Rumination and Rebound from Failure as a Function of Gender and Time on Task
Abstract
:1. Introduction
2. Method
2.1. Subjects
2.2. Design and Procedure
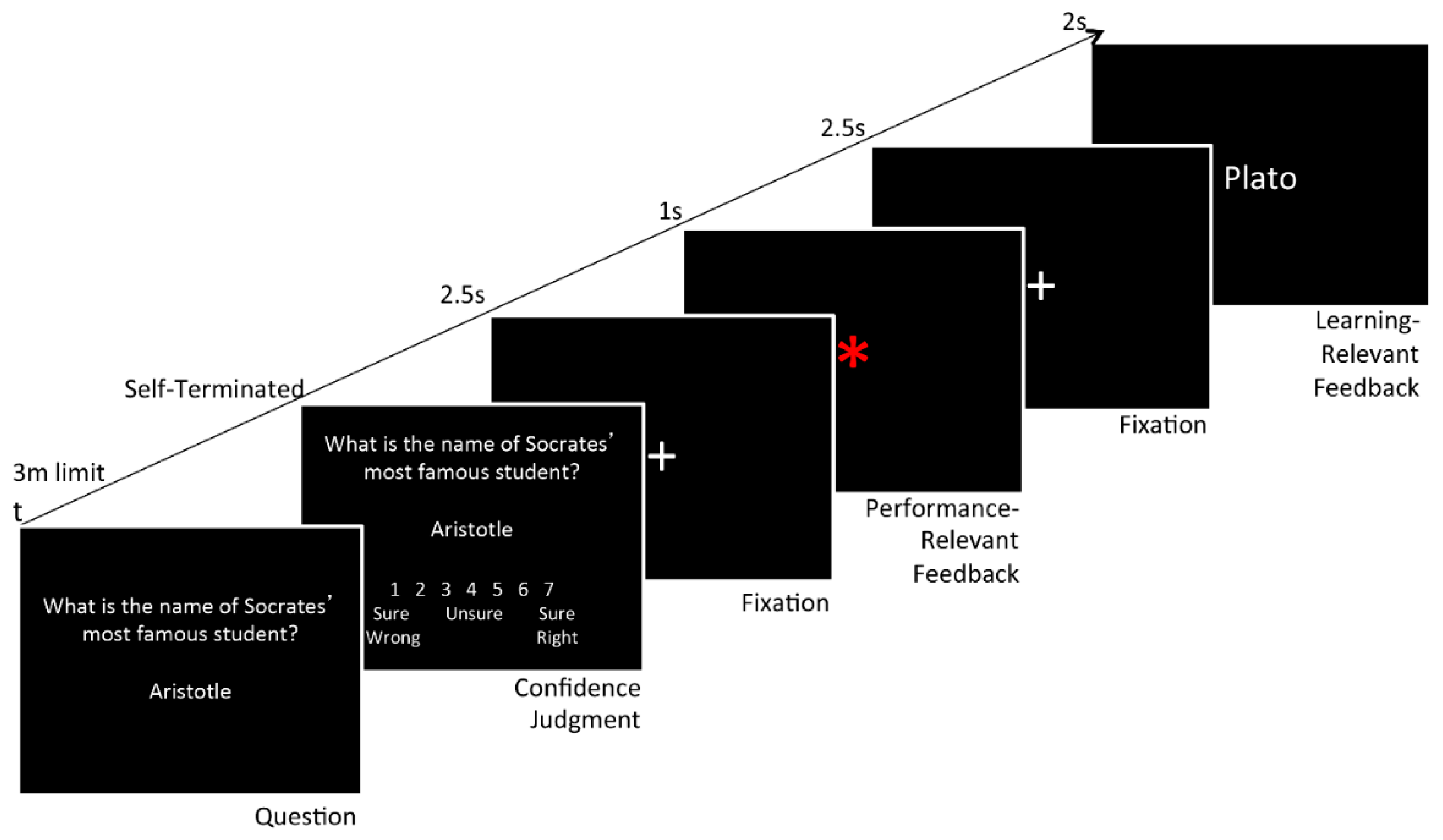
2.2.1. Overview
2.2.2. First Day of Testing
2.2.3. Second Day of Testing
2.3. EEG Recording
2.4. Data Analysis
2.4.1. Overview
2.4.2. ERP Responses to Performance-Relevant Feedback
The Feedback-Related Negativity (FRN)
The Late Positive Potential (LPP)
2.4.3. ERP Response to Learning-Relevant Feedback
3. Results
3.1. Memory Performance
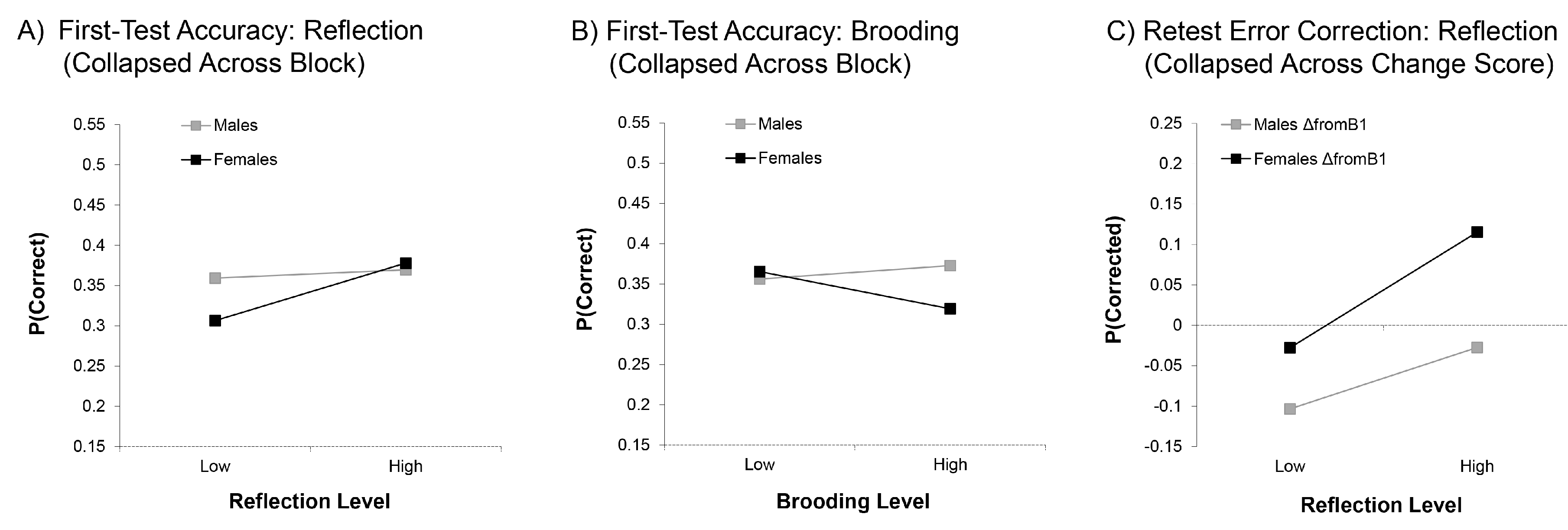
3.1.1. First-Test Accuracy
3.1.2. Error Correction
3.2. Subjective Experiences
3.2.1. Recurring Negative Thoughts (RNTs)
3.2.2. Feelings After Errors (FAEs)
3.3. Event-Related Potentials (ERPs)
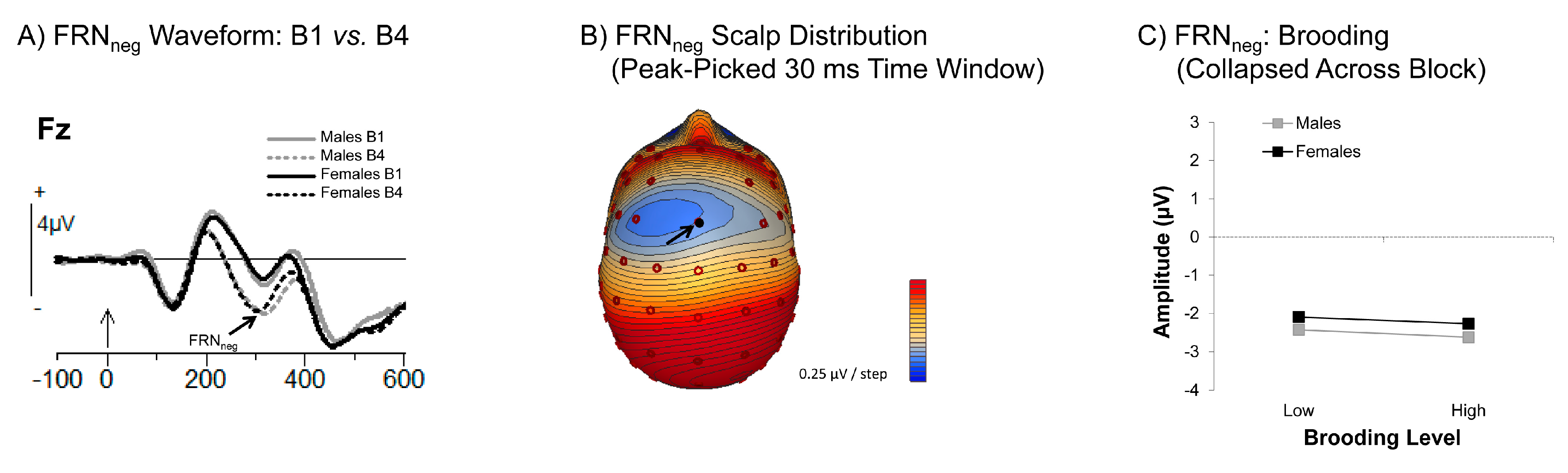
3.3.1. Feedback Related Negativity (FRN)
3.3.2. Late Positive Potential (LPP)
The Early LPPneg
The Late LPPneg
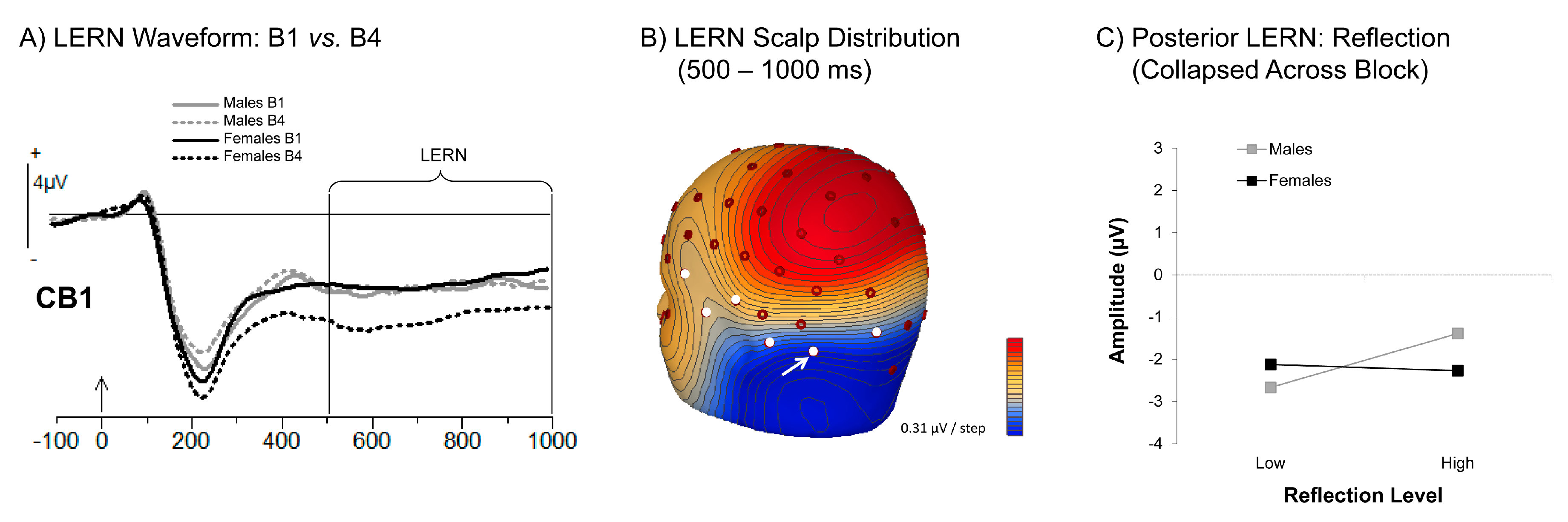
3.3.3. Learning Error Related Negativity (LERN)
4. Discussion
4.1. Reflection Benefits First-Test and Retest Memory
4.2. Brooding and Reflection Influence Thoughts and Feelings in Response to Negative Feedback
4.3. Differential Effects of Brooding and Reflection as a Function of Time on Task
4.4. Differential Effects of Brooding and Reflection as a Function of Gender
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bandura, S.A. Perceived self-efficacy in cognitive development and functioning. Educ. Psychol. 1993, 28, 117–148. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Peterson, C.; Matthews, M.D.; Kelly, D.R. Grit: Perseverence and passion for long-term goals. Personal. Process. Individ. Differ. 2007, 92, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.J. Emotion, cognition, and behavior. Science 2002, 298, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. Emergent processes in cognitive-emotional interactions. Dialogues Clin. Neurosci. 2010, 12, 433–448. [Google Scholar] [PubMed]
- Nolen-Hoeksema, S.; Morrow, J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 loma prieta earthquake. J. Personal. Soc. Psychol. 1991, 61, 115–121. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Wisco, B.E.; Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci. 2008, 3, 400–424. [Google Scholar] [CrossRef] [PubMed]
