Individual Differences in Anticipatory Somatosensory Cortex Activity for Shock is Positively Related with Trait Anxiety and Multisensory Integration
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Design and Procedure
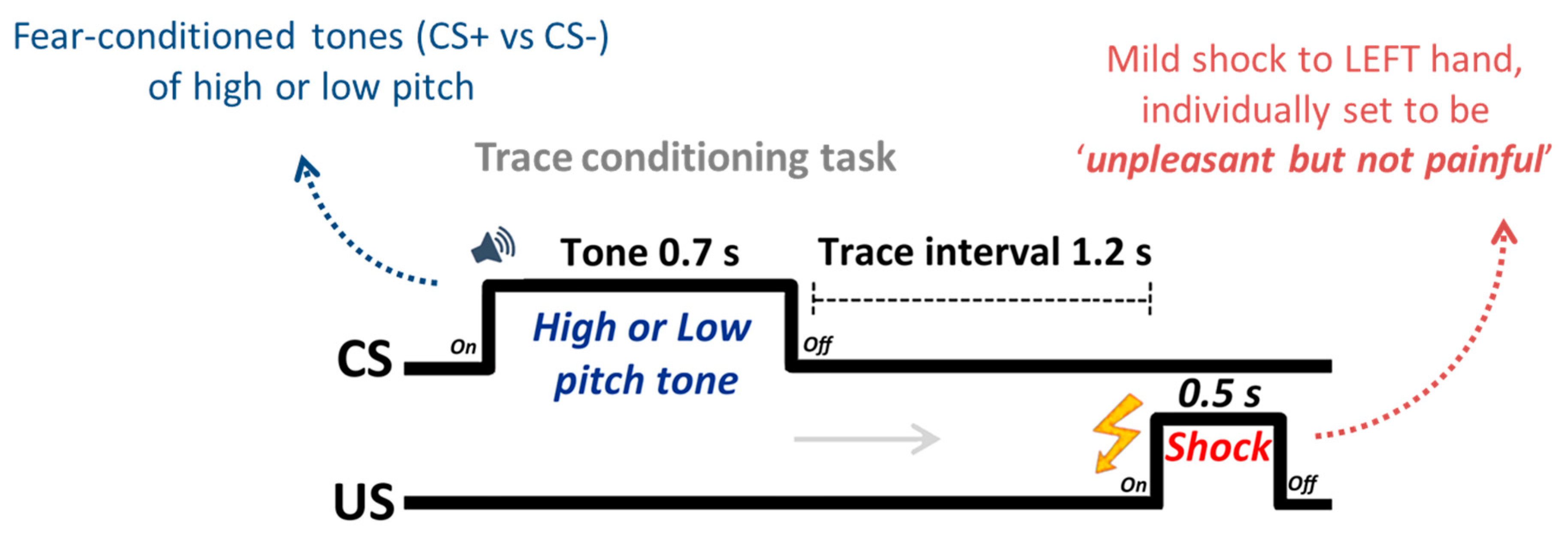
2.3. Physiological Recordings
2.4. MRI Data Acquisition
2.5. General fMRI Data Analysis
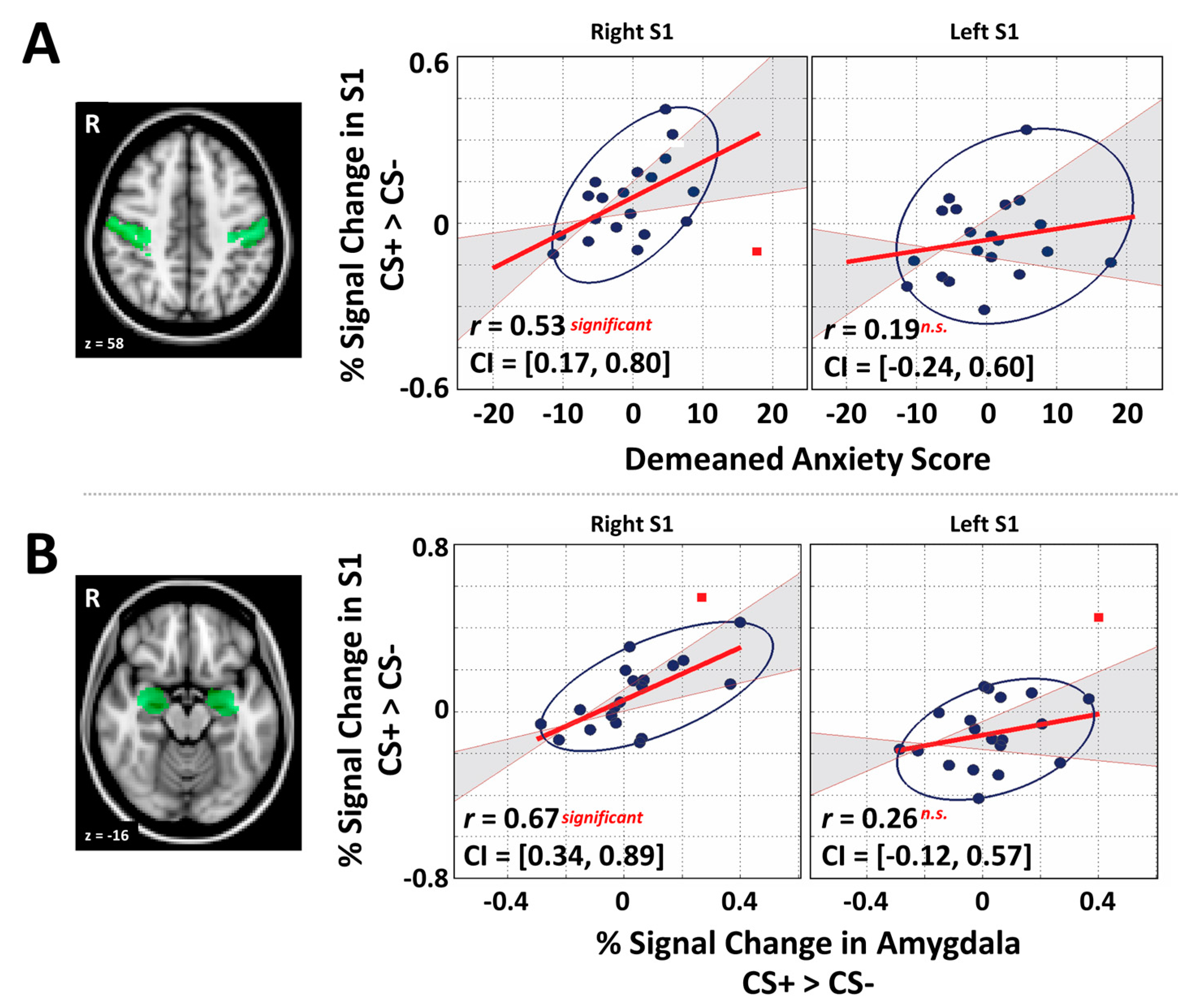
2.6. General Region of Interest Analysis and Correlation
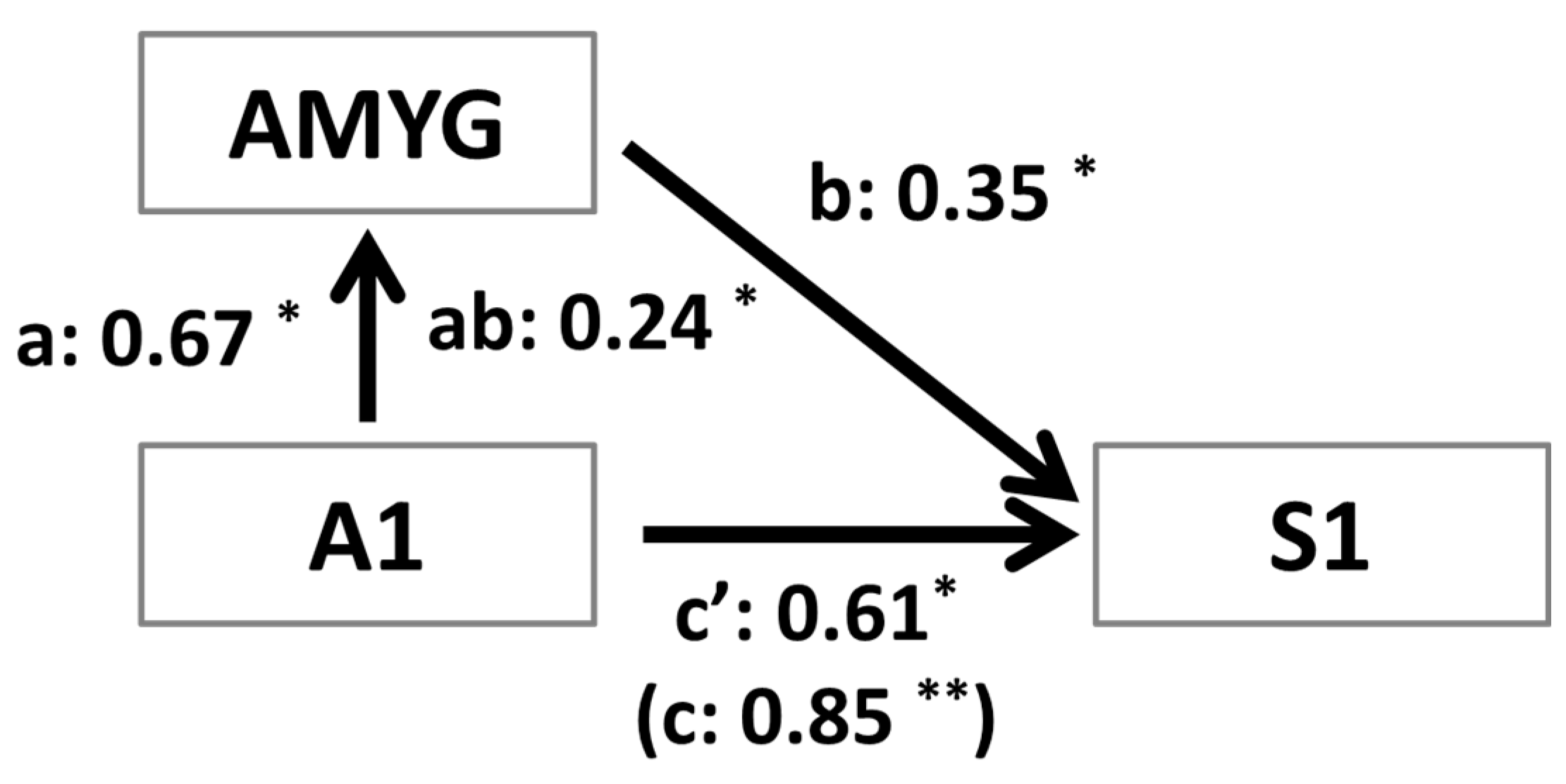
2.7. Mediation Analysis
2.8. Functional Connectivity Analysis
3. Results
3.1. Physiological Index of Expectancy
3.2. ROI Univariate Effects
3.3. Primary Somatosensory Cortex and Individual Differences Analyses
3.4. Mediation Analysis
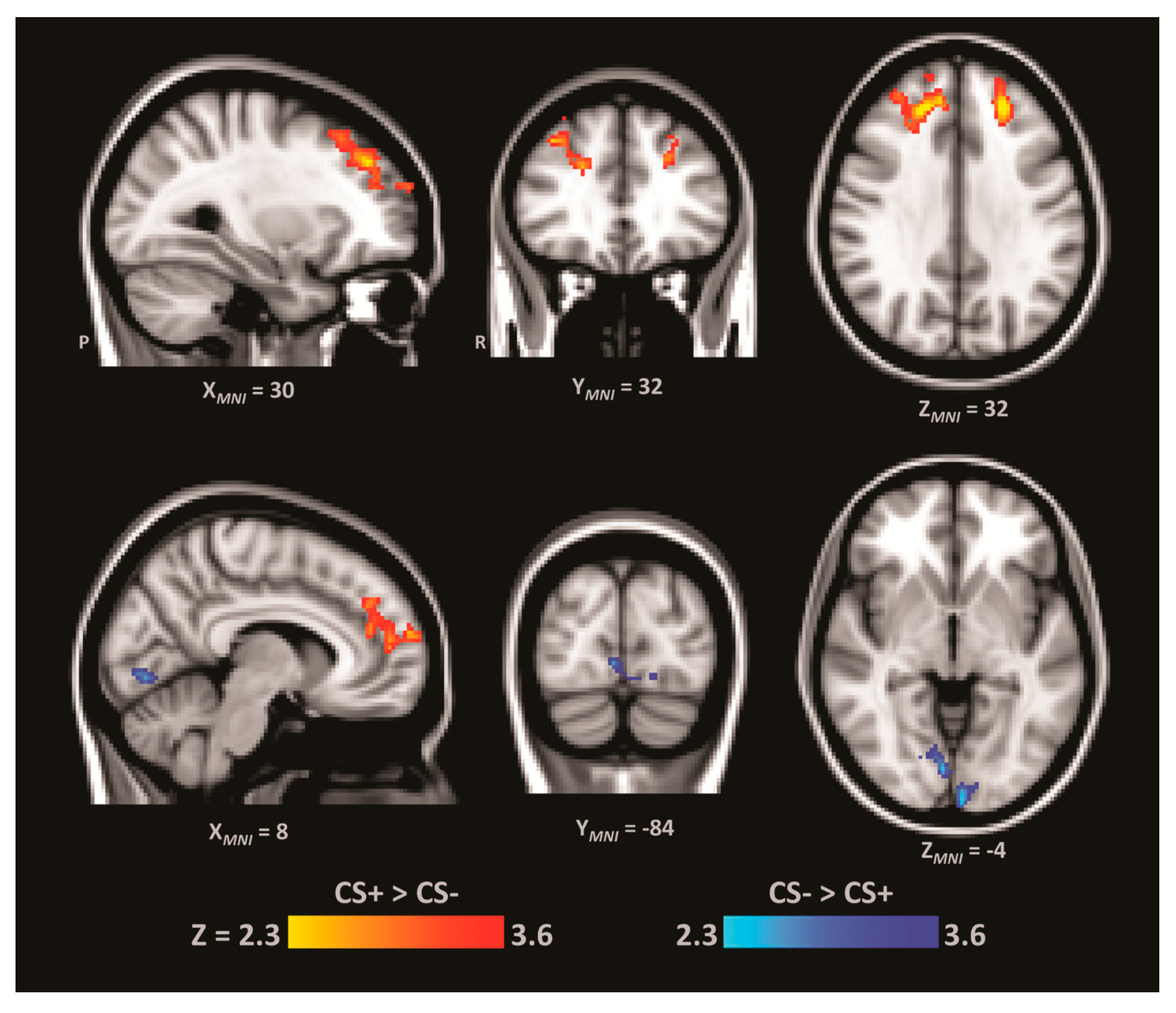
3.5. Functional Connectivity Analysis
| Cluster # | Cluster k | Region of Local Maxima | Z | p-Val | H | MNI | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| CS+ > CS− | ||||||||
| 1 | 1369 | Superior/Middle Frontal Gyrus (dlPFC) | 3.41 | <0.001 | R | 34 | 46 | 28 |
| Paracingulate | 3.06 | <0.005 | R | 6 | 52 | 10 | ||
| Middle Frontal Gyrus | 3.02 | <0.005 | R | 38 | 30 | 42 | ||
| 2 | 577 | Superior/Middle Frontal Gyrus (dlPFC) | 3.89 | <0.001 | L | −24 | 44 | 36 |
| CS− > CS+ | ||||||||
| 1 | 660 | Occipital pole | 3.80 | <0.001 | L | −2 | −98 | 10 |
| Lingual gyrus | 3.18 | <0.001 | R | 10 | −76 | −6 | ||
3.6. Whole-Brain Analysis
| Cluster # | Cluster k | Region of Local Maxima | Z | p-Val | H | MNI | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| CS+ > CS− | ||||||||
| 1 | 9034 | Frontal Orbital Cortex | 5.10 | <0.001 | R | 38 | 24 | −6 |
| Insula | 4.72 | <0.001 | R | 38 | 18 | −6 | ||
| Caudate | 4.57 | <0.001 | R | 10 | 12 | 2 | ||
| Caudate | 4.28 | <0.001 | L | −8 | 8 | 2 | ||
| Heschl’s Gyrus | 3.79 | <0.001 | R | 50 | −20 | 10 | ||
| Temporal Pole | 3.55 | <0.001 | R | 50 | 16 | −12 | ||
| Thalamus | 3.49 | <0.001 | R | 8 | −16 | 6 | ||
| Angular Gyrus | 3.36 | <0.001 | R | 54 | −50 | 18 | ||
| Putamen | 3.21 | <0.001 | R | 32 | −14 | 2 | ||
| Inferior Frontal Gyrus | 3.19 | <0.001 | R | 54 | 16 | 8 | ||
| Thalamus | 3.05 | <0.005 | L | −12 | −18 | 6 | ||
| Middle Temporal Gyrus | 2.79 | <0.005 | R | 68 | −34 | −12 | ||
| Supramarginal Gyrus | 2.71 | <0.005 | R | 64 | −24 | 28 | ||
| Putamen | 2.48 | <0.01 | L | −18 | 6 | −2 | ||
| 2 | 4936 | Anterior Cingulate Cortex | 4.37 | <0.001 | 4 | 6 | 38 | |
