Functional Neuroimaging Correlates of Autobiographical Memory Deficits in Subjects at Risk for Depression
Abstract
:1. Introduction
2. Results
2.1. Behavioral Results
| Demographics | HC | HR | rMDD |
|---|---|---|---|
| N (number females) | 20 [13] | 20 [14] | 20 [12] |
| Age in years | 29 (9) | 30 (9) | 30 (12) |
| IQ (as determined by the WASI) | 109 (13.9) | 109 (8.11) | 109 (9.58) |
| HDRS | 0.81 (2.05) | 0.75 (1.48) | 3.40 (4.17) *, # |
| MADRS | 0.79 (2.08) | 0.95 (2.35) | 4.55 (4.98) *, # |
| POMS Total Mood Disturbance | −30.3 (19.9) | −33.7 (15.5) | −28.3 (16.8) |
| State Anxiety | 24.9 (4.17) | 26.7 (6.02) | 28.3 (6.16) |
| Trait Anxiety | 27.0 (5.09) | 26.9 (3.73) | 32.9 (7.02) |
| Pre-Post scan Change in POMS | 3.95 (8.84) | 3.15 (10.4) | 5.60 (19.1) |
| Pre-post scan change in State Anxiety | 0.85 (3.92) | 0.50 (5.38) | 0.85 (5.69) |
| Pre-post scan change in Trait Anxiety | −1.35 (3.08) | −1.50 (2.04) | -2.15 (2.83) |
| Control Tasks | |||
| Percent correct—Riser detection task | 78.3 (11.5) | 74.7 (11.1) | 75.7 (10.4) |
| Number of Examples Positive Categories | 5.95 (0.99) | 5.75 (1.03) | 5.47 (0.81) |
| Number of Examples Negative Categories | 5.64 (1.25) | 5.36 (0.91) | 5.05 (0.91) |
| Number of Examples Neutral Categories | 6.16 (0.83) | 6.14 (0.82) | 5.83 (0.70) |
| Percent of Positive Examples Easy to Generate | 87.8 (12.3) | 84.7 (13.1) | 85.8 (26.0) |
| Percent of Negative Examples Easy to Generate | 83.3 (18.8) | 77.1 (19.9) | 79.1 (19.8) |
| Percent of Neutral Examples Easy to Generate | 83.5 (22.1) | 86.3 (10.4) | 83.5 (20.4) |
| Memory Type (% recalled) | |||
| Specific | 65.0 (10.9) | 48.5 (13.9) * | 42.2 (10.9) * |
| Categorical | 16.5 (6.56) | 28.0 (11.4) * | 32.9 (9.99) * |
| Extended | 3.14 (2.64) | 5.12 (3.52) | 4.12 (3.12) |
| Semantic | 4.43 (3.36) | 7.01 (5.18) | 6.85 (5.62) |
| No Memory | 1.93 (2.48) | 2.08 (3.58) | 3.31 (4.31) |
| Can’t Recall Post Scan | 9.25 (6.67) | 9.45 (5.85) | 10.5 (7.43) |
2.2. Functional MRI Results
| Contrast | Specific Memories vs. Example Generation | Categorical Memories vs. Example Generation | Example Generation vs. Riser Baseline | |||
|---|---|---|---|---|---|---|
| Area | Cluster Size | x, y, z | Cluster Size | x, y, z | Cluster Size | x, y, z |