- Lyubomirsky, S.; Nolen-Hoeksema, S. Effects of self-focused rumination on negative thinking and interpersonal problem solving. J. Personal. Soc. Psychol. 1995, 69, 176–190. [Google Scholar] [CrossRef]
- Martin, L.; Tesser, A. Toward a motivational and structural theory of ruminative thought. In Unintened Thought; Uleman, J.S., Bargh, J.A., Eds.; Guilford: New York, NY, USA, 1989; pp. 306–326. [Google Scholar]
- Martin, L.; Tesser, A. Some ruminative thoughts. In Ruminative Thoughts; Wyer, R.S., Ed.; Erlbaum: Hillsdale, NJ, USA, 1996; pp. 1–47. [Google Scholar]
- Martin, L.L. Set/reset: Use and disuse of concepts in impression formation. J. Personal. Soc. Psychol. 1986, 51, 493–504. [Google Scholar] [CrossRef]
- Treynor, W.; Gonzalez, R.; Nolen-Hoeksema, S. Rumination reconsidered: A psychometric analysis. Cogn. Ther. Res. 2003, 27, 247–259. [Google Scholar] [CrossRef]
- Donaldson, C.; Lam, D.; Mathews, A. Rumination and attention in major depression. Behav. Res. Ther. 2007, 45, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Vanderhasselt, M.A.; Kuhn, S.; de Raedt, R. Healthy brooders employ more attentional resources when disengaging from the negative: An event-related fMRI study. Cogn. Affect. Behav. Neurosci. 2011, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Daches, S.; Mor, N.; Winquist, J.; Gilboa-Schechtman, E. Brooding and attentional control in processing self-relevant information: Evidence from a modified garner task. Cogn. Emot. 2010, 24, 876–885. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S. Sex differences in unipolar depression: Evidence and theory. Psychol. Bull. 1987, 101, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Morrow, J.; Fredrickson, B.L. Response styles and the duration of episodes of depressed mood. J. Abnorm. Psychol. 1993, 102, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Parker, L.E.; Larson, J. Ruminative coping with depressed mood following loss. J. Personal. Soc. Psychol. 1994, 67, 92–104. [Google Scholar] [CrossRef]
- Sarin, S.; Abela, J.R.; Auerbach, R.P. The response styles theory of depression: A test of specificity and causal mediation. Cogn. Emot. 2005, 19, 751–761. [Google Scholar] [CrossRef]
- Butterfield, B.; Mangels, J.A. Neural correlates of error detection and correction in a semantic retrieval task. Brain Res. Cogn. Brain Res. 2003, 17, 793–817. [Google Scholar] [CrossRef]
