Cryoprotective Effects of Protein Hydrolysates Prepared from By-Products of Silver Carp (Hypophthalmichthys Molitrix) on Freeze-Thawed Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Hydrolysates Preparation
2.3. Characterization of the Hydrolysates
2.3.1. DH
2.3.2. Zeta Potential
2.3.3. Molecular Weight Distribution
2.3.4. Amino Acid Composition
2.4. Preparation of Freeze-Thawed Surimi for Cryoprotection Study
2.5. Extraction of Actomyosin from Unfrozen and Freeze-Thawed Surimi
2.6. Investigation of the Actomyosin Samples
2.6.1. Ca2+-ATPase Activity
2.6.2. Total Sulfhydryl Content
2.6.3. Surface Hydrophobicity
2.7. Texture Analysis of Heat-Set Unfrozen and Freeze-Thawed Surimi Gels
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Hydrolysates (PH and AH) Characterization
3.2. Amino Acid Analysis of PH and AH
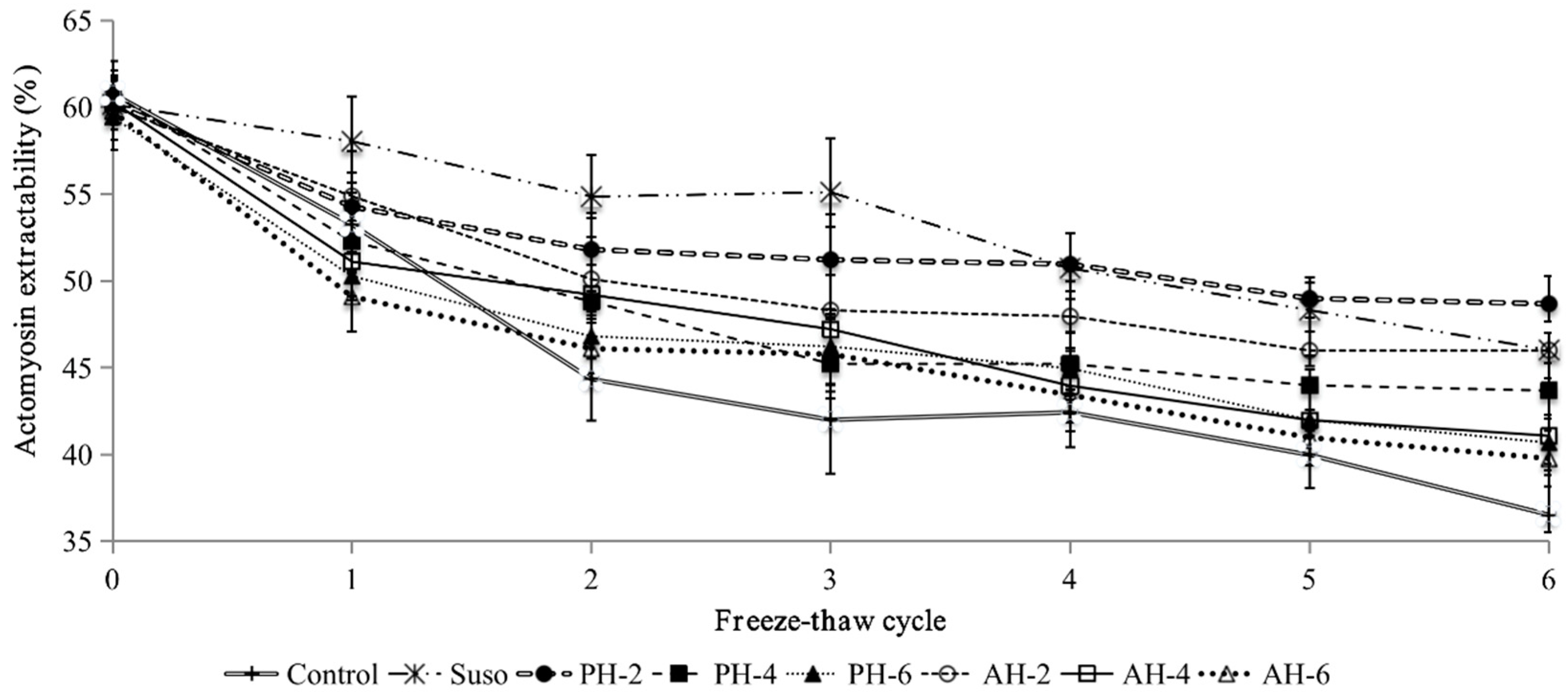
3.3. Actomyosin Extractability of Unfrozen and Freeze-Thawed Surimi
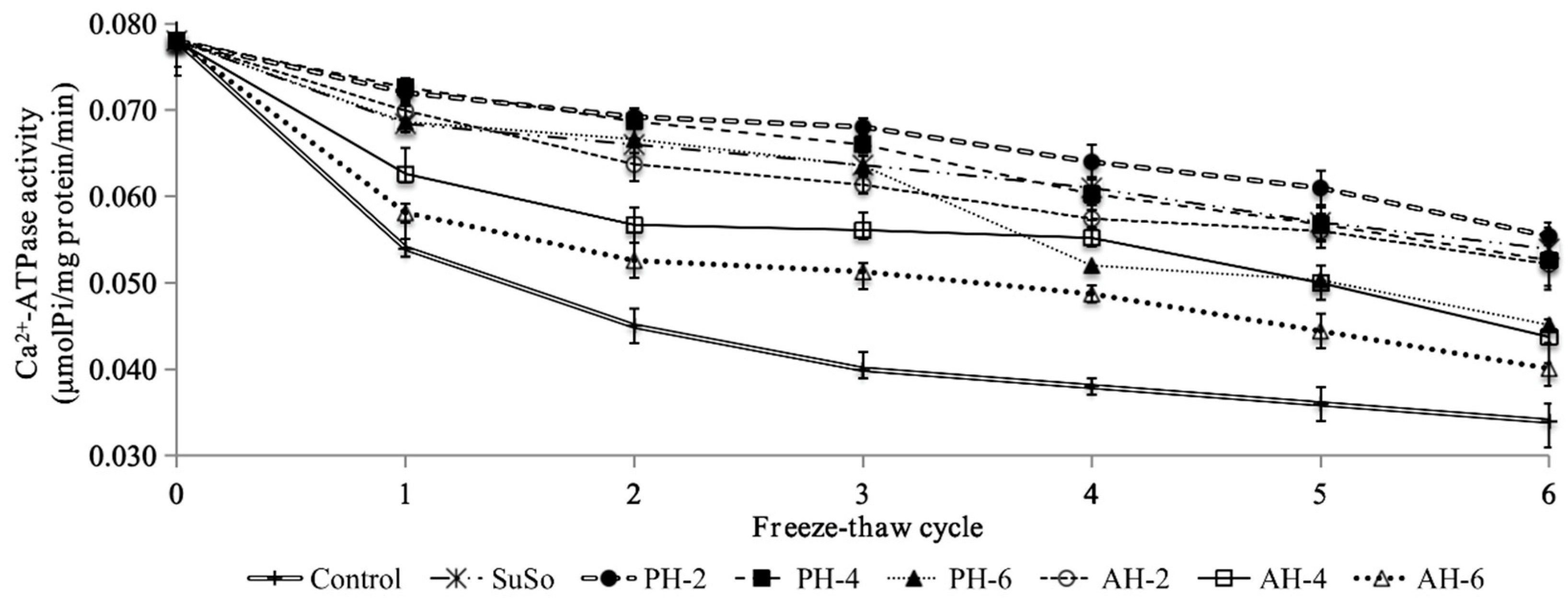
3.4. Actomyosin Ca2+-ATPase Activity
3.5. Total Sulfhydryl Content of Actomyosin
3.6. Surface Hydrophobicity of Actomyosin
3.7. Textural Properties of Heat-Set Unfrozen and Freeze-Thawed Surimi Gels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. FAO-Yearbook of Fishery Statistics: Aquaculture Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; p. 30. [Google Scholar]
- Paker, I.; Beamer, S.; Jaczynski, J.; Matak, K.E. Compositional characteristics of materials recovered from headed gutted silver carp (Hypophthalmichthys molitrix) by isoelectric solubilization and precipitation using organic acids. J. Food Sci. 2013, 78, E445–E451. [Google Scholar] [CrossRef]
- Torres, J.A.; Chen, Y.C.; Rodrigo-Garcia, J.; Jaczynski, J. Recovery of by-products from sea food processing streams. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 65–90. [Google Scholar]