| Superior Parietal Lobule | 4.16 | <0.001 | R | 32 | −44 | 68 | ||
| Posterior Cingulate Cortex | 3.69 | <0.001 | 6 | −22 | 44 | |||
| Precentral Gyrus | 3.25 | <0.001 | R | 46 | −6 | 54 | ||
| Paracingulate | 3.18 | <0.001 | 6 | 40 | 26 | |||
| Supplementary Motor Cortex | 3.07 | <0.005 | R | 6 | −6 | 58 | ||
| Supplementary Motor Cortex | 2.42 | <0.01 | L | −2 | 4 | 58 | ||
| Superior Frontal Gyrus | 2.41 | <0.01 | R | 4 | 28 | 52 | ||
| 3 | 1198 | Frontal Orbital Cortex | 4.36 | <0.001 | L | −32 | 24 | −8 |
| Insula | 3.32 | <0.001 | L | −40 | 10 | −6 | ||
| Temporal Pole | 2.69 | <0.005 | L | −54 | 8 | −6 | ||
| CS− > CS+ | ||||||||
| 1 | 2450 | Middle Frontal Gyrus | 3.12 | <0.001 | L | −34 | 24 | 48 |
| Subcallosal Cortex | 2.62 | <0.005 | L | −6 | 24 | −12 | ||
| Superior Frontal Gyrus | 2.52 | <0.01 | L | −12 | 28 | 58 | ||
| Superior Frontal Gyrus | 2.35 | <0.01 | R | 22 | 26 | 58 | ||
| 2 | 1362 | Temporal Fusiform Cortex | 3.36 | <0.001 | L | −36 | −40 | −22 |
| Inferior Temporal Gyrus | 2.88 | <0.005 | L | −50 | −56 | −14 | ||
| Hippocampus | 2.73 | <0.005 | L | −28 | −38 | −4 | ||
| Parahippocampal Gyrus | 2.62 | <0.005 | L | −22 | −36 | −18 | ||
| Temporal Occipital Fusiform | 2.31 | <0.05 | L | −38 | −50 | −20 | ||
| 3 | 886 | Postcentral Gyrus | 3.59 | <0.001 | L | −54 | −8 | 26 |
| 4 | 877 | Middle Frontal Gyrus | 3.24 | <0.001 | R | 34 | 30 | 44 |
| Frontal Pole | 3.06 | <0.005 | R | 22 | 44 | 46 | ||
| Frontal Pole | 2.46 | <0.01 | L | −10 | 44 | 48 | ||
4. Discussion
4.1. Interactions between Anxiety and Sensory Processing
4.2. Fear-Conditioning and the Importance of Multisensory Integration
4.3. Anxiety and Multisensory Integration
4.4. The Role of Higher-Order Processes and Related Regions
4.5. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barlow, D.H. Disorders of emotion. Psychol. Inq. 1991, 2, 58–71. [Google Scholar] [CrossRef]
- Knight, D.C.; Nguyen, H.T.; Bandettini, P.A. Expression of conditional fear with and without awareness. Proc. Natl. Acad. Sci. USA 2003, 100, 15280–15283. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Nguyen, H.T.; Bandettini, P.A. The role of awareness in delay and trace fear conditioning in humans. Cogn. Affect. Behav. Neurosci. 2006, 6, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Lovibond, P.F. Expectancy bias in trait anxiety. J. Abnorm. Psychol. 