- Mangels, J.A.; Butterfield, B.; Lamb, J.; Good, C.; Dweck, C.S. Why do beliefs about intelligence influence learning success? A social cognitive neuroscience model. Soc. Cogn. Affect. Neurosci. 2006, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Miltner, W.H.; Braun, C.H.; Coles, M.G. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. J. Cogn. Neurosci. 1997, 9, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.F. The way of our errors: Theme and variations. Psychophysiology 2010, 47, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Foti, D.; Hajcak, G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. J. Cogn. Neurosci. 2008, 20, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.; Cuthbert, B.; Bradley, M.; Hillman, C.; Hamm, A.; Lang, P. Brain processes in emotional perception: Motivated attention. Cogn. Emot. 2004, 18, 593–611. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Coles, M.G. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002, 109, 679–709. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.S.; Hajcak, G.; Bukay, E.; Simons, R.F. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology 2006, 43, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Olvet, D.M. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion 2008, 8, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.M.; Birbaumer, N.; Lang, P.J. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef]
- Mangels, J.A.; Good, C.; Whiteman, R.C.; Maniscalco, B.; Dweck, C.S. Emotion blocks the path to learning under stereotype threat. Soc. Cogn. Affect. Neurosci. 2012, 7, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Beilock, S.L.; Rydell, R.J.; McConnell, A.R. Stereotype threat and working memory: Mechanisms, alleviation, and spillover. J. Exp. Psychol. Gen. 2007, 136, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Schmader, T.; Johns, M.; Forbes, C. An integrated process model of stereotype threat effects on performance. Psychol. Rev. 2008, 115, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Marco-Pallares, J.; Kramer, U.M.; Strehl, S.; Schroder, A.; Munte, T.F. When decisions of others matter to me: An electrophysiological analysis. BMC Neurosci. 2010, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Hertel, P. Memory for emotional and nonemotional events in depression. Mem. Emot. 2004, 186–216. [Google Scholar]