- Liu, Y.L.; Li, X.H.; Chen, Z.J.; Yu, J.; Wang, F.X.; Wang, J.H. Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem. 2014, 151, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.W.Y.; Liceaga, A.M.; Li-Chan, E.C.Y. Pacific Hake (Merluccius Productus) hydrolysates as cryoprotective agents in frozen Pacific Cod fillet mince. J. Food Sci. 2009, 74, C588–C594. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. Inhibition of ice crystal growth in ice cream mix by gelatin hydrolysate. J. Agr. Food Chem. 2007, 55, 10918–10923. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S. Cryoprotective and antioxidative effects of gelatin hydrolysate from unicorn leatherjacket skin. Int. J. Refrig. 2015, 49, 69–78. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Cryoprotective effect of gelatin hydrolysate from blacktip shark skin on surimi subjected to different freeze-thaw cycles. LWT Food Sci. Technol. 2012, 47, 437–442. [Google Scholar] [CrossRef]
- Mueller, J.P.; Liceaga, A.M. Characterization and cryoprotection of invasive silver carp (Hypophthalmicthys molitrix) protein hydrolysates. J. Aquat. Food Prod. T. 2014. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.F.; Yang, N.; Xu, B.C.; Jin, Z.Y.; Xu, X.M. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhao, J.; Chen, L.; Zhou, Y.F.; Wu, J.H. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Wu, J.H.; Rong, Y.Z.; Wang, Z.W.; Zhou, Y.F.; Wang, S.Y.; Zhao, B. Isolation and characterisation of sericin antifreeze peptides and molecular dynamics modelling of their ice-binding interaction. Food Chem. 2015, 174, 621–629. [Google Scholar] [CrossRef]
- Korzeniowska, M.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Effects of fish protein hydrolysate and freeze–thaw treatment on physicochemical and gel properties of natural actomyosin from Pacific cod. Food Chem. 2013, 138, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins, 1st ed.; Elsevier Applied Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Wang, L.; Xiong, G.Q.; Peng, Y.B.; Wu, W.J.; Li, X.; Wang, J.; Qiao, Y.; Liao, L.; Ding, A.Z. The cryoprotective effect of different Konjac Glucomannan (KGM) hydrolysates on the glass carp (Ctenopharyngodon idella) myofibrillar during frozen storage. Food Bioprocess Tech. 2014, 7, 3398–3406. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Vasyukova, I.; Muratov, D.; Rybkin, I.; Bratashov, D.; Lapanje, A.; Il’inikh, I.; Kolesnikov, E.; Kuznetsov, D. Effect of GO on bacterial cells: Role of the medium type and electrostatic interactions. Mater. Sci. Eng. C. 2019. [Google Scholar] [CrossRef]
- Wang, W.L.; Chen, M.S.; Wu, J.H.; Wang, S.Y. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. LWT Food Sci. Technol. 2015, 63, 878–885. [Google Scholar] [CrossRef]
- Kim, J.S.; Damodaran, S.; Yethiraj, A. Retardation of ice crystallization by short peptides. J. Phys. Chem. A. 2009, 113, 4403–4407. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Zhou, Y.F.; Wang, S.Y.; Wang, Z.W.; Wu, Y.; Guo, X.Q. Laboratory-scale extraction and characterization of ice-binding sericin peptides. Eur. Food Res. Technol. 2013, 236, 637–646. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.H.; Zhang, L.; Chen, X.; Wu, Y.; Liu, J.H.; Geng, X.Q.; Wang, Z.W.; Wang, S.Y. Investigation of the physiochemical properties, cryoprotective activity and possible action mechanisms of sericin peptides derived from membrane separation. LWT Food Sci. Technol. 2017, 77, 532–541. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, A.K.; Do, H.; Park, K.S.; Moh, S.H.; Chi, Y.M.; Kim, H.J. Structural Basis for antifreeze activity of ice-binding protein from arctic yeast. J. Biol. Chem. 2012, 287, 11460–11468. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Morikawa, J.; Hashimoto, T. Effect of amino acids on the eutectic behavior of NaCl solutions studied by DSC. Cryobiology 2005, 50, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Badii, F.; Howell, N.K. Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (Gadus morhua). J. Agric. Food Chem. 2002, 50, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- Godiksen, H.; Hyldig, G.; Jessen, F. Sarcoplasmic reticulum Ca2+-ATPase and cytochrome oxidase as indicators of frozen storage in cod (Gadus morhua). J. Food Sci. 2003, 68, 2579–2585. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X.M. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef]
