ≥4000 | ≥4000 | 14.3 | ||
| 51 | Berberis thunbergii | Whole | EtOH | ≥4000 | 2553 | 78.5 |
| 52 | EtAc | ≥4000 | ≥4000 | 36.9 | ||
| 53 | Hex | ≥4000 | ≥4000 | 13.6 | ||
| 54 | Ipomoea quamoclit | Whole | EtOH | 3460 | ≥4000 | 24.1 |
| 55 | EtAc | 3210 | ≥4000 | 42.3 | ||
| 56 | Oldenlandia corymbosa L. | Whole | EtOH | 3740 | ≥4000 | 35.6 |
| 57 | EtAc | ≥4000 | ≥4000 | 15.3 | ||
| 58 | Hex | ≥4000 | ≥4000 | 8.6 | ||
| 59 | Bidens pilosa L. | Whole | EtOH | 720 | 730 | 83.4 |
| 60 | EtAc | 900 | 900 | 105.0 | ||
| 61 | Justicia betonica L. | Whole | EtOH | ≥4000 | 3040 | 28.6 |
| 62 | EtAc | ≥4000 | ≥4000 | 40.1 | ||
| 63 | Hex | ≥4000 | ≥4000 | 6.5 | ||
| 64 | Clerodendrum paniculatum | Whole | EtOH | 1720 | 2300 | 71.7 |
| 65 | EtAc | 3190 | ≥4000 | 34.5 | ||
| 66 | Hex | ≥4000 | ≥4000 | 6.1 | ||
| 67 | Ardisia punctata Jack | Whole | EtOH | 710 | 810 | 101.7 |
| 68 | EtAc | 1310 | 3130 | 83.8 | ||
| 69 | Hex | 2460 | ≥4000 | 78.0 | ||
| 70 | Clerodendron nutans Jack | Whole | EtOH | 1000 | 1040 | 67.1 |
| 71 | EtAc | 1320 | 1990 | 56.4 | ||
| 72 | Hex | ≥4000 | ≥4000 | 9.2 | ||
| 73 | Marsypopetallum pallidum (Bl.) Kurz | Leaf | Water | 420 | 710 | 128.3 |
| 74 | EtOH | 1530 | 1380 | 76.9 | ||
| 75 | EtAc | ≥4000 | ≥4000 | 19.2 | ||
| 76 | Hex | ≥4000 | ≥4000 | 16.7 | ||
| 77 | Archidendron ellipticum | Leaf | EtOH | 1310 | 1130 | 97.3 |
| 78 | EtAc | 1930 | 2420 | 60.0 | ||
| 79 | Hex | ≥4000 | ≥4000 | 8.6 | ||
| 80 | Bark | EtOH | 1970 | 2310 | 32.3 | |
| 81 | EtAc | ≥4000 | ≥4000 | 25.0 | ||
| 82 | Hex | ≥4000 | ≥4000 | 9.8 | ||
| 83 | Pipturus argenteus (Forst. f.) | Leaf | EtOH | 340 | 940 | 119.2 |
| 84 | EtAc | 3520 | ≥4000 | 19.3 | ||
| 85 | Hex | ≥4000 | ≥4000 | 12.0 | ||
| 86 | Bark | EtOH | 540 | 550 | 174.6 | |
| 87 | EtAc | ≥4000 | ≥4000 | 28.7 | ||
| 88 | Hex | ≥4000 | ≥4000 | 6.4 | ||
| 89 | Duabanga grandiflora | Leaf | Water | 140 | 210 | 251.9 |
| 90 | EtOH | 50.0 | 110 | 476.8 | ||
| 91 | EtAc | 150 | 280 | 192.3 | ||
| 92 | Hex | ≥4000 | ≥4000 | 10.4 | ||
| 93 | Bark | Water | 130 | 260 | 363.7 | |
| 94 | EtOH | 80.0 | 210 | 404.6 | ||
| 95 | EtAc | 240 | 570 | 135.4 | ||
| 96 | Hex | ≥4000 | ≥4000 | 18.9 | ||
