Hippocampal Cortactin Levels are Reduced Following Spatial Working Memory Formation, an Effect Blocked by Chronic Calpain Inhibition
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Apparatus
2.3. Behavioral Procedure
2.4. Surgery
2.5. Immunoblotting
2.6. Behavioral Procedure
2.6.1. Experiment 1
2.6.2. Experiment 2
3. Results and Discussion
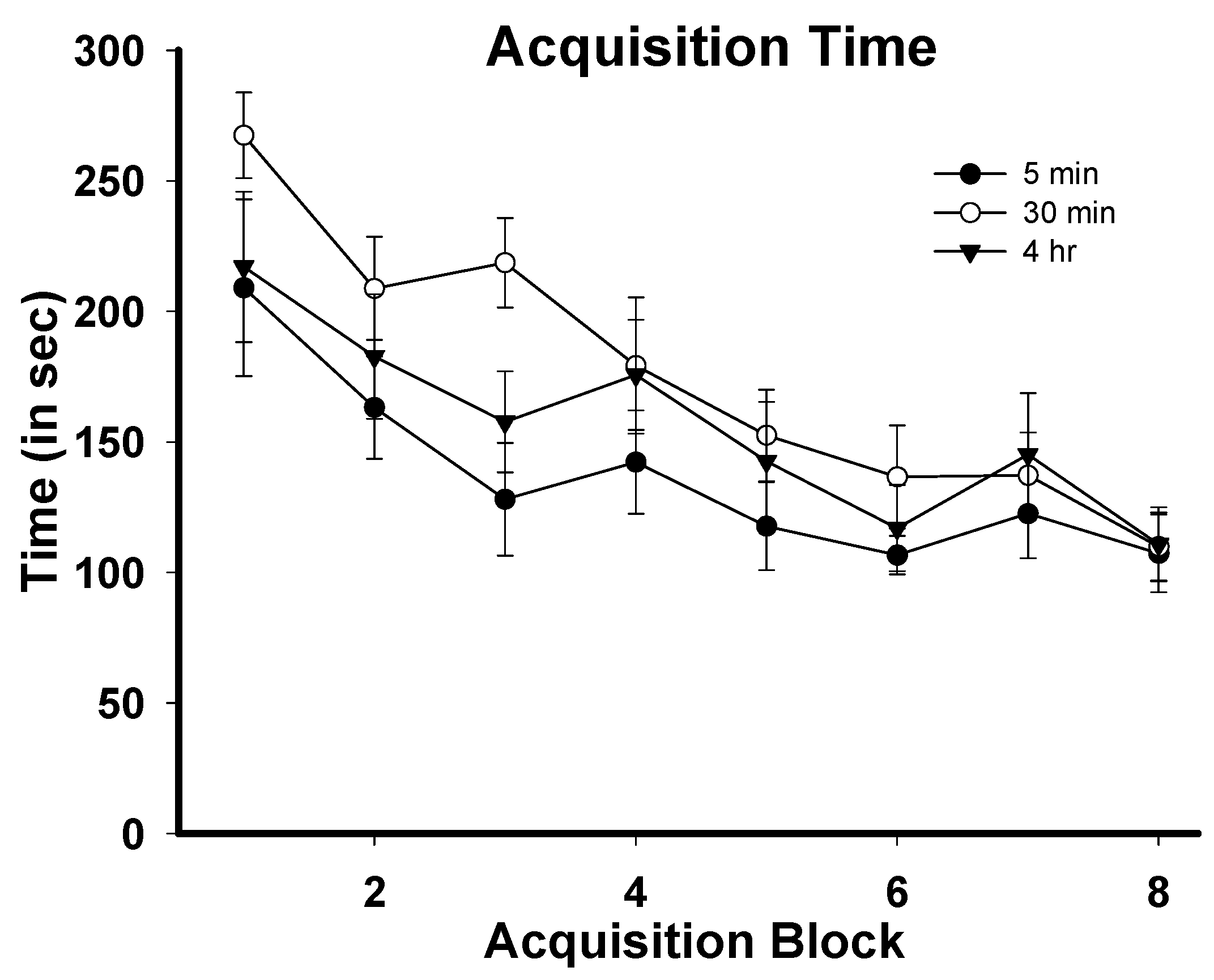
3.1. Task Acquisition
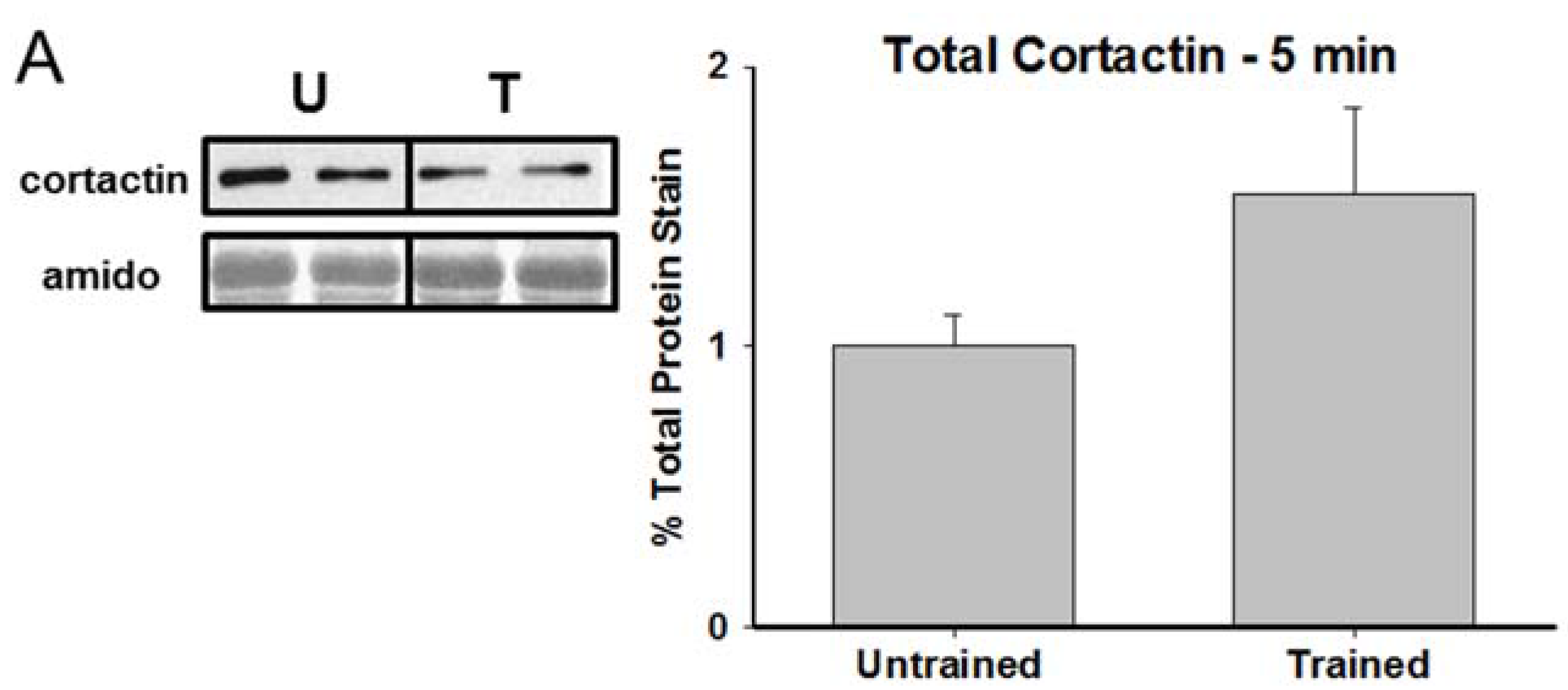
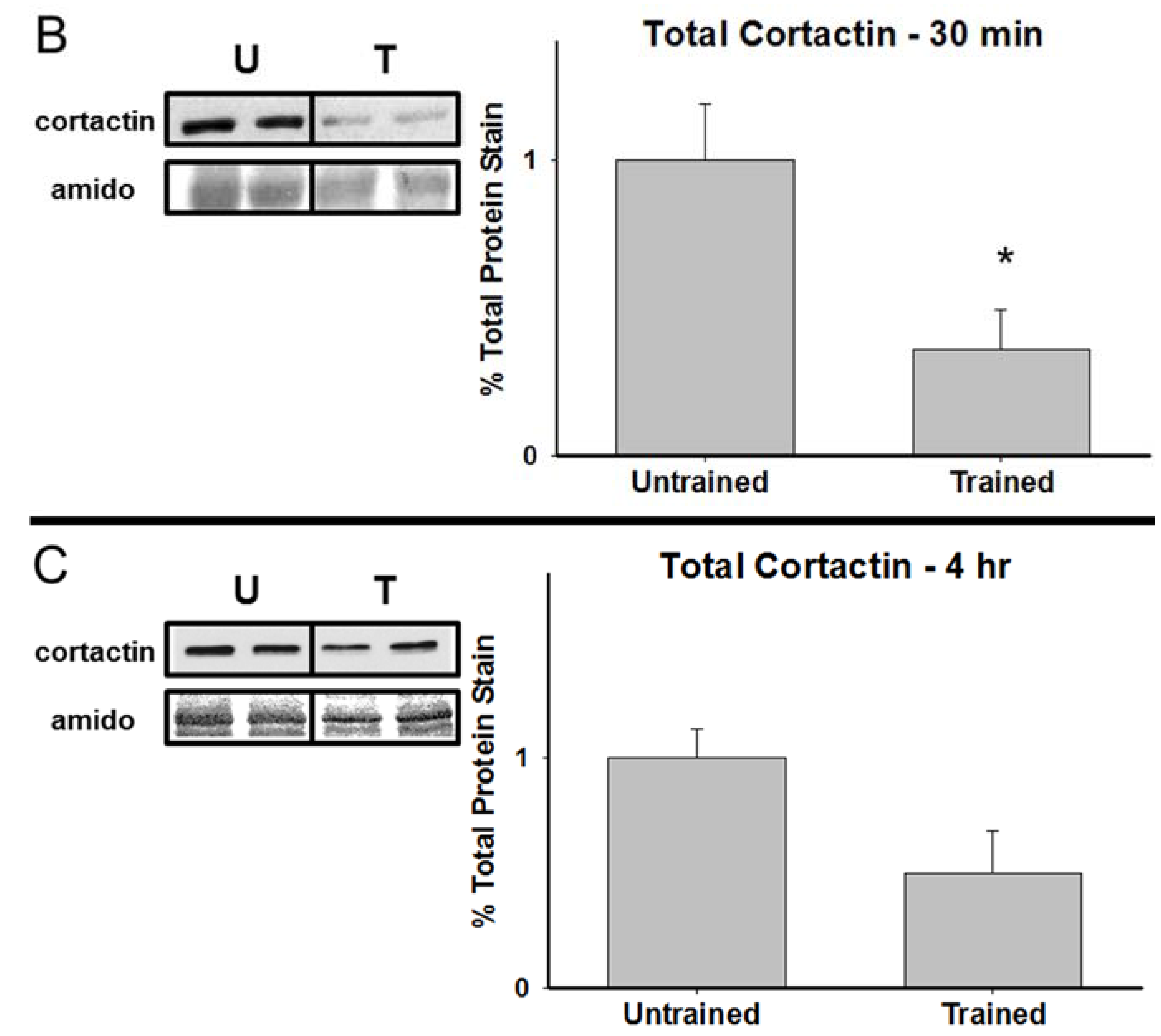
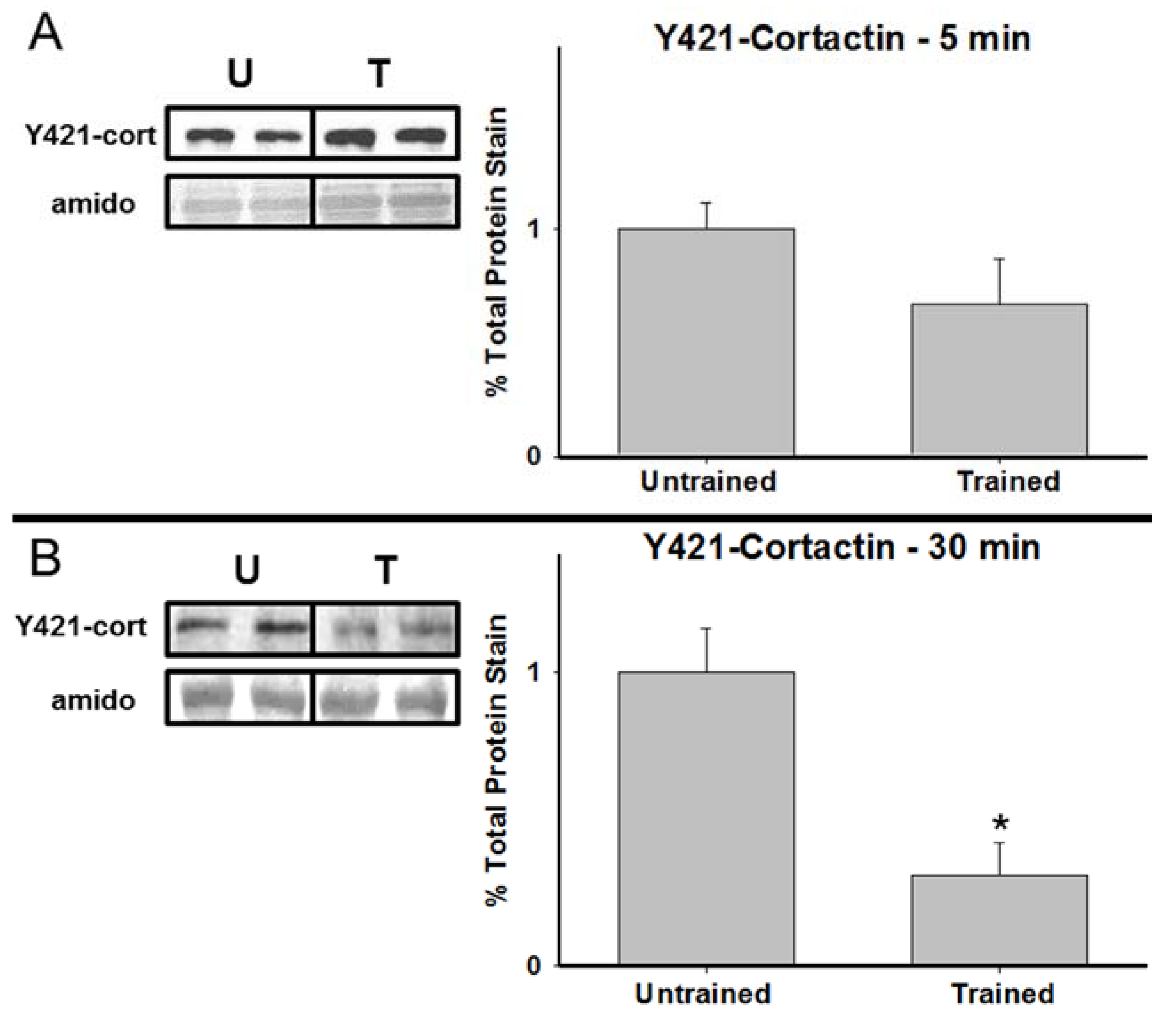
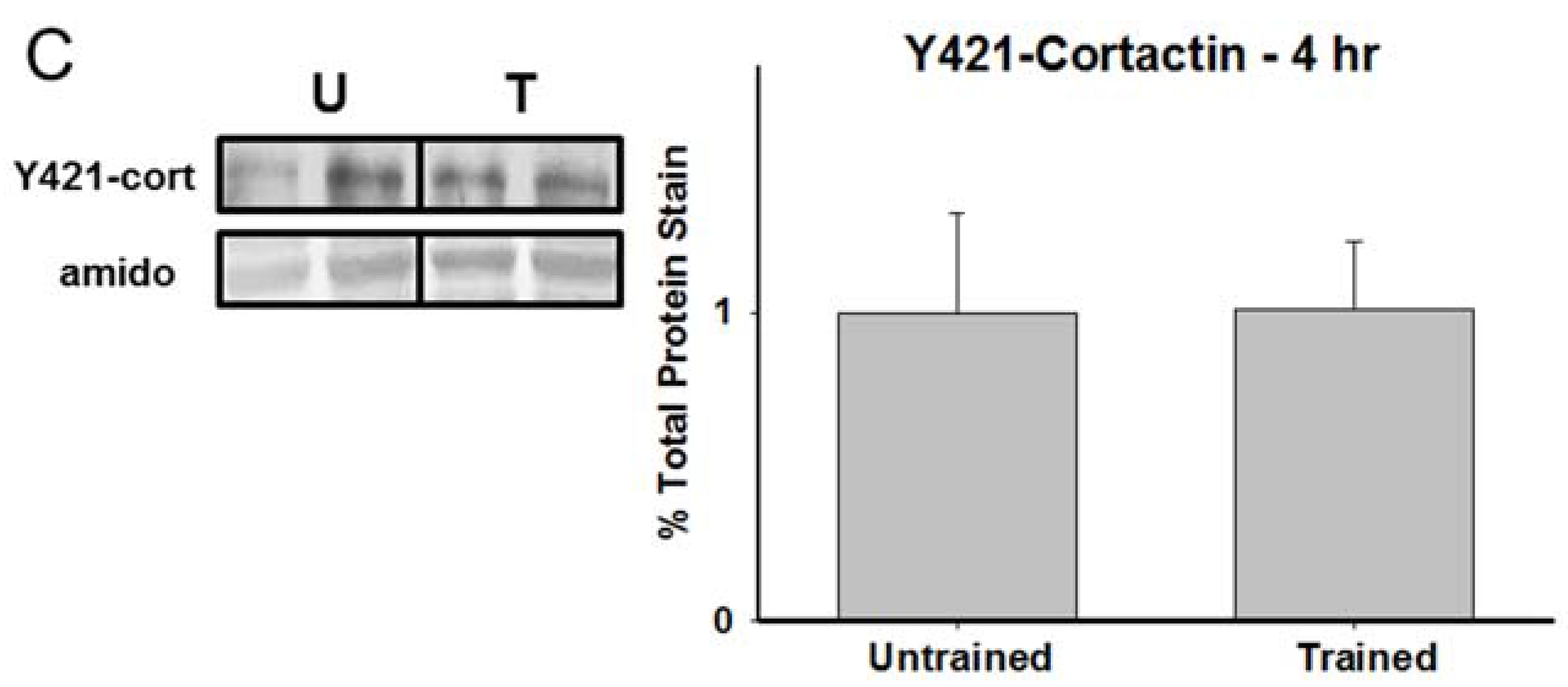
3.2. Total Cortactin and Y421-Phosphorylated Cortactin are Transiently Reduced Following RAM Training
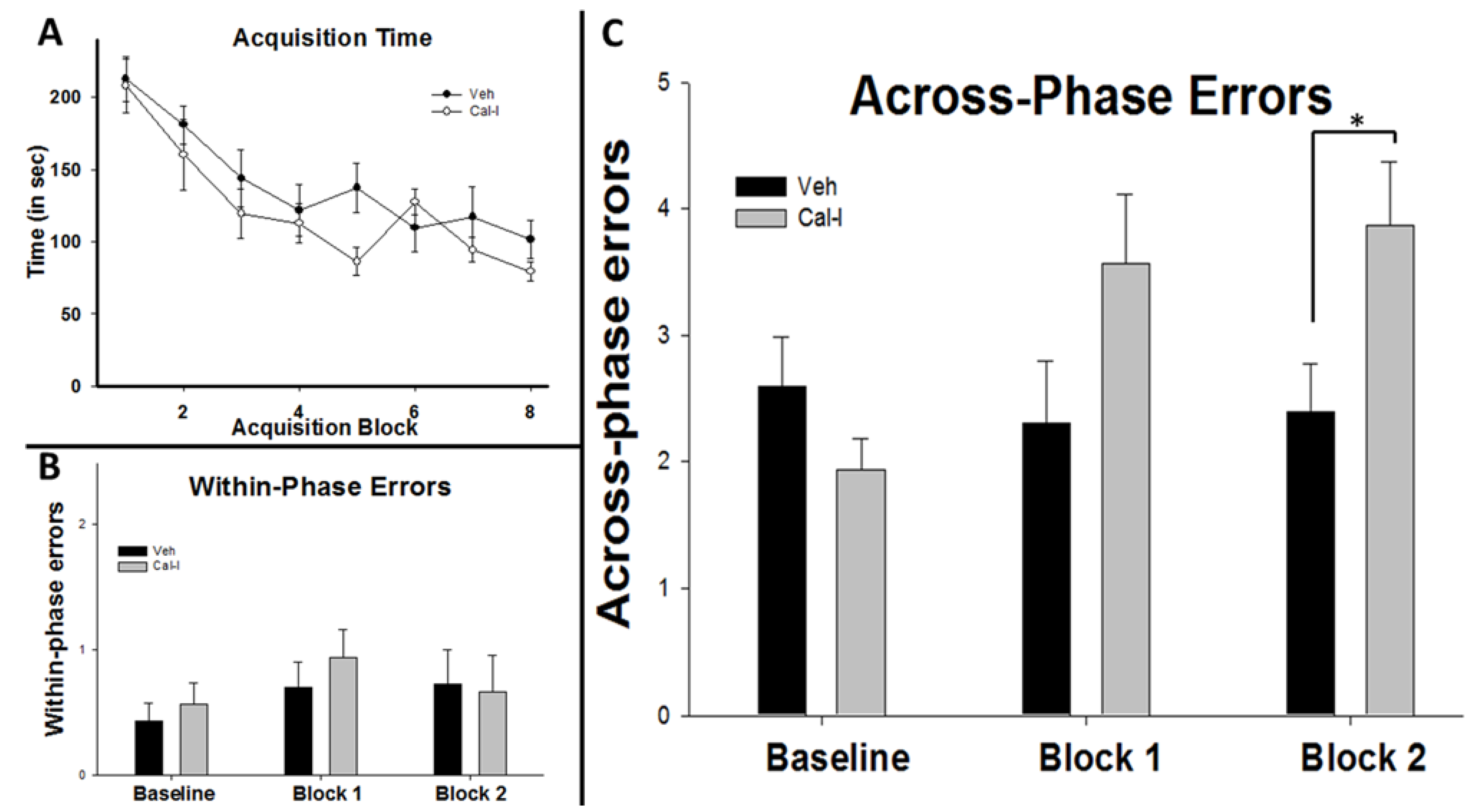
3.3. Chronic Calpain Inhibition Impairs Spatial Working Memory Formation on the SWSh Version of the RAM
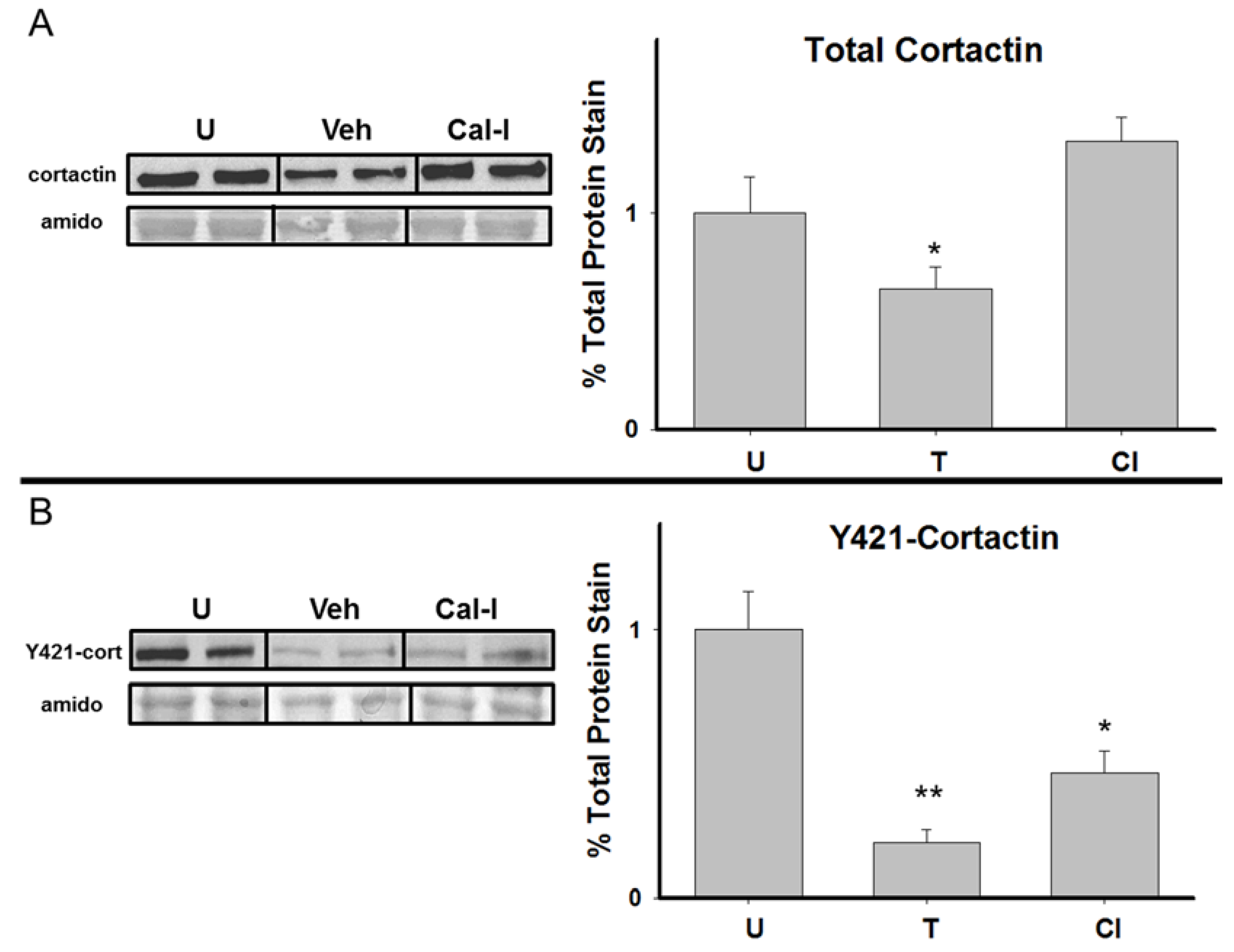
3.4. Chronic Calpain Inhibition Blocks the Training-Induced Reduction in Cortactin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Murphy, E.S.; Elijah, I.E.; Holtfreter, K.L.; Davis, C.J.; Olson, M.L.; Muhunthan, K.; Harding, J.W. Influence of hippocampectomy on habituation, exploratory behavior, and spatial memory in rats. Brain