- Benjakul, S.; Sutthipan, N. Muscle changes in hard and soft shell crabs during frozen storage. LWT Food Sci. Technol. 2009, 42, 723–729. [Google Scholar] [CrossRef]




| Sample | Crude Protein (g/100 g) | Ash (g/100 g) | Moisture (g/100 g) |
|---|---|---|---|
| Defatted by-products | 89.7 ± 2.52 | 9.75 ± 0.88 | 3.72 ± 0.61 |
| PH | 85.2 ± 3.11 | 9.74 ± 0.12 | 3.52 ± 0.14 |
| AH | 84.3 ± 3.25 | 9.79 ± 0.45 | 3.66 ± 0.10 |
| Sample | DH (%) | ζ Potential (mV) | Relative Proportion (%) of Each Molecular Weight | ||||
|---|---|---|---|---|---|---|---|
| ≥7500 Da | 2027 Da | ~1420 Da | 286–780 Da | ≤138 Da | |||
| PH | 13.2 (±1.5) a | −23.8 (±2.4) a | 7.7 (±1.6) a | 32.6 (±2.8) a | 37.4 (±3.1) b | 16.8 (±2.4) b | 4.7 (±1.2) a |
| AH | 12.9 (±1.1) a | −23.1 (±2.8) a | 5.1 (±0.9) a | - | 50.6 (±5.2) a | 39.1 (±2.6) a | 3.5 (±0.9) a |
| AA (mg/g) | PH | AH | ||||||
|---|---|---|---|---|---|---|---|---|
| TAA | Content (%) | FAA | Content (%) | TAA | Content (%) | FAA | Content (%) | |
| Asp | 99.8 (±3.2) | 12.33 (±0.40) | 0.8 (±0.4) | 2.75 (±1.37) | 96.7 (±2.8) | 11.85 (±0.34) | 1.2 (±0.5) | 5.91 (±2.46) |
| Glu | 120.9 (±4.5) | 14.93 (±0.56) | 1.5 (±0.2) | 5.15 (±0.69) | 123.4 (±3.3) | 15.13 (±0.40) | 0.7 (±0.2) | 3.45 (±0.98) |
| Asn | 0.4 (±0.3) | 0.05 (±0.03) | 0.4 (±0.3) | 1.37 (±1.03) | 0.5 (±0.2) | 0.06 (±0.02) | 0.5 (±0.2) | 2.46 (±0.98) |
| Gln | 1.8 (±0.4) | 0.22 (±0.05) | 1.8 (±0.4) | 6.18 (±1.37) | 1.0 (±0.2) | 0.12 (±0.02) | 1.0 (±0.2) | 4.93 (±0.98) |
| Thr | 33.1 (±1.8) | 4.09 (±0.22) | 0.8 (±0.3) | 2.75 (±1.03) | 38.4 (±2.9) | 4.71 (±0.36) | 0.2 (±0.1) | 0.98 (±0.49) |
| Ser | 35.4 (±4.1) | 4.37 (±0.51) | 1.0 (±0.1) | 3.44 (±0.34) | 38.6 (±4.4) | 4.73 (±0.54) | 0.3 (±0.1) | 1.48 (±0.49) |