- Watkins, E.R.; Nolen-Hoeksema, S. A habit-goal framework of depressive rumination. J. Abnorm. Psychol. 2014, 123, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gu, R.; Tang, P.; Luo, Y.J. How does cognitive reappraisal affect the response to gains and losses? Psychophysiology 2013, 50, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef] [PubMed]
- Paller, K.A.; Wagner, A.D. Observing the transformation of experience into memory. Trends Cogn. Sci. 2002, 6, 93–102. [Google Scholar] [CrossRef]
- Kutas, M.; Federmeier, K.D. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn. Sci. 2000, 4, 463–470. [Google Scholar] [CrossRef]
- Nobre, A.C.; McCarthy, G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. J. Neurosci. 1995, 15, 1090–1098. [Google Scholar] [PubMed]
- Stern, E.R.; Mangels, J.A. An electrophysiological investigation of preparatory attentional control in a spatial stroop task. J. Cogn. Neurosci. 2006, 18, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R., Jr.; Nessler, D.; Friedman, D. Temporally specific divided attention tasks in young adults reveal the temporal dynamics of episodic encoding failures in elderly adults. Psychol. Aging 2013, 28, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Mangels, J.A.; Picton, T.W.; Craik, F.I. Attention and successful episodic encoding: An event-related potential study. Brain Res. Cogn. Brain Res. 2001, 11, 77–95. [Google Scholar] [CrossRef]
- Nessler, D.; Johnson, R., Jr.; Bersick, M.; Friedman, D. On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal erp activity. NeuroImage 2006, 30, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Manual for Beck Depression Inventory II (bdi-ii); Psychology Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- De Lissnyder, E.; Koster, E.H.; Goubert, L.; Onraedt, T.; Vanderhasselt, M.A.; de Raedt, R. Cognitive control moderates the association between stress and rumination. J. Behav. Ther. Exp. Psychiatry 2012, 43, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetsche, U.; Joormann, J. Components of interference control predict depressive symptoms and rumination cross-sectionally and at six months follow-up. J. Behav. Ther. Exp. Psychiatry 2011, 42, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.G.; Peltier, S.; Nee, D.E.; Kross, E.; Deldin, P.J.; Jonides, J. Depression, rumination and the default mode network. Soc. Cogn. Affect. Neurosci. 2010, 6, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bernblum, R.; Mor, N. Rumination and emotion-related biases in refreshing information. Emotion 2010, 10, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.N.; Nolen-Hoeksema, S. Cognitive inflexibility among ruminators and nonruminators. Cogn. Ther. Res. 2000, 24, 699–711. [Google Scholar] [CrossRef]