| L DLPFC | 503 | −39, 3, 50 | 94 | 45, 11, 46 | 225 | 35, −21, 60 |
| R DLPFC | 227 | 39, 5, 48 | 84 | −41, 11, 50 | ||
| L DMPFC | 37 | −9, 11, 60 | ||||
| R DMPFC | 44 | 11, 53, 34 | ||||
| R VLPFC | 515 | 59, 19, 14 | 173 | 57, 23, 10 | ||
| R OFC | 65 | 53, 21, 4 | ||||
| L Medial PFC | 7809 | −1, 59, 34 | 5123 | −1, 59, −2 | ||
| R Postcentral G/BA 3 | 35 | 21, −35, 56 | ||||
| L PCC | 154 | −1, −27, 32 | 714 | −3, −37, 32 | ||
| L Superior Temporal G | 2351 | −49, −59, 20 | 91 | 65, −41, 12 | ||
| R Superior Temporal G | 2690 | 21, 13, −36 | ||||
| L Middle Temporal G | 2291 | −55, −3, −12 | 1251 | −63, −11, −8 | 492 | −47, −71, 28 |
| R Middle Temporal G | 2895 | 53, −9, −14 | 1238 | 61, −1, −12 | ||
| L Hippocampus/Parahippocampus | 98 | −21, −21, −12 | 4221 | −30, −24, −9 | ||
| R Hippocampus/Parahippocampus | 61 | 17, −11, −24 | 815 | 19, −19, −14 | ||
| L Precuneus | 1676 | −33, −81, 34 | 3846 | −33, −81, 34 | ||
| R Precuneus | 1698 | 45, −71, 36 | 989 | 45, −73, 34 | ||
| L Cuneus | 5364 | −1, −67, 32 | 3631 | −1, −65, 32 | ||
| Area | Cluster Size | x, y, z | F value | β Weight | ||
|---|---|---|---|---|---|---|
| HC | HR | rMDD | ||||
| Specific AMs vs. Example Generation | ||||||
| HR > HC > rMDD | ||||||
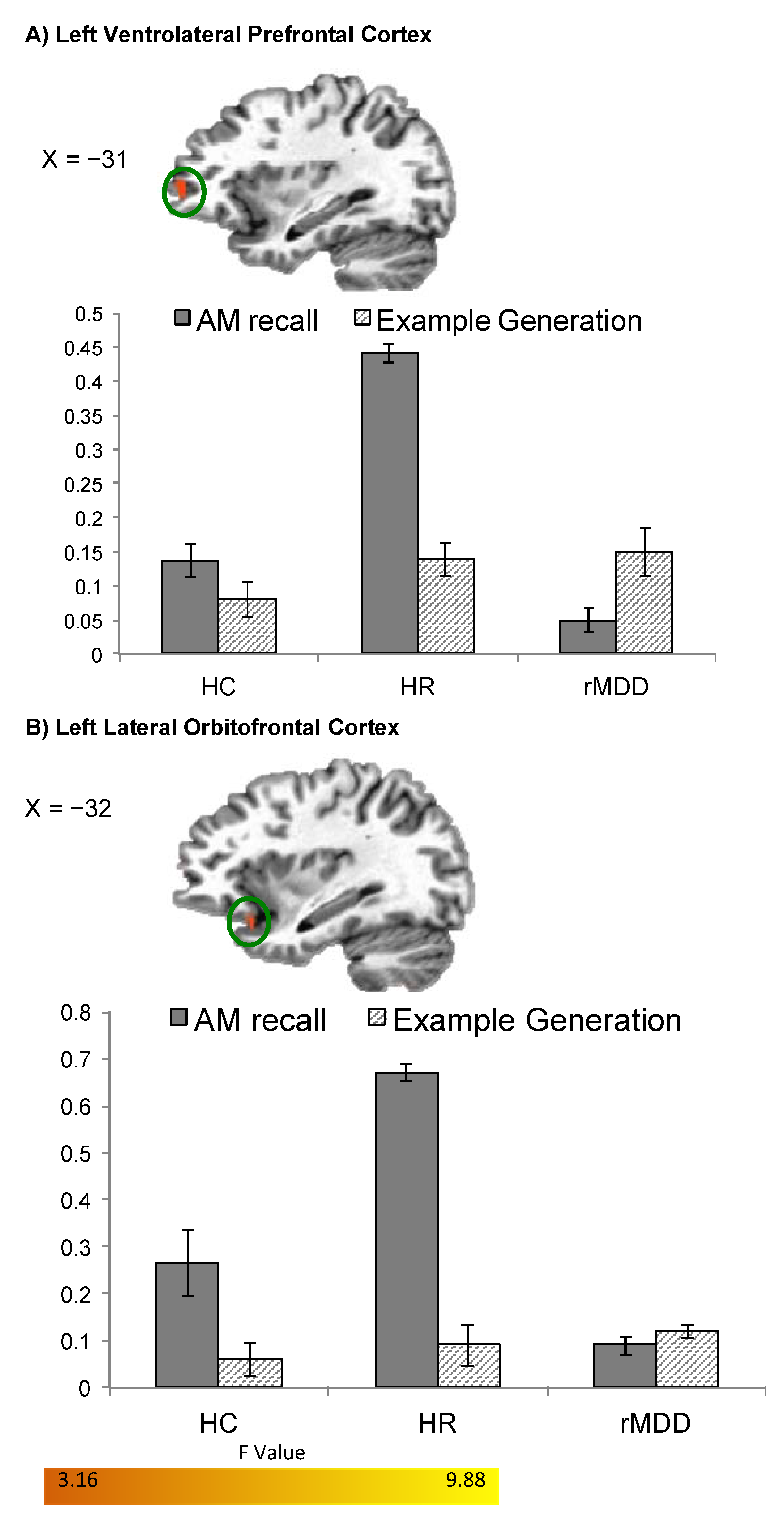
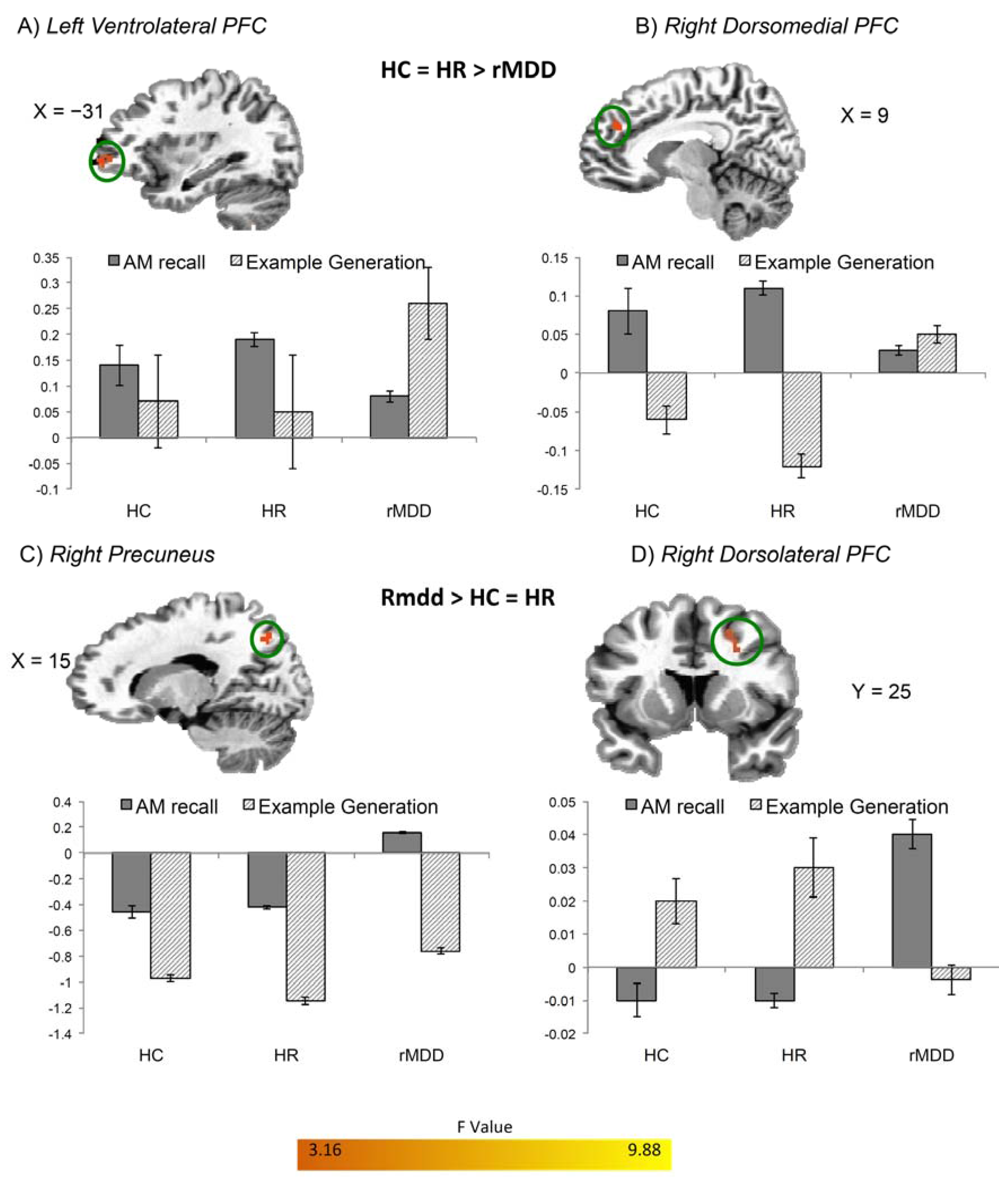
| L VLPFC | 30 | −31, 51, 2 | 6.96 | 0.17 (0.02) | 0.20 (0.03) | −0.04 (0.04) |
| L Lateral OFC/BA 47 | 33 | −33, 17, −16 | 7.94 | 0.21 (0.04) | 0.26 (0.03) | 0.02 (0.02) |
| Positive Specific AMs v Positive Example Generation | ||||||
| HR = HC > rMDD | ||||||
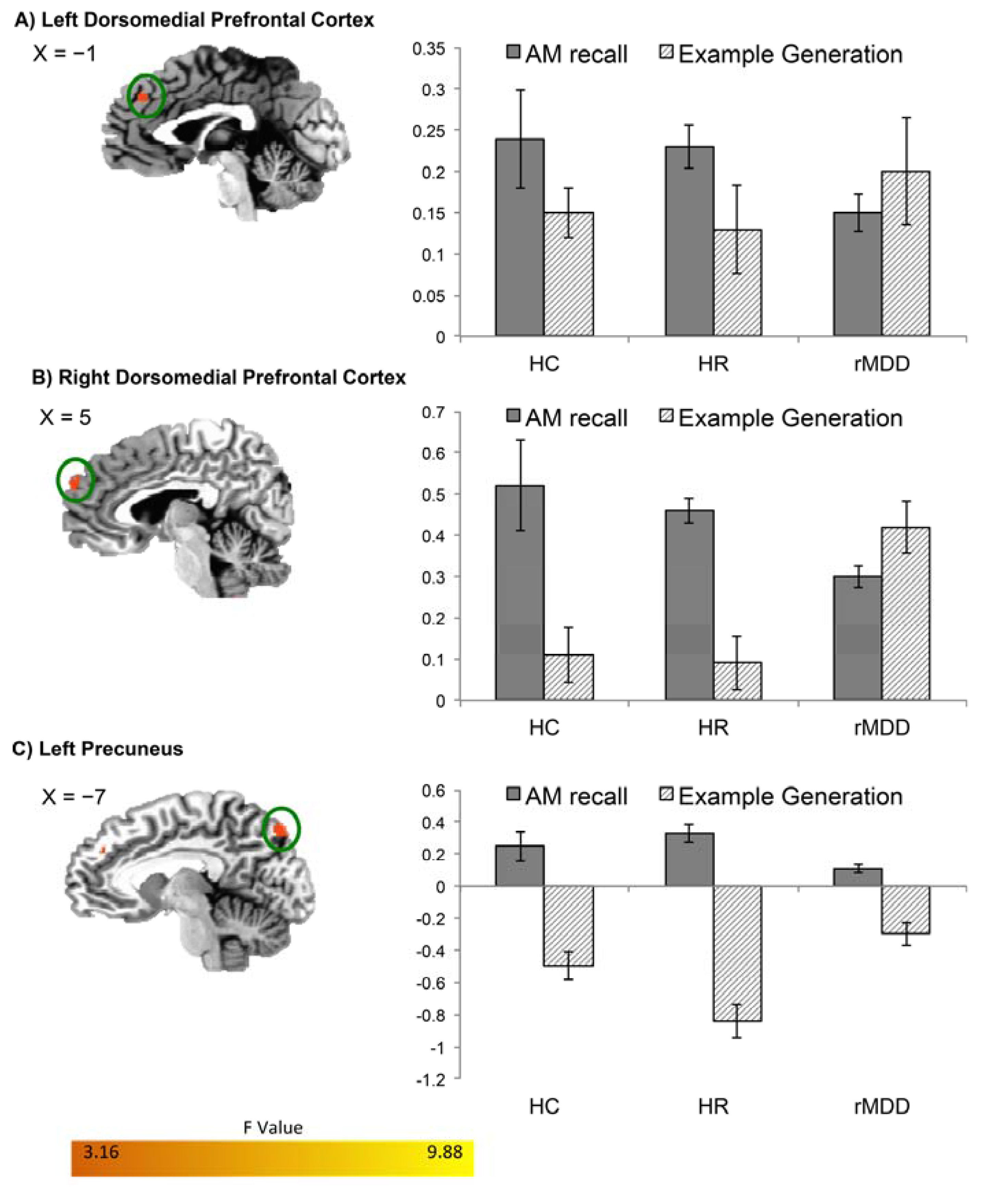
| L DMPFC/BA 9 | 62 | −1, 39, 34 | 8.51 | 0.24 (0.03) | 0.22 (0.06) | 0.02 (0.02) |
| R DMPFC/BA 9 | 44 | 5, 57, 28 | 7.32 | 0.38 (0.05) | 0.37 (0.07) | 0.07 (0.04) |
| L Precuneus | 32 | −7, −79, 44 | 7.51 | 0.29 (0.04) | 0.26 (0.17) | 0.05 (0.05) |
| Negative Specific AMs v Negative Example Generation | ||||||
| HR > HC > rMDD | ||||||
| L VLPFC | 37 | −31, 47, 0 | 9.34 | 0.11 (0.03) | 0.21 (0.06) | 0.02 (0.02) |
| R DMPFC/BA 9 | 30 | 9, 41, 28 | 8.55 | 0.08 (0.01) | 0.14 (0.04) | 0.01 (0.02) |
| rMDD > HR = HC | ||||||
| R DLPFC | 36 | 25, 7, 34 | 9.01 | −0.04 (0.03) | −0.03 (0.02) | 0.05 (0.02) |