| Gly | 30.0 (±2.2) | 3.71 (±0.27) | 0.5 (±0.2) | 1.72 (±0.69) | 32.3 (±1.5) | 3.96 (±0.18) | 0.5 (±0.4) | 2.46 (±1.96) |
| Ala | 48.4 (±3.6) | 5.98 (±0.44) | 0.7 (±0.2) | 2.41 (±0.69) | 51.3 (±3.5) | 6.29 (±0.43) | 0.4 (±0.3) | 1.97 (±1.48) |
| Val | 39.2 (±5.1) | 4.84 (±0.63) | 1.9 (±0.7) | 6.53 (±2.40) | 36.6 (±2.8) | 4.49 (±0.34) | 2.3 (±0.2) | 11.33 (±0.98) |
| Cys | 2.9 (±1.4) | 0.36 (±0.17) | - | - | 3.5 (±0.9) | 0.43 (±0.11) | - | - |
| Met | 22.7 (±4.3) | 2.80 (±0.53) | 1.3 (±0.4) | 4.47 (±1.37) | 17.6 (±4.7) | 2.16 (±0.58) | 2.2 (±0.2) | 10.84 (±0.98) |
| Ile | 29.8 (±3.0) | 3.68 (±0.37) | 1.4 (±0.6) | 4.81 (±2.06) | 31.5 (±4.7) | 3.86 (±0.58) | 0.5 (±0.1) | 2.46 (±0.49) |
| Leu | 61.4 (±5.5) | 7.58 (±0.68) | 1.8 (±0.5) | 6.18 (±1.72) | 63.7 (±2.1) | 7.81 (±0.26) | 0.9 (±0.3) | 4.43 (±1.48) |
| Tyr | 24.7 (±3.6) | 3.05 (±0.44) | 2.1 (±0.8) | 7.22 (±2.75) | 20.6 (±1.5) | 2.53 (±0.18) | 1.3 (±0.3) | 6.40 (±1.48) |
| Phe | 30.0 (±2.7) | 3.71 (±0.33) | 1.9 (±0.3) | 6.53 (±1.03) | 27.9 (±2.4) | 3.42 (±0.29) | 1.3 (±0.1) | 6.40 (±0.49) |
| Lys | 73.2 (±4.0) | 9.04 (±0.49) | 6.1 (±0.5) | 20.96 (±1.72) | 75.6 (±5.5) | 9.27 (±0.67) | 0.9 (±0.2) | 4.43 (±0.98) |
| Trp | - | - | - | - | - | - | - | - |
| His | 16.5 (±3.9) | 2.04 (±0.48) | 1.0 (±0.2) | 3.44 (±0.69) | 17.2 (±2.0) | 2.11 (±0.24) | 1.5 (±0.4) | 7.39 (±1.96) |
| Arg | 39.6 (±1.1) | 4.89 (±0.14) | 2.7 (±0.3) | 9.28 (±1.03) | 33.8 (±1.4) | 4.15 (±0.17) | 3.1 (±0.5) | 15.27 (±2.46) |
| Pro | 102.0 (±6.2) | 12.60 (±0.76) | 1.4 (±0.4) | 4.81 (±1.37) | 106.7 (±3.6) | 13.09 (±0.44) | 1.5 (±0.2) | 7.39 (±0.98) |
| Total | 809.6 | 100 | 29.1 | 100 | 815.4 | 100 | 20.3 | 100 |
| Sample | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) | ||||
|---|---|---|---|---|---|---|---|---|
| UF | FT | UF | FT | UF | FT | UF | FT | |
| Control | 1328 (±38.00) cB | 1889 (±67.00) aA* | 0.9220 (±0.02) aB | 1.4380 (±0.05) aA* | 0.6340 (±0.03) aB | 0.9590 (±0.03) aA* | 702 (±47.00) cB | 1117 (±85.00) aA* |