Res. 2004, 1023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Papas, B.C. Spatial memory and hippocampal function. Neuropsychologia 1979, 17, 669–682. [Google Scholar] [CrossRef]
- Okamoto, K.; Nagai, T.; Miyawaki, A.; Hayashi, Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 2004, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, M.; Hoogenraad, C.C. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kesner, R.P. Differential contribution of nmda receptors in hippocampal subregions to spatial working memory. Nat. Neurosci. 2002, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- McHugh, S.B.; Niewoehner, B.; Rawlins, J.N.; Bannerman, D.M. Dorsal hippocampal N-methyl-d-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the t-maze. Behav. Brain Res. 2008, 186, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sakimura, K.; Kutsuwada, T.; Ito, I.; Manabe, T.; Takayama, C.; Kushiya, E.; Yagi, T.; Aizawa, S.; Inoue, Y.; Sugiyama, H.; et al. Reduced hippocampal ltp and spatial learning in mice lacking nmda receptor epsilon 1 subunit. Nature 1995, 373, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.B.; Frerking, M.; Zhou, Q. Spine expansion and stabilization associated with long-term potentiation. J. Neurosci. 2008, 28, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Cosen-Binker, L.I.; Kapus, A. Cortactin: The gray eminence of the cytoskeleton. Physiology (Bethesda) 2006, 21, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lua, B.L.; Low, B.C. Filling the gaps in cell dynamics control: Bpgap1 promotes cortactin translocation to the cell periphery for enhanced cell migration. Biochem. Soc. Trans. 2004, 32, 1110–1112. [Google Scholar] [PubMed]
- Hering, H.; Sheng, M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 2003, 23, 11759–11769. [Google Scholar] [PubMed]
- Seese, R.R.; Babayan, A.H.; Katz, A.M.; Cox, C.D.; Lauterborn, J.C.; Lynch, G.; Gall, C.M. Ltp induction translocates cortactin at distant synapses in wild-type but not fmr1 knock-out mice. J. Neurosci. 2012, 32, 7403–7413. [Google Scholar] [CrossRef] [PubMed]
- Uruno, T.; Liu, J.; Zhang, P.; Fan, Y.; Egile, C.; Li, R.; Mueller, S.C.; Zhan, X. Activation of arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001, 3, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Weed, S.A.; Karginov, A.V.; Schafer, D.A.; Weaver, A.M.; Kinley, A.W.; Cooper, J.A.; Parsons, J.T. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with f-actin and the arp2/3 complex. J. Cell Biol. 2000, 151, 29–40. [Google Scholar] [CrossRef] [PubMed]