| R Precuneus | 35 | 15, −67, 42 | 8.21 | −0.38 (0.10) | −0.42 (0.06) | 0.17 (0.04) |
| Categorical AMs vs. Example Generation | ||||||
| no significant clusters | ||||||
| Example Generation vs. Riser Baseline | ||||||
| no significant clusters | ||||||
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Image Acquisition
4.3. fMRI Autobiographical Memory Task
4.4. Assessment of Behavioral Performance during fMRI
4.5. fMRI Processing and Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Williams, J.M.; Barnhofer, T.; Crane, C.; Herman, D.; Raes, F.; Watkins, E.; Dalgleish, T. Autobiographical memory specificity and emotional disorder. Psychol. Bull. 2007, 133, 122–148. [Google Scholar] [CrossRef] [PubMed]
- Mackinger, H.; Pachinger, M.; Leibetseder, M.; Fartacek, R. Autobiographical memories in women remitted from major depression. J. Abnorm. Psychol. 2000, 109, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Spinhoven, P.; Bockting, C.L.; Schene, A.H.; Koeter, M.W.; Wekking, E.M.; Williams, J.M. Autobiographical memory in the euthymic phase of recurrent depression. J. Abnorm. Psychol. 2006, 115, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Nandrino, J.L.; Pezard, L.; Poste, A.; Reveillere, C.; Beaune, D. Autobiographical memory in major depression: A comparison between first-episode and recurrent patients. Psychopathology 2002, 35, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Bellgowan, P.; Bodurka, J.; Drevets, W.C. Behavioral and neurophysiological correlates of autobiographical memory deficits in patients with depression and individuals at high risk for depression. JAMA Psychiatry 2013, 70, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Erickson, K.; Nugent, A.C.; Fromm, S.J.; Mallinger, A.G.; Furey, M.L.; Drevets, W.C. Functional anatomy of autobiographical memory recall deficits in depression. Psychol. Med. 2012, 42, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Bellgowan, P.; Bodurka, J.; Drevets, W. Neurophysiological correlates of autobiographical memory deficits in currently and formerly depressed subjects. Psychol. Med. 2014, 44, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Hach, S.; Tippett, L.J.; Addis, D.R. Neural chances associated with the generation of specific past and future events in depression. Neuropsychologia 2014, 65, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Keedwell, P.; Andrew, C.; Williams, S.; Brammer, M.; Phillips, M. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol. Psychiatry 2005, 58, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Whalley, M.G.; Rugg, M.D.; Brewin, C.R. Autobiographical memory in depression: An FMRI study. Psychiatry Res. 2012, 201, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kerestes, R.; Ladoucer, C.D.; Meda, S.; Nathan, P.J.; Blumberg, H.P.; Maloney, K.; Ruf, B.; Saricicek, A.; Pearlson, G.D.; Bhagwagar, Z.; et al. Abormal prefonrtal activity subserving attentional control of emotion in remitted depressed pateitns during a working memory task with emotional distracters. Psychol. Med. 2012, 42, 29–40. [Google Scholar]
- Liotti, M.; Mayberg, H.; McGinnis, S.; Brannan, S.; Jerabek, P. Unmasking disease-specific cerebral blood flow abnormalities: Mood challenge in patients with remitted unipolar depression. Am. J. Psychiatry 2002, 159, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Dichter, G.S.; Kozink, R.V.; McClernon, F.J.; Smoski, M.J. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J. Affect. Disord. 2012, 136, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System—An Approach to Cerebral Imaging; Thieme Medical Publishers: New York, NY, USA, 1988. [Google Scholar]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, M.; Postle, B.R.; Rypma, B. Prefrontal cortical contributions to working memory: Evidence from event-related fmri studies. Exp. Brain Res. 2000, 133, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Masui, K.; Onoda, K.; Furutani, K.; Nomura, M.; Yoshida, H.; Ura, M. The effects of the behavioral inhibition and activation systems on social inclusion and exclusion. J. Exp. Soc. Psychol. 2011, 47, 502–505. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000, 126, 413–431. [Google Scholar] [PubMed]
- Drevets, W.C. Neuroimaging studies of mood disorders. Biol. Psychiatry 2000, 48, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001, 11, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.; Raichle, M.E. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cogn. Emotion. 1998, 12, 353–385. [Google Scholar] [CrossRef]