| SuSo | 1565 (±52.00) aA | 1422 (±70.00) bB | 0.9300 (±0.03) aA | 0.9280 (±0.06) bA | 0.6760 (±0.02) aA | 0.6690 (±0.04) bA | 943 (±36.00) aA | 818 (±72.00) bA |
| PH-2 | 1417 (±27.00) bcA | 1389 (±36.00) bA | 0.9210 (±0.06) aA | 0.9180 (±0.06) bA | 0.6780 (±0.03) aA | 0.6600 (±0.07) bA | 815 (±42.00) bcA | 781 (±68.00) bA |
| PH-4 | 1506 (±44.00) abA | 1426 (±51.00) bA | 0.9250 (±0.02) aA | 0.9210 (±0.07) bA | 0.6800 (±0.06) aA | 0.6650 (±0.05) bA | 877 (±27.00) abA | 802 (±48.00) bA |
| PH-6 | 1555 (±29.00) aA | 1485 (±39.00) bA* | 0.9280 (±0.03) aA | 0.8325 (±0.06) bA | 0.6910 (±0.02) aA | 0.6760 (±0.06) bA | 916 (±31.00) abA | 845 (±52.00) bA* |
| AH-2 | 1438 (±32.00) bcA | 1387 (±76.00) bA | 0.9230 (±0.05) aA | 0.7915 (±0.07) bA* | 0.6780 (±0.05) aA | 0.6650 (±0.06) bA | 835 (±52.00) abA | 789 (±39.00) bA |
| AH-4 | 1522 (±61.00) abA | 1415 (±72.00) bA | 0.9270 (±0.02) aA | 0.8325 (±0.05) bB | 0.6880 (±0.01) aA | 0.6720 (±0.09) bA | 882 (±74.00) abA | 820 (±45.00) bA* |
| AH-6 | 1598 (±27.00) aA | 1473 (±55.00) bB* | 0.9370 (±0.06) aA | 0.7995 (±0.07) bB* | 0.6990 (±0.06) aA | 0.6830 (±0.08) bA | 951 (±26.00) aA | 836 (±56.00) bB* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.-J.; Wang, F.-X.; Yu, J.; Li, X.-H.; Liu, Y.-L. Cryoprotective Effects of Protein Hydrolysates Prepared from By-Products of Silver Carp (Hypophthalmichthys Molitrix) on Freeze-Thawed Surimi. Appl. Sci. 2019, 9, 563. https://doi.org/10.3390/app9030563
Zhou W-J, Wang F-X, Yu J, Li X-H, Liu Y-L. Cryoprotective Effects of Protein Hydrolysates Prepared from By-Products of Silver Carp (Hypophthalmichthys Molitrix) on Freeze-Thawed Surimi. Applied Sciences. 2019; 9(3):563. https://doi.org/10.3390/app9030563
Chicago/Turabian StyleZhou, Wen-Juan, Fa-Xiang Wang, Jian Yu, Xiang-Hong Li, and Yong-Le Liu. 2019. "Cryoprotective Effects of Protein Hydrolysates Prepared from By-Products of Silver Carp (Hypophthalmichthys Molitrix) on Freeze-Thawed Surimi" Applied Sciences 9, no. 3: 563. https://doi.org/10.3390/app9030563