| Quercetin | 59.8 ± 0.75 3 | 49.9 ± 1.02 3 | N/A |
2.2. Chemicals
2.3. DPPH Assay
2.4. FRAP Assay
2.5. TPC Assay
2.6. Data Analysis
3. Results and Discussion
3.1. Results
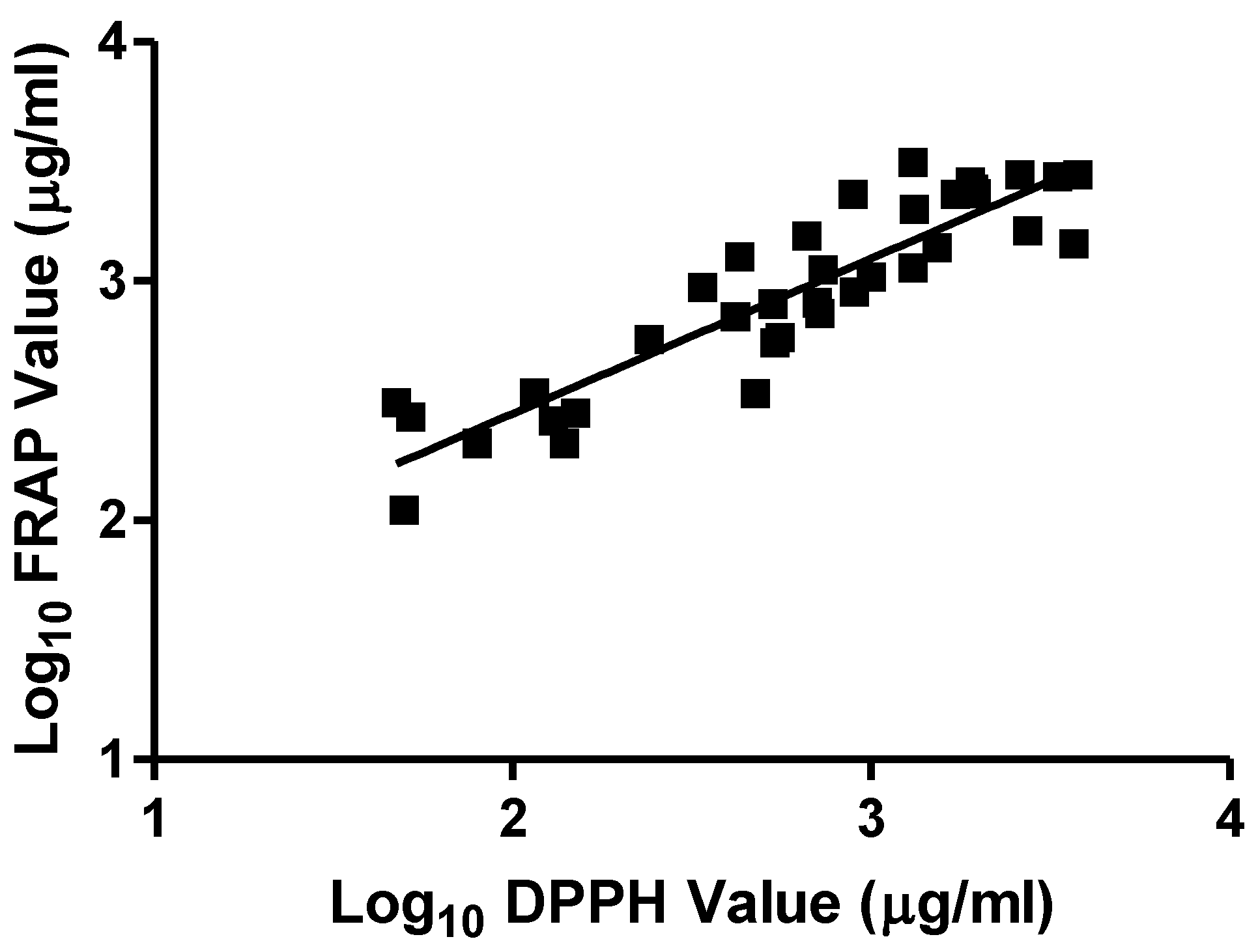

3.2. Discussion
4. Conclusions
References
- Ting, K.N.; Othman, M.; Telford, G.; Clarke, G.; Bradshaw, T.D.; Khoo, T.J.; Loh, H.S.; Wiart, C.; Pritchard, D.; Fry, J.R. Antioxidant, cytoprotective, growth inhibitory and immunomodulatory activities of extracts of Dysoxylum cauliflorum Hiern., a Malaysian Meliaceae. J. Med. Plants Res. 2011, 5, 5867–5872. [Google Scholar]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Nat. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 54, 1841–1856. [Google Scholar]
- Othman, M.; Loh, H.S.; Wiart, C.; Khoo, T.J.; Lim, K.H.; Ting, K.N. Optimal methods for evaluating antimicrobial activities from plant extracts. J. Microbiol. Methods 2011, 84, 161–166. [Google Scholar] [CrossRef]
- Xu, H.-X.; Chen, J.-W. Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) fruits. J. Sci. Food Agric. 2011, 91, 1057–1063. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Bartkowiak-Broda, I.; Karlović, I.; Karlovits, G.; Szłyk, E. Antioxidant capacity, total phenolics, glucosinolates and colour parameters of rapeseed cultivars. Food Chem. 2011, 127, 556–563. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Blomhoff, R. Validation of a quantitative assay for the total content of lipophilic and hydrophilic antioxidants in foods. Food Chem. 2011, 127, 761–768. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenlic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Clarke, G.; Fry, J.R. Unpublished work, University of Nottingham: Nottingham, UK, 2011.
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants 2013, 2, 1-10. https://doi.org/10.3390/antiox2010001
Clarke G, Ting KN, Wiart C, Fry J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants. 2013; 2(1):1-10. https://doi.org/10.3390/antiox2010001
Chicago/Turabian StyleClarke, Garry, Kang Nee Ting, Christophe Wiart, and Jeffrey Fry. 2013. "High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest" Antioxidants 2, no. 1: 1-10. https://doi.org/10.3390/antiox2010001