- Drevets, W.C.; Videen, T.O.; Price, J.L.; Preskorn, S.H.; Carmichael, S.T.; Raichle, M.E. A functional anatomical study of unipolar depression. J. Neurosci. 1992, 12, 3628–3641. [Google Scholar] [PubMed]
- Brody, A.L.; Saxena, S.; Mandelkern, M.A.; Fairbanks, L.A.; Ho, M.L.; Baxter, L.R. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 2001, 50, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Harel, E.V.; Roth, Y.; Braw, Y.; Most, D.; Katz, L.N.; Sheer, A.; Gersner, R.; Zangen, A. Deep transcranial magnetic stimulation over the prefrontal cortex: Evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. 2009, 2, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Isserles, M.; Rosenberg, O.; Dannon, P.; Levkovitz, Y.; Kotler, M.; Deutsch, F.; Lerer, B.; Zangen, A. Cognitive-emotional reactivation during deep transcranial magnetic stimulation over the prefrontal cortex of depressive patients affects antidepressant outcome. J. Affect. Disord. 2011, 128, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.J.; Fletcher, P.; Morris, J.; Kapur, N.; Deakin, J.F.; Frith, C.D. Neural activation during covert processing of positive emotional facial expressions. Neuroimage 1996, 4, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Drevets, W.; Rauch, S.; Lane, R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Drevets, W.; Rauch, S.; Lane, R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 2003, 54, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Salvadore, G.; Nugent, A.C.; Lemaitre, H.; Luckenbaugh, D.A.; Tinsley, R.; Cannon, D.M.; Neumeister, A.; Zarate, C.A., Jr.; Drevets, W.C. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage 2011, 54, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.C.; Meisenzahl, E.; Scheuerecker, J.; Rose, E.; Schoepf, V.; Wiesmann, M.; Frodl, T. Brain activation predicts treatment improvement in patients with major depressive disorder. J. Psychiatr. Res. 2011, 45, 1214–1222. [Google Scholar] [PubMed]