- Joormann, J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: Evidence from a negative priming task. Cogn. Ther. Res. 2006, 30, 149–160. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 65–70. [Google Scholar]
- Olvet, D.M.; Hajcak, G. The stability of error-related brain activity with increasing trials. Psychophysiology 2009, 46, 957–961. [Google Scholar] [CrossRef] [PubMed]
- San Martín, R. Event-related potential studies of outcome processing and feedback-guided learning. Front. Hum. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.B.; Pakzad-Vaezi, K.L.; Krigolson, O.E. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology 2008, 45, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, S.; Slagter, H.A.; Geusau, V.; Alting, N.J.; Heslenfeld, D.J.; Holroyd, C.B. Knowing good from bad: Differential activation of human cortical areas by positive and negative outcomes. Eur. J. Neurosci. 2005, 21, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Moser, J.S.; Holroyd, C.B.; Simons, R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006, 71, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Dunning, J.P.; Foti, D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clin. Neurophysiol. 2009, 120, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Hilgard, J.; Bartholow, B.D.; Hajcak, G. Emotional targets: Evaluative categorization as a function of context and content. Int. J. Psychophysiol. 2012, 84, 149–154. [Google Scholar] [CrossRef] [PubMed]
- De Lissnyder, E.; Koster, E.H.; Derakshan, N.; de Raedt, R. The association between depressive symptoms and executive control impariments in response to emotional and non-emotional information. Cogn. Emot. 2010, 24, 264–280. [Google Scholar] [CrossRef] [Green Version]
- De Lissnyder, E.; Derakshan, N.; de Raedt, R.; Koster, E.H. Depressive symptoms and attentional control in a mixed antisaccade task: Specific effects of rumination. Cogn. Emot. 2011, 25, 886–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertel, P.T.; Benbow, A.A.; Geraerts, E. Brooding deficits in memory: Focusing attention improves subsequent recall. Cogn. Emot. 2012, 26, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Ciarocco, N.J.; Vohs, K.D.; Baumeister, R.F. Some good news about rumination: Task-focused thinking after failure facilitates performance improvement. J. Soc. Clin. Psychol. 2010, 29, 1057–1073. [Google Scholar] [CrossRef]