1996, 105, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Cabeleira, C.M.; Steinman, S.A.; Burgess, M.M.; Bucks, R.S.; MacLeod, C.; Melo, W.; Teachman, B.A. Expectancy bias in anxious samples. Emotion 2014, 14, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Buchel, C.; Dolan, R.J.; Armony, J.L.; Friston, K.J. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J. Neurosci. 1999, 19, 10869–10876. [Google Scholar] [PubMed]
- Knight, D.C.; Smith, C.N.; Cheng, D.T.; Stein, E.A.; Helmstetter, F.J. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn. Affect. Behav. Neurosci. 2004, 4, 317–325. [Google Scholar] [CrossRef] [PubMed]
- LaBar, K.S.; Gatenby, J.C.; Gore, J.C.; LeDoux, J.E.; Phelps, E.A. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 1998, 20, 937–945. [Google Scholar] [CrossRef]
- Carlson, J.M.; Greenberg, T.; Rubin, D.; Mujica-Parodi, L.R. Feeling anxious: Anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc. Cogn. Affect. Neurosci. 2011, 6, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.M.; Hofstotter, C.; Tsuchiya, N.; Koch, C. Working memory and fear conditioning. Proc. Natl. Acad. Sci. USA 2003, 100, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, M.; Geffen, M.N. Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nat. Neurosci. 2013, 16, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, T.W.; Gentile, C.G.; Romanski, L.M.; McCabe, P.M.; Schneiderman, N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 1987, 412, 285–294. [Google Scholar] [CrossRef]
- Oleson, T.D.; Ashe, J.H.; Weinberger, N.M. Modification of auditory and somatosensory system activity during pupillary conditioning in the paralyzed cat. J. Neurophysiol. 1975, 38, 1114–1139. [Google Scholar] [PubMed]
- Weinberger, N.M. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn. Mem. 2007, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.M.; O’Doherty, J.P.; Seymour, B.; Koch, C.; Dolan, R.J. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage 2006, 29, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C. d-Cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol. Psychiatry 2009, 66, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; O’Tuathaigh, C.M.; van Trigt, L.; Quinn, J.J.; Fanselow, M.S.; Mongeau, R.; Koch, C.; Anderson, D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Buchel, C.; Dolan, R.J. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage 2001, 13, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Tabbert, K.; Stark, R.; Kirsch, P.; Vaitl, D. Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int. J. Psychophysiol. 2005, 57, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Apergis-Schoute, A.M.; Schiller, D.; LeDoux, J.E.; Phelps, E.A. Extinction resistant changes in the human auditory association cortex following threat learning. Neurobiol. Learn. Mem. 2014, 113, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Armony, J.L.; Dolan, R.J. Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport 2001, 12, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Howard, J.D.; Parrish, T.B.; Gottfried, J.A. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science 2008, 319, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; Prince, S.E.; Murty, V.P.; Kragel, P.A.; LaBar, K.S. Neurobehavioral mechanisms of human fear generalization. Neuroimage 2011, 55, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Bradford, D.E.; Alvarez, R.P.; Burton, P.; Espensen-Sturges, T.; Reynolds, R.C.; Grillon, C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc. Cogn. Affect. Neurosci. 2014, 9, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, M.R.; Balderston, N.L.; Helmstetter, F.J. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; LaBar, K.S. Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiol. Learn. Mem. 2012, 97, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Miltner, W.H.; Braun, C.; Arnold, M.; Witte, H.; Taub, E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature 1999, 397, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Mathias, C.J.; Dolan, R.J. Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 2002, 33, 653–663. [Google Scholar] [CrossRef]
- Sehlmeyer, C.; Schoning, S.; Zwitserlood, P.; Pfleiderer, B.; Kircher, T.; Arolt, V.; Konrad, C. Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE 2009, 4, e5865. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Sakaki, M.; Cheng, R.; Velasco, R.; Mather, M. Emotional arousal amplifies the effects of biased competition in the brain. Soc. Cogn. Affect. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Bishop, S.J.; Duncan, J.; Lawrence, A.D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004, 24, 10364–10368. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.J.; Jenkins, R.; Lawrence, A.D. Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cereb. Cortex 2007, 17, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Nunez Elizalde, A.O.; Castle, E.; Bishop, S.J. Unraveling the anxious mind: Anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb. Cortex 2015, 25, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Indovina, I.; Robbins, T.W.; Nunez-Elizalde, A.O.; Dunn, B.D.; Bishop, S.J. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 2011, 69, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G. The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Greening, S.G.; Mather, M. Encoding of goal-relevant stimuli is strengthened by emotional arousal in memory. Front. Psychol. 2015, 6, 1173. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; Smith, S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 2004, 23, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J. Statistical Analysis of Activation Images; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Kriegeskorte, N.; Simmons, W.K.; Bellgowan, P.S.; Baker, C.I. Circular analysis in systems neuroscience: The dangers of double dipping. Nat. Neurosci. 2009, 12, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Vul, E.; Harris, C.; Winkielman, P.; Pashler, H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009, 4, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Wilcox, R.; Rousselet, G.A. Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Front. Psychol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R. Introduction to Robust Estimation and Hypothesis Testing; Academic Press: Waltham, MA, USA, 2012. [Google Scholar]
- Wager, T.D.; Davidson, M.L.; Hughes, B.L.; Lindquist, M.A.; Ochsner, K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008, 59, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Gazzaley, A.; Rissman, J.; Cooney, J.; Rutman, A.; Seibert, T.; Clapp, W.; D’Esposito, M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex 2007, 17, i125–i135. [Google Scholar] [CrossRef] [PubMed]
- Rissman, J.; Gazzaley, A.; D’Esposito, M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 2004, 23, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Drabant, E.M.; McRae, K.; Manuck, S.B.; Hariri, A.R.; Gross, J.J. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol. Psychiatry 2009, 65, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Klemenhagen, K.C.; Dudman, J.T.; Rogan, M.T.; Hen, R.; Kandel, E.R.; Hirsch, J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 2004, 44, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Greening, S.; Norton, L.; Virani, K.; Ty, A.; Mitchell, D.; Finger, E. Individual differences in the anterior insula are associated with the likelihood of financially helping versus harming others. Cogn. Affect. Behav. Neurosci. 2014, 14, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Pauli, P.; Wiedemann, G.; Dengler, W.; Kuhlkamp, V. A priori expectancy bias and its relation to shock experience and anxiety: A naturalistic study in patients with an automatic implantable cardioverter defibrillator. J. Behav. Ther. Exp. Psychiatry 2001, 32, 159–171. [Google Scholar] [CrossRef]
- Boddez, Y.; Vervliet, B.; Baeyens, F.; Lauwers, S.; Hermans, D.; Beckers, T. Expectancy bias in a selective conditioning procedure: Trait anxiety increases the threat value of a blocked stimulus. J. Behav. Ther. Exp. Psychiatry 2012, 43, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Letkiewicz, A.M.; Overstreet, C.; Ernst, M.; Grillon, C. The effect of induced anxiety on cognition: Threat of shock enhances aversive processing in healthy individuals. Cogn. Affect. Behav. Neurosci. 2011, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Krusemark, E.A.; Li, W. Enhanced olfactory sensory perception of threat in anxiety: An event-related fMRI study. Chemosens. Percept. 2012, 5, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.; Dickinson, A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000, 23, 473–500. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Overstreet, C.; Charney, D.R.; Vytal, K.; Grillon, C. Stress increases aversive prediction error signal in the ventral striatum. Proc. Natl. Acad. Sci. USA 2013, 110, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Sarinopoulos, I.; Grupe, D.W.; Mackiewicz, K.L.; Herrington, J.D.; Lor, M.; Steege, E.E.; Nitschke, J.B. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb. Cortex 2010, 20, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Manber, T.; Hakimi, S.; Canli, T.; Gross, J.J. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry 2009, 66, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Doehrmann, O.; Ghosh, S.S.; Polli, F.E.; Reynolds, G.O.; Horn, F.; Keshavan, A.; Triantafyllou, C.; Saygin, Z.M.; Whitfield-Gabrieli, S.; Hofmann, S.G.; et al. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 2013, 70, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Siegle, G.J.; Silk, J.S.; Ladouceur, C.D.; McFarland, A.; Dahl, R.E.; Ryan, N.D. Looking under the hood of the dot-probe task: An fMRI study in anxious youth. Depress. Anxiety 2014, 31, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Krusemark, E.A.; Novak, L.R.; Gitelman, D.R.; Li, W. When the sense of smell meets emotion: Anxiety-state-dependent olfactory processing and neural circuitry adaptation. J. Neurosci. 2013, 33, 15324–15332. [Google Scholar] [CrossRef] [PubMed]
- Greening, S.G.; Mitchell, D.G.V. A network of amygdala connections predict individual differences in trait anxiety. Hum. Brain Mapp. 2015, 36, 4819–4830. [Google Scholar] [CrossRef]
- Britton, J.C.; Bar-Haim, Y.; Clementi, M.A.; Sankin, L.S.; Chen, G.; Shechner, T.; Norcross, M.A.; Spiro, C.N.; Lindstrom, K.M.; Pine, D.S. Training-associated changes and stability of attention bias in youth: Implications for attention bias modification treatment for pediatric anxiety. Dev. Cogn. Neurosci. 2013, 4, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Eldar, S.; Apter, A.; Lotan, D.; Edgar, K.P.; Naim, R.; Fox, N.A.; Pine, D.S.; Bar-Haim, Y. Attention bias modification treatment for pediatric anxiety disorders: A randomized controlled trial. Am. J. Psychiatry 2012, 169, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Squire, L.R. Classical conditioning and brain systems: The role of awareness. Science 1998, 280, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Raio, C.M.; Carmel, D.; Carrasco, M.; Phelps, E.A. Nonconscious fear is quickly acquired but swiftly forgotten. Curr. Biol. 2012, 22, R477–R479. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Phelps, E.A. Learned fear of “unseen” faces after pavlovian, observational, and instructed fear. Psychol. Sci. 2004, 15, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Vrana, S.R.; Lang, P.J. Fear imagery and the startle-probe reflex. J. Abnorm. Psychol. 1990, 99, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.J.; Charney, D.R.; Overstreet, C.; Vytal, K.; Grillon, C. The adaptive threat bias in anxiety: Amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage 2012, 60, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Ray, R.D.; Cooper, J.C.; Robertson, E.R.; Chopra, S.; Gabrieli, J.D.; Gross, J.J. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004, 23, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Feng, T.; Chen, Z.; Lei, X. Memory consolidation of fear conditioning: Bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2014, 9, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; Bandettini, P.A.; Knight, D.C. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav. Neurosci. 2007, 121, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Greening, S.G.; Finger, E.C.; Mitchell, D.G. Parsing decision making processes in prefrontal cortex: Response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage 2011, 54, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G.; Luo, Q.; Avny, S.B.; Kasprzycki, T.; Gupta, K.; Chen, G.; Finger, E.C.; Blair, R.J. Adapting to dynamic stimulus-response values: Differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. J. Neurosci. 2009, 29, 10827–10834. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn. Sci. 2010, 14, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav. Brain Res. 2011, 217, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Greening, S.G.; Lee, T.H.; Mather, M. A dual process for the cognitive control of emotional significance: Implications for emotion regulation and disorders of emotion. Front. Hum. Neurosci. 2014, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Greening, S.G.; Osuch, E.A.; Williamson, P.C.; Mitchell, D.G. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc. Cogn. Affect. Neurosci. 2014, 9, 628–637. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greening, S.G.; Lee, T.-H.; Mather, M. Individual Differences in Anticipatory Somatosensory Cortex Activity for Shock is Positively Related with Trait Anxiety and Multisensory Integration. Brain Sci. 2016, 6, 2. https://doi.org/10.3390/brainsci6010002
Greening SG, Lee T-H, Mather M. Individual Differences in Anticipatory Somatosensory Cortex Activity for Shock is Positively Related with Trait Anxiety and Multisensory Integration. Brain Sciences. 2016; 6(1):2. https://doi.org/10.3390/brainsci6010002
Chicago/Turabian StyleGreening, Steven G., Tae-Ho Lee, and Mara Mather. 2016. "Individual Differences in Anticipatory Somatosensory Cortex Activity for Shock is Positively Related with Trait Anxiety and Multisensory Integration" Brain Sciences 6, no. 1: 2. https://doi.org/10.3390/brainsci6010002






