Significant Tic Reduction in An Otherwise Treatment-Resistant Patient with Gilles de la Tourette Syndrome Following Treatment with Nabiximols
Abstract
:1. Introduction
2. A Case of Severe TS
3. Treatment with Nabiximols
4. Limitations
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Robertson, M.M.; Eapen, V.; Cavanna, A.E. The international prevalence, epidemiology, and clinical phenomenology of Tourette syndrome: A cross-cultural perspective. J. Psychosom. Res. 2009, 67, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cubo, E.; Trejo Gabriel y Galán, J.M.; Villaverde, V.A.; Sáez Velasco, S.; Delgado Benito, V.; Vicente Macarrón, J.; Guevara, J.C.; Louis, E.D.; Benito-León, J. Prevalence of Tics in Schoolchildren in Central Spain: A Population-Based Study. Pediatr. Neurol. 2011, 45, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.D.; Fast, D.K.; Burd, L.; Kerbeshian, J.; Robertson, M.M.; Sandor, P. An international perspective on Tourette syndrome: Selected findings from 3,500 individuals in 22 countries. Dev. Med. Child Neurol. 2000, 42, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M. Annotation: Gilles de la Tourette syndrome-an update. J. Child Psychol. Psychiatry 1994, 35, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Tourette syndrome. Neurol. Clin. 1997, 15, 395–402. [Google Scholar] [CrossRef]
- Roessner, V.; Plessen, K. J.; Rothenberger, A.; Ludolph, A.G.; Rizzo, R.; Skov, L.; Strand, G.; Stern, J.S.; Termine, C.; Hoekstra, P.J. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: Pharmacological treatment. Eur. Child Adolesc. Psychiatry 2011, 20, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.; Yellowlees, P.M. Effective treatment of Tourette’s syndrome with marijuana. J. Psychopharmacol. 1993, 7, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R.; Awerbuch, G. Marijuana and Tourette’s syndrome. J. Clin. Psychopharmacol 1988, 8, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Schneider, U.; Prevedel, H.; Theloe, K.; Kolbe, H.; Daldrup, T.; Emrich, H.M. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: A 6-week randomized trial. J. Clin. Psychiatry 2003, 64, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Schneider, U.; Koblenz, A.; Jöbges, M.; Kolbe, H.; Daldrup, T.; Emrich, H.M. Treatment of Tourette’s syndrome with Delta 9-tetrahydrocannabinol (THC): A randomized crossover trial. Pharmacopsychiatry 2002, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.A.; Freeman, T.P.; Schafer, G.L.; Curran, H.V. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.A.; Schafer, G.; Freeman, T.P.; Curran, H.V. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study. Br. J. Psychiatry J. Ment. Sci. 2010, 197, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Wippermann, S.; Ahrens, J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 2010, 70, 2409–2438. [Google Scholar] [CrossRef] [PubMed]
- Trainor, D.; Evans, L.; Bird, R. Severe motor and vocal tics controlled with Sativex®. Australas. Psychiatry 2016, 24, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.; Shapiro, E.; Young, J.; Feinberg, T. Signs, symptoms, and clinical course. In Gilles de la Tourette Syndrome; Shapiro, A., Shapiro, E., Young, J., Feinberg, T., Eds.; Raven: Albany, NY, USA, 1988; pp. 127–193. [Google Scholar]