- MacGrath, S.M.; Koleske, A.J. Cortactin in cell migration and cancer at a glance. J. Cell Sci. 2012, 125, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, J.; Haudenschild, C.C.; Zhan, X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998, 273, 25770–25776. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ni, Y.; Wang, T.; Gao, Y.; Haudenschild, C.C.; Zhan, X. Down-regulation of the filamentous actin cross-linking activity of cortactin by src-mediated tyrosine phosphorylation. J. Biol. Chem. 1997, 272, 13911–13915. [Google Scholar] [CrossRef] [PubMed]
- Meighan, S.E.; Meighan, P.C.; Choudhury, P.; Davis, C.J.; Olson, M.L.; Zornes, P.A.; Wright, J.W.; Harding, J.W. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 2006, 96, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J.; Meighan, P.C.; Taishi, P.; Krueger, J.M.; Harding, J.W.; Wright, J.W. Rem sleep deprivation attenuates actin-binding protein cortactin: A link between sleep and hippocampal plasticity. Neurosci. Lett. 2006, 400, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.J.; Amann, K.J.; Huttenlocher, A. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol. Biol. Cell 2006, 17, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Del Cerro, S.; Arai, A.; Kessler, M.; Bahr, B.A.; Vanderklish, P.; Rivera, S.; Lynch, G. Stimulation of nmda receptors activates calpain in cultured hippocampal slices. Neurosci. Lett. 1994, 167, 149–152. [Google Scholar] [CrossRef]
- Shimizu, K.; Phan, T.; Mansuy, I.M.; Storm, D.R. Proteolytic degradation of scop in the hippocampus contributes to activation of map kinase and memory. Cell 2007, 128, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Baudry, M.; Lynch, G. Leupeptin, a thiol proteinase inhibitor, causes a selective impairment of spatial maze performance in rats. Behav. Neural Biol. 1984, 40, 58–69. [Google Scholar] [CrossRef]
- Zadran, S.; Bi, X.; Baudry, M. Regulation of calpain-2 in neurons: Implications for synaptic plasticity. Mol. Neurobiol. 2010, 42, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Del Cerro, S.; Larson, J.; Oliver, M.W.; Lynch, G. Development of hippocampal long-term potentiation is reduced by recently introduced calpain inhibitors. Brain Res. 1990, 530, 91–95. [Google Scholar] [CrossRef]