- Hajcak, G.; MacNamara, A.; Olvet, D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol. 2010, 35, 129–155. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Jackson, B. Mediators of the gender difference in rumination. Psychol. Women Q. 2001, 25, 37–47. [Google Scholar] [CrossRef]
- Borkovec, T.D.; Hazlett-Stevens, H.; Diaz, M.L. The role of positive beliefs about worry in generalized anxiety disorder and its treatment. Clin. Psychol. Psychother. 1999, 6, 126–138. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Borkovec, T.D.; Sibrava, N.J. The effects of worry and rumination on affect states and cognitive activity. Behav. Ther. 2007, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Santesso, D.L.; Steele, K.T.; Bogdan, R.; Holmes, A.J.; Deveney, C.M.; Meites, T.M.; Pizzagalli, D.A. Enhanced negative feedback responses in remitted depression. Neuroreport 2008, 19, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Luu, P.; Frishkoff, G.; Quiring, J.; Poulsen, C. Frontolimbic response to negative feedback in clinical depression. J. Abnorm. Psychol. 2003, 112, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Sabatinelli, D.; Lang, P.J.; Keil, A.; Bradley, M.M. Emotional perception: Correlation of functional mri and event-related potentials. Cereb. Cortex 2007, 17, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Siegle, G.J.; Steinhauer, S.R.; Thase, M.E.; Stenger, V.A.; Carter, C.S. Can’t shake that feeling: Event-related fmri assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry 2002, 51, 693–707. [Google Scholar] [CrossRef]
- Ray, R.D.; Ochsner, K.N.; Cooper, J.C.; Robertson, E.R.; Gabrieli, J.D.; Gross, J.J. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 2005, 5, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, M.; Ferrari, V.; Bradley, M.M. Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. J. Cogn. Neurosci. 2007, 19, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Moser, J.S.; Simons, R.F. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion 2006, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, G.D.; Pezzo, M.V.; Apple, K.J. The transmitter-persistence effect: A confounded discovery? Psychol. Sci. 1993, 4, 208–210. [Google Scholar] [CrossRef]