- Goetz, C.G.; Pappert, E.J.; Louis, E.D.; Raman, R.; Leurgans, S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 502–506. [Google Scholar] [CrossRef]
- Woods, D.W.; Piacentini, J.; Himle, M.B.; Chang, S. Premonitory Urge for Tics Scale (PUTS): Initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J. Dev. Behav. Pediatr. JDBP 2005, 26, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Schrag, A.; Morley, D.; Orth, M.; Robertson, M.M.; Joyce, E.; Critchley, H.D.; Selai, C. The Gilles de la Tourette syndrome-quality of life scale (GTS-QOL): Development and validation. Neurology 2008, 71, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Schneider, U.; Kolbe, H.; Emrich, H.M. Treatment of Tourette’s syndrome with delta-9-tetrahydrocannabinol. Am. J. Psychiatry 1999, 156, 495. [Google Scholar] [PubMed]
- Brunnauer, A.; Segmiller, F.M.; Volkamer, T.; Laux, G.; Müller, N.; Dehning, S. Cannabinoids improve driving ability in a Tourette’s patient. Psychiatry Res. 2011, 190, 382. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Rothenberger, A.; Münchau, A.; Wobrock, T.; Falkai, P.; Roessner, V. Oral delta 9-tetrahydrocannabinol improved refractory Gilles de la Tourette syndrome in an adolescent by increasing intracortical inhibition: A case report. J. Clin. Psychopharmacol. 2010, 30, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Kolbe, H.; Schneider, U.; Emrich, H.M. Cannabinoids: Possible role in patho-physiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatr. Scand. 1998, 98, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K. Tourette-Syndrom und andere Tic-Erkrankungen im Kindes—Und Erwachsenenalter, 2nd ed.; Auflage; Medizinisch Wissenschaftliche Verlagsgesellschaft: Berlin, Germany, 2014. [Google Scholar]
- Gerasch, S.; Kanaan, A.S.; Jakubovski, E.; Müller-Vahl, K.R. Aripiprazole improves associated comorbid Conditions in addition to Tics in adult patients with Gilles de la Tourette Syndrome. Front. Neurosci. 2016, 10, 416. [Google Scholar] [CrossRef] [PubMed]
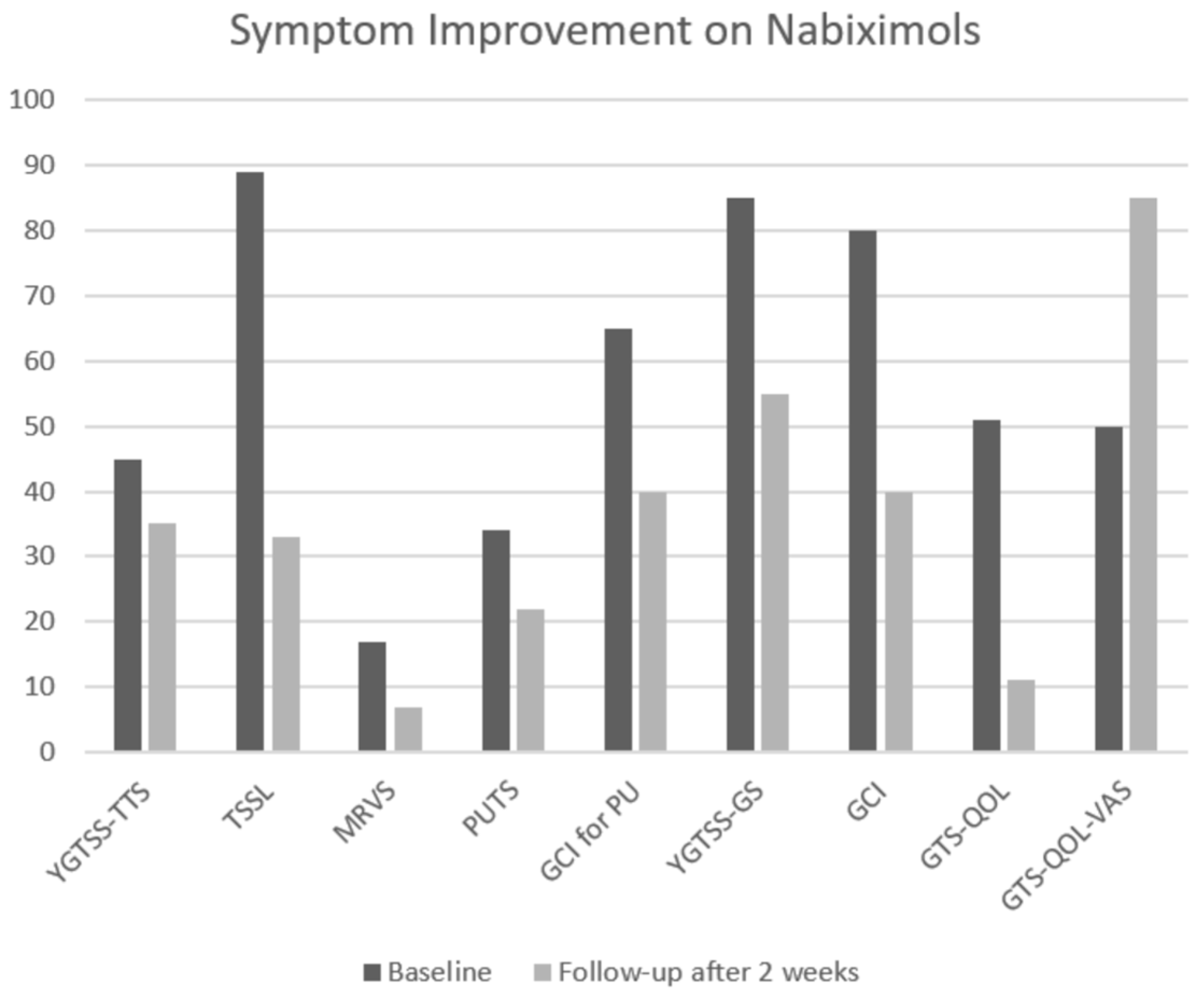
| Symptom | Scale | Baseline | Follow-Up after 2 Weeks | Percentage Improvement |
|---|---|---|---|---|
| Tics | YGTSS-TTS | 45 | 35 | −22.2% |
| TSSL | 89 | 33 | −62.9% | |
| MRVS | 17 | 7 | −58.8% | |
| Premonitory urges | PUTS | 34 | 22 | −35.3% |
| GCI for PU | 65 | 40 | −38.5% | |
| Global impairment | YGTSS-GS | 85 | 55 | −35.2% |
| GCI | 80 | 40 | −50.0% | |
| Quality of life | GTS-QOL | 51 | 11 | −78.4% |
| GTS-QOL-VAS a | 50 | 85 | 70% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanaan, A.S.; Jakubovski, E.; Müller-Vahl, K. Significant Tic Reduction in An Otherwise Treatment-Resistant Patient with Gilles de la Tourette Syndrome Following Treatment with Nabiximols. Brain Sci. 2017, 7, 47. https://doi.org/10.3390/brainsci7050047
Kanaan AS, Jakubovski E, Müller-Vahl K. Significant Tic Reduction in An Otherwise Treatment-Resistant Patient with Gilles de la Tourette Syndrome Following Treatment with Nabiximols. Brain Sciences. 2017; 7(5):47. https://doi.org/10.3390/brainsci7050047
Chicago/Turabian StyleKanaan, Ahmad Seif, Ewgeni Jakubovski, and Kirsten Müller-Vahl. 2017. "Significant Tic Reduction in An Otherwise Treatment-Resistant Patient with Gilles de la Tourette Syndrome Following Treatment with Nabiximols" Brain Sciences 7, no. 5: 47. https://doi.org/10.3390/brainsci7050047