- Thompson, R.J.; Mata, J.; Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Gotlib, I.H. The role of attention to emotion in recovery from major depressive disorder. Depress. Res. Treat. 2013, 2013. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Freton, M.; Lemonge, C.; Delaveau, P.; Guionnet, S.; Wright, E.; Wiernik, E.; Bertasi, E.; Fossati, P. The dark side of self-focus: Brain activity during self-focus in low and high brooders. Soc. Cogn. Affect. Neurosci. 2014, 9, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Scheuerecker, J.; Meisenzahl, E.M.; Koutsouleris, N.; Roesner, M.; Schopf, V.; Linn, J.; Wiesmann, M.; Bruckmann, H.; Moller, H.J.; Frodl, T. Orbitofrontal volume reductions during emotion recognition in patients with major depression. J. Psychiatry Neurosci. 2010, 35, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.W.; Kawahara-Baccus, T.N.; Johnson, S.C. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage 2004, 22, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal limbic-cortical function and negative mood: Converging pet findings in depression and normal sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar]
- Joormann, J.; Cooney, R.E.; Henry, M.L.; Gotlib, I.H. Neural correlates of automatic mood regulation in girls at high risk for depression. J. Abnorm. Psychol. 2012, 121, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.K.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A.; et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar]
- Thompson, R.J.; Mata, J.; Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Gotlib, I.H. Maladaptive coping, adaptive coping, and depressive symptoms: Variations across age and depressive state. Behav. Res. Ther. 2010, 48, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Oertel-Knochel, V.; Reinke, B.; Feddern, R.; Knake, A.; Knochel, C.; Prvulovic, D.; Fusser, F.; Karakaya, T.; Loellgen, D.; Freitag, C.; et al. Verbal episodic memory deficits in remitted bipolar patients: A combined behavioural and FMRI study. J. Affect. Disord. 2013, 150, 430–440. [Google Scholar]
- Dietsche, B.; Backes, H.; Stratmann, M.; Konrad, C.; Kircher, T.; Krug, A. Altered neural function during episodic memory encoding and retrieval in major depression. Hum. Brain Mapp. 2014, 35, 4293–4302. [Google Scholar] [CrossRef] [PubMed]
- APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, Text Revision; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Frank, E.; Prien, R.F.; Jarrett, R.B.; Keller, M.B.; Kupfer, D.J.; Lavori, P.W.; Rush, A.J.; Weissman, M.M. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch. Gen. Psychiatry 1991, 48, 851–855. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P); New York State Psychiatric Institute, Biometrics Research: New York, NY, USA, 2002. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence (Wasi); Harcourt Assessment: San Antonio, TX, USA, 1999. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.; Lorr, M.; Dropplemen, L. Edits Manual: Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Williams, J.M.; Dritschel, B.H. Emotional disturbance and the specificity of autobiographical memory. Cogn. Emot. 1988, 2, 221–234. [Google Scholar] [CrossRef]
- AFNI. Available online: http://afni.nimh.nih.gov/afni (accessed on 15 April 2015).
- Lemogne, C.; Piolino, P.; Friszer, S.; Claret, A.; Girault, N.; Jouvent, R.; Allilaire, J.; Fossati, P. Episodic autobiographical memory in depression: Specificity, autonoetic consciousness, and self-perspective. Conscious. Cogn. 2006, 15, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Scott, J. Autobiographical memory in depression. Psychol. Med. 1988, 18, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.; Teasdale, J.D. Adaptive and maladaptive self-focus in depression. J. Affect. Disord. 2004, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, J.D.; Segal, Z.V.; Williams, J.M.; Ridgeway, V.A.; Soulsby, J.M.; Lau, M.A. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol. 2000, 68, 615–623. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.D.; Bellgowan, P.S.F.; Bodurka, J.; Drevets, W.C. Functional Neuroimaging Correlates of Autobiographical Memory Deficits in Subjects at Risk for Depression. Brain Sci. 2015, 5, 144-164. https://doi.org/10.3390/brainsci5020144
Young KD, Bellgowan PSF, Bodurka J, Drevets WC. Functional Neuroimaging Correlates of Autobiographical Memory Deficits in Subjects at Risk for Depression. Brain Sciences. 2015; 5(2):144-164. https://doi.org/10.3390/brainsci5020144
Chicago/Turabian StyleYoung, Kymberly D., Patrick S. F. Bellgowan, Jerzy Bodurka, and Wayne C. Drevets. 2015. "Functional Neuroimaging Correlates of Autobiographical Memory Deficits in Subjects at Risk for Depression" Brain Sciences 5, no. 2: 144-164. https://doi.org/10.3390/brainsci5020144