- McIntosh, W.D.; Martin, L.L. The cybernetics of happiness: The relation betewen goal attainment, rumination, and affect. In Review of Personality and Social Psychology; Clark, M.S., Ed.; Sage: Newbury Park, CA, USA, 1992; Volume 14, pp. 222–246. [Google Scholar]
- Nolen-Hoeksema, S.; Larson, J.; Grayson, C. Explaining the gender difference in depressive symptoms. J. Personal. Soc. Psychol. 1999, 77, 1061–1072. [Google Scholar] [CrossRef]
- Johnson, D.P.; Whisman, M.A. Gender differences in rumination: A meta-analysis. Personal. Individ. Differ. 2013, 55, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S. Sex Differences in Depression; Stanford University Press: Redwood City, CA, USA, 1990. [Google Scholar]
- Thayer, J.; Johnsen, B.H. Sex differences in judgement of facial affect: A multivariate analysis of recognition errors. Scand. J. Psychol. 2000, 41, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Rossy, L.A.; Ruiz-Padial, E.; Johnsen, B.H. Gender differences in the relationship between emotional regulation and depressive symptoms. Cogn. Ther. Res. 2003, 27, 349–364. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kambara, M.; Tanno, Y. Response styles and cognitive and affective symptoms of depression. Personal. Individ. Differ. 2001, 31, 1053–1065. [Google Scholar] [CrossRef]


| Overall | Range | Males | Females | |
|---|---|---|---|---|
| n | 40 | - | 20 | 20 |
| Age | 20.43 (0.38) | 18.16–29.91 | 20.87 (0.66) | 19.99 (0.38) |
| Years of Education | 14.20 (0.18) | 13–16 | 14.20 (0.25) | 14.20 (0.27) |
| BDI-II | 7.45 (0.74) | 0–17 | 7.80 (1.03) | 7.10 (1.07) |
| RRS Total | 39.23 (1.65) | 23–69 | 38.80 (2.66) | 39.65 (2.03) |
| Brooding | 9.05 (0.46) | 5–16 | 8.90 (0.76) | 9.20 (0.52) |
| Reflection | 9.28 (0.56) | 5–19 | 9.50 (0.93) | 9.05 (0.64) |
| Behavior | Overall | Block 1 | Block 2 | Block 3 | Block 4 |
|---|---|---|---|---|---|
| First-Test Accuracy | |||||
| Females | 0.340 (0.007) | 0.346 (0.006) | 0.349 (0.008) | 0.335 (0.012) | 0.328 (0.016) |
| Males | 0.363 (0.007) | 0.363 (0.006) | 0.360 (0.008) | 0.377 (0.012) | 0.352 (0.016) |
| Retest Error Correction | |||||
| Females | 0.606 (0.021) | 0.578 (0.025) | 0.643 (0.030) | 0.605 (0.026) | 0.598 (0.031) |