- Denny, J.B.; Polan-Curtain, J.; Ghuman, A.; Wayner, M.J.; Armstrong, D.L. Calpain inhibitors block long-term potentiation. Brain Res. 1990, 534, 317–320. [Google Scholar] [CrossRef]
- Vanderklish, P.; Bednarski, E.; Lynch, G. Translational suppression of calpain blocks long-term potentiation. Learn. Mem. 1996, 3, 209–217. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; White, N.M. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993, 107, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B.; Seamans, J.K.; Phillips, A.G. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997, 17, 1880–1890. [Google Scholar] [PubMed]
- Nagai, T.; Kamei, H.; Dohniwa, M.; Takayanagi, M.; Suzuki, M.; Matsuya, T.; Nabeshima, T.; Takuma, K.; Yamada, K. Involvement of hippocampal extracellular signal-regulated kinase 1/2 in spatial working memory in rats. Neuroreport 2006, 17, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.L.; Meighan, P.C.; Brown, T.E.; Asay, A.L.; Benoist, C.C.; Harding, J.W.; Wright, J.W. Hippocampal mmp-3 elevation is associated with passive avoidance conditioning. Regul. Pept. 2008, 146, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N. Equithesin without chloral hydrate as an anaesthetic for rats. Psychopharmacology (Berl) 1996, 124, 288–290. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The use of total protein stains as loading controls: An alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Olevska, A.; Gordon, J.; Taylor, J.R. Cytoskeletal determinants of stimulus-response habits. J. Neurosci. 2013, 33, 11811–11816. [Google Scholar] [CrossRef] [PubMed]
- Iki, J.; Inoue, A.; Bito, H.; Okabe, S. Bi-directional regulation of postsynaptic cortactin distribution by bdnf and nmda receptor activity. Eur. J. Neurosci. 2005, 22, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tandon, N.N.; Greco, N.J.; Ni, Y.; Wang, T.; Zhan, X. Proteolysis of platelet cortactin by calpain. J. Biol. Chem. 1997, 272, 19248–19252. [Google Scholar] [CrossRef] [PubMed]
- Mingorance-Le Meur, A.; O’Connor, T.P. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009, 28, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, F.; Fa, M.; Liu, S.; Zhang, H.; Hidalgo, A.; Schmidt, S.D.; Yamaguchi, H.; Yoshii, N.; Mathews, P.M.; Nixon, R.A.; et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of alzheimer disease. J. Clin. Invest. 2008, 118, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, M.L.; Ingebretson, A.E.; Harmelink, K.M. Hippocampal Cortactin Levels are Reduced Following Spatial Working Memory Formation, an Effect Blocked by Chronic Calpain Inhibition. Brain Sci. 2015, 5, 241-257. https://doi.org/10.3390/brainsci5020241
Olson ML, Ingebretson AE, Harmelink KM. Hippocampal Cortactin Levels are Reduced Following Spatial Working Memory Formation, an Effect Blocked by Chronic Calpain Inhibition. Brain Sciences. 2015; 5(2):241-257. https://doi.org/10.3390/brainsci5020241
Chicago/Turabian StyleOlson, Mikel L., Anna E. Ingebretson, and Katherine M. Harmelink. 2015. "Hippocampal Cortactin Levels are Reduced Following Spatial Working Memory Formation, an Effect Blocked by Chronic Calpain Inhibition" Brain Sciences 5, no. 2: 241-257. https://doi.org/10.3390/brainsci5020241
APA StyleOlson, M. L., Ingebretson, A. E., & Harmelink, K. M. (2015). Hippocampal Cortactin Levels are Reduced Following Spatial Working Memory Formation, an Effect Blocked by Chronic Calpain Inhibition. Brain Sciences, 5(2), 241-257. https://doi.org/10.3390/brainsci5020241