| Males | 0.545 (0.021) | 0.597 (0.025) | 0.573 (0.030) | 0.499 (0.026) | 0.513 (0.031) |
| Subjective Experience | Overall | Block 1 | Block 2 | Block 3 | Block 4 |
|---|---|---|---|---|---|
| Recurring Negative Thoughts | |||||
| Females | 4.50 (0.40) | 4.45 (0.40) | 4.46 (0.42) | 4.78 (0.51) | 4.29 (0.51) |
| Males | 3.97 (0.40) | 4.55 (0.40) | 4.09 (0.42) | 3.62 (0.51) | 3.61 (0.51) |
| Feelings After Errors | |||||
| Females | 3.44 (0.24) | 3.53 (0.27) | 3.45 (0.35) | 3.19 (0.29) | 3.59 (0.31) |
| Males | 3.66 (0.24) | 2.98 (0.27) | 3.48 (0.35) | 3.96 (0.29) | 4.21 (0.31) |
| ERP | Overall | Block 1 | Block 2 | Block 3 | Block 4 |
|---|---|---|---|---|---|
| FRNneg | |||||
| Females | −2.18 (0.45) | −1.58 (0.53) | −1.83 (0.44) | −2.34 (0.54) | −2.97 (0.49) |
| Males | −2.53 (0.45) | −1.82 (0.53) | −2.48 (0.44) | −2.78 (0.54) | −3.06 (0.49) |
| FRNdiff | |||||
| Females | −1.91 (0.33) | −1.18 (0.51) | −1.72 (0.47) | −2.38 (0.39) | −2.34 (0.42) |
| Males | −1.87 (0.33) | −1.71 (0.51) | −2.07 (0.47) | −1.83 (0.39) | −1.84 (0.42) |
| Early LPPneg | |||||
| Females | 3.05 (0.29) | 3.43 (0.32) | 3.10 (0.31) | 3.06 (0.34) | 2.61 (0.31) |
| Males | 1.96 (0.29) | 2.43 (0.32) | 2.02 (0.31) | 1.70 (0.34) | 1.68 (0.31) |
| Late LPPneg | |||||
| Females | 2.09 (0.21) | 1.98 (0.24) | 2.26 (0.24) | 2.09 (0.27) | 2.02 (0.24) |
| Males | 1.68 (0.21) | 1.83 (0.24) | 1.96 (0.24) | 1.48 (0.27) | 1.44 (0.24) |
| Post LERN | |||||
| Females | −3.22 (0.39) | −2.33 (0.34) | −3.47 (0.42) | −3.60 (0.43) | −3.47 (0.48) |
| Males | −2.75 (0.39) | −2.77 (0.34) | −2.94 (0.42) | −2.78 (0.43) | −2.51 (0.48) |
| Left LERN | |||||
| Females | −2.18 (0.28) | −1.50 (0.26) | −2.39 (0.30) | −2.32 (0.32) | −2.52 (0.33) |
| Males | −2.03 (0.28) | −1.82 (0.26) | −2.08 (0.30) | −2.22 (0.32) | −1.99 (0.33) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whiteman, R.C.; Mangels, J.A. Rumination and Rebound from Failure as a Function of Gender and Time on Task. Brain Sci. 2016, 6, 7. https://doi.org/10.3390/brainsci6010007
Whiteman RC, Mangels JA. Rumination and Rebound from Failure as a Function of Gender and Time on Task. Brain Sciences. 2016; 6(1):7. https://doi.org/10.3390/brainsci6010007
Chicago/Turabian StyleWhiteman, Ronald C., and Jennifer A. Mangels. 2016. "Rumination and Rebound from Failure as a Function of Gender and Time on Task" Brain Sciences 6, no. 1: 7. https://doi.org/10.3390/brainsci6010